Abstract
Excessive exposure to ultraviolet (UV) radiation causes premature aging of the skin, known as photoaging. UV radiation induces DNA damage, oxidative stress, inflammatory reactions, and degradation of extracellular matrix (ECM) proteins, contributing to the aged skin phenotype. The skin synthesizes vitamin D upon UVB exposure, which plays a pivotal role in the proper function of multiple body systems. Vitamin D protects skin from photo-damage by repairing cyclobutane pyrimidine dimers, reversing oxidative stress, and reducing chronic inflammation. Moreover, various epidemiological studies have identified vitamin D deficiency as a marker for common dermatological disorders. Improvement of clinical outcomes with vitamin D supplementation further suggests its protective role against skin pathologies. This review comprehensively covers the involvement of vitamin D in combating UV-induced photoaging and various skin disorders, highlighting the significance of maintaining vitamin D adequacy for healthy skin.
Keywords
Vitamin D, calcipotriol, calcitriol, photoaging, skin diseases, 1,25(OH)2D, 25(OH)D, UV radiationIntroduction
Vitamin D is a lipid-soluble essential micronutrient that gets converted into a multifunctional seco-steroid molecule in the human body. The two main forms, vitamin D2 (ergocalciferol), and D3 (cholecalciferol) can be obtained in limited quantities (~20%) from plant and animal-based diets [1]. However, ultraviolet (UV) radiation-activated cutaneous synthesis is considered the main source of vitamin D. Cutaneously generated pre-vitamin D gets converted to vitamin D3 via thermal isomerization and enters the blood circulation bound to vitamin D binding protein [2, 3]. Once in the circulation, vitamin D is hydroxylated into 25-hydroxyvitamin D [25(OH)D; calcidiol] in the liver, and then in the kidney to 1,25-dihydroxyvitamin D [1,25(OH)2D; calcitriol], the physiologically active form of vitamin D [4]. Calcitriol can also be generated within peripheral target cells, functioning as a localized cytokine and a signaling molecule. The active 1,25(OH)2D that gets circulated through the bloodstream exerts its biological actions by binding to the vitamin D receptor (VDR) in the tissues through genomic and non-genomic pathways [5]. The generation of calcitriol from sunlight exposure and dietary sources is illustrated in Figure 1.
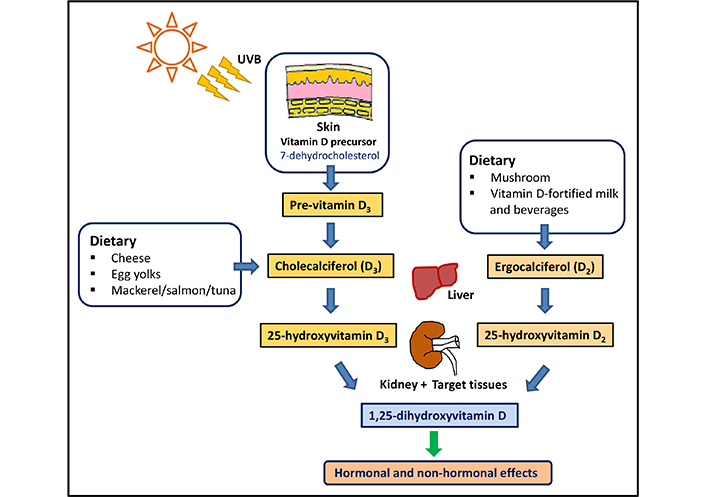
Generation of calcitriol in human body. Vitamins D2 and D3 are synthesized in the human body and converted in to 25-hydroxyvitamin D [25(OH)D] in the liver and to the active form 1,25-dihydroxyvitamin D [1,25(OH)2D] (calcitriol) in the kidney and target tissues. UVB: ultraviolet B
Note. Adapted from “Controlling chronic diseases and acute infections with vitamin D sufficiency’’ by Wimalawansa SJ. Nutrients. 2023;15:3623 (https://www.mdpi.com/2072-6643/15/16/3623). CC BY.
The physiological effect of the hormonal form of calcitriol is the regulation of calcium and phosphorous homeostasis via VDR-genomic interactions and non-genomic membrane effects. With the regulatory impact of the parathyroid hormone (PTH), calcitriol promotes normal bone development and ossification, preventing rickets, osteomalacia, and osteoporosis [6]. Further, vitamin D improves muscle size, cell growth, strength, and neuromuscular performance [7]. In addition to these musculoskeletal effects, vitamin D exerts a pleiotropic effect by controlling multiple body systems including respiratory, reproductive, nervous, gastrointestinal, and renal systems [5]. It plays a key role in modulating the immune system, overcoming acute infections such as sepsis and COVID-19, and preventing autoimmunity [8]. Therefore, vitamin D deficiency has been associated with a large range of diseases including autoimmune, cardiovascular, neurological disorders, and even cancer. The beneficial roles of vitamin D in human health are summarized in Figure 2.
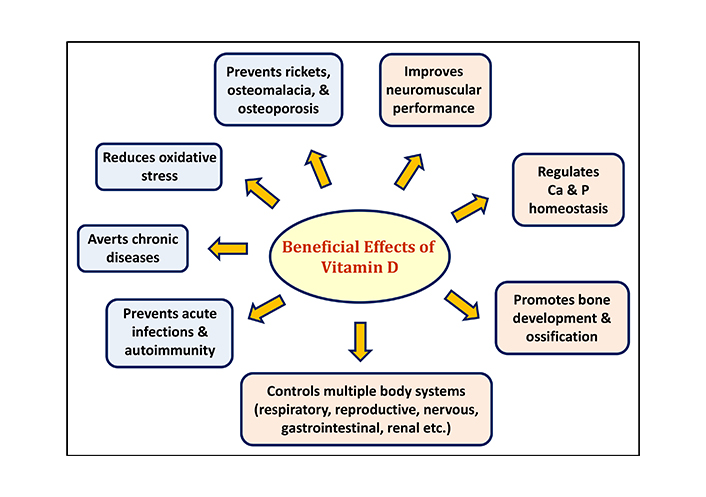
The role of vitamin D in health and diseases [9]
Note. Adapted from “Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging’’ by Wimalawansa SJ. Biology. 2019;8:30 (https://www.mdpi.com/2079-7737/8/2/30). CC BY.
Vitamin D deficiency is a global health problem affecting individuals of all ages, across the world. Based on a systematic review by Hilger et al. [10], 37.3% of 195 studies in 44 countries reported a mean 25(OH)D levels below 20 ng/mL (50 nmol/L). Vitamin D levels are generally higher in North and Latin Americans, Australians, and Europeans living in northern latitudes when compared with tenants in southern latitudes and close to the equator [11].
Humans are expected to generate more than 80% of vitamin D from direct exposure to sunlight, specifically to UVB radiation (280–315 nm). UVB photons photolyze the precursor 7-dehydrocholesterol (DHC) in the skin to previtamin D3, which gets isomerized later to form vitamin D3 [12, 13]. The amount of vitamin D that gets synthesized depends on the melanin content of the skin, duration of sun exposure, intensity of sunlight, and the ability of the skin to generate vitamin D [3, 5]. Due to increased UV absorption by melanin, more intense sun exposure is needed to produce vitamin D in darker skin [9]. Despite this beneficial effect, UV radiation also causes harm to the skin by inducing photoaging and skin cancers. This review provides knowledge on skin aging, mechanisms of skin photoaging, and the protective role of vitamin D against skin photoaging. The impact of vitamin D on skin diseases is also discussed giving emphasis on psoriasis, rosacea, seborrheic dermatitis (SD), atopic dermatitis (AD), congenital ichthyosis, vitiligo, and alopecia areata (AA).
Vitamin D and photoaging
Skin aging
Skin aging refers to changing the structural, cellular, and molecular characteristics in the dermis and epidermis of the skin, with age. Age-related structural skin changes include reduced thickness, increased wrinkles, loss of elasticity, changes in pigmentation, dryness, and sagging appearance. Aging also causes cellular level alterations such as flattening the dermo-epidermal junction, reducing melanocyte production, and declining Langerhans cells [14, 15]. Intrinsic factors including genetics and the natural aging process, and extrinsic factors such as exposure to sun, smoking, alcohol, environmental pollutants, and stressors contribute to skin aging [16, 17]. Intrinsic aging occurs due to programmed aging and cellular senescence associated with oxidative stress. Oxidative stress arises when there is an imbalance between reactive oxygen species (ROS) and the ability of a biological system to neutralize the reactive products. The increased levels of ROS are attributed to the leakage of electrons from damaged mitochondria, decreased protection from anti-oxidants, reduced growth factors, and hormonal activity. Intrinsic aging causes fine wrinkles and thinning of epidermis, whereas extrinsically damaged skin shows deep wrinkles, thickened epidermis, laxity, dryness, mottled discoloration, dullness and roughness [18–20].
Skin photoaging
UV radiation is the most prominent exogenous factor, giving rise to cutaneous photo-damage on areas of prolonged sun exposure. UV radiation consists of three major regions: UVA, the weakest, (λ = 320−400 nm), UVB (λ = 280−320 nm), and UVC, the strongest (λ = 100−280 nm). However, UVC is completely absorbed by the ozone layer and does not reach the earth. Both UVA and UVB reach the earth at sufficient levels and can cause photoaging of the human skin [21]. UVB majorly causes UV-induced erythema and direct DNA damage contributing to mutagenesis and skin cancers [22]. The low-energy UVA is twenty times more abundant in the earth’s atmosphere and does not get blocked by glass. Although UVB gets predominantly absorbed by the skin’s epidermis, UVA penetrates deep into dermal layers. Further, UVA causes indirect DNA damage and degradation of collagen and elastin fibers via oxidative stress pathways, making it the primary driver of photoaging [23].
Chromophores in human skin absorb UVA and undergo photo-excitation to initiate different reactions with molecular oxygen (O2) generating ROS species such as singlet oxygen (1O2), superoxide radical anion (O2·−), hydroxyl radical (OH·), and hydrogen peroxide (H2O2) [24]. UVA also induces the release of free iron (Fe) which indirectly generates (OH·), via the Fenton reaction, leading to oxidative stress in all skin cells [24].
UV radiation also facilitates the degradation of dermal extracellular matrix (ECM) components such as collagen, elastin, and glycoproteins. Collagen becomes fragmented and degraded primarily providing a wrinkled appearance of photoaged skin. The expression and secretion of matrix metalloproteinases (MMPs) from dermal fibroblasts are also enhanced, further degrading ECM and causing solar elastosis [16, 25].
MMPs are a family of endopeptidases participating in inflammatory processes regulating barrier function, inflammatory cytokines, and chemokine activity [26]. Previous studies have demonstrated that the expression of MMP-1, a key protease involved in photoaging, increased in the UV-irradiated skin cells. MMPs are directly responsible for collagen degradation, promoting photoaging of skin [27, 28].
Both the damaged DNA and degraded compounds of ECM trigger cellular inflammation in the skin. The two major signaling pathways, nuclear factor kappa B (NF-κB), and activator protein-1 (AP-1) pathways get activated by UV irradiation. Inflammatory cytokines are stimulated by the NF-κB pathway whereas MMPs are regulated by AP-1 [29]. A genome-wide RNA sequencing analysis identified a few cytokines [interleukin 6 (IL-6) and IL-24] and chemokines (CCL3, CCL20, CXCL1, CXCL2, CXCL3, and CXCL5) as the most highly up-regulated genes in human skin upon UV exposure [30]. Further, many cytokines including interleukins (IL-1, IL-3, IL-6, IL-8, and IL-33), tumor necrosis factor-alpha (TNF-α), transforming growth factor α (TGF-α), TGF-β, colony-stimulating factors (GM-CSF, M-CSF, G-CSF), high-mobility group box 1 (HMGB1), and platelet-derived growth factor (PDGF) are secreted from UV-irradiated epidermal keratinocytes [31].
UV irradiation also activates toll-like receptor 9 (TLR9), cyclic GMP-AMP synthase (cGAS), and stimulator of interferon genes (cGAS-STING) pathways in human keratinocytes. TLR9 is a transmembrane receptor that regulates innate immunity and cellular stress responses. Upon UV irradiation TLR9 expression gets activated in human keratinocytes by the two transcription factors, p53 and c-Jun [32]. Hours after UVB induced DNA damage, keratinocytes display an innate immune response activating cGAS-STING pathway, inducing apoptosis [33, 34].
Skin possesses diverse immune-competent cells, including Langerhans cells, macrophages, and mast cells. While triggering inflammation and senescence, both UVA and UVB also induce local and systemic immunosuppression of the skin, thus promoting photoaging [25]. UV-irradiated skin increases the expansion and activity of Treg cells, promoting the release of immunosuppressive cytokine IL-10 to induce a general immunosuppression. UVB radiation also converts L-tryptophan into 6-formylindolo (3,2-b) carbazole (FICZ), activating aryl hydrocarbon receptor (AhR), to regulate many immunosuppressive activities [25, 31].
Vitamin D against photo-damage
Vitamin D imposes a plethora of beneficial biological actions by mediating numerous endocrine and peripheral target cell functions. Skin, the location where vitamin D3 synthesis takes place, also benefits from the protective mechanisms of vitamin D in mitigating the impact of skin photoaging [5, 17, 19, 35].
Long-term exposure to solar radiation can result in a range of skin damages including acute sunburns, erythema, inflammation, increased melanogenesis, photoaging, and skin cancer [25]. Sunburn is an inflammatory response to erythemogenic doses of UVR, causing redness due to dermal vasodilation, edema, and increased inflammatory cell infiltration [36]. Many studies have investigated the efficacy of topical and oral vitamin D treatments against sunburns with promising results. In a double blinded, placebo-controlled interventional trial, Scott et al. [36] supplemented healthy individuals either with high doses of vitamin D3 or placebo, one hour after experimental sunburn induced by an erythemogenic dose of UVR. Based on the results, participants who received 200,000 IU vitamin D3 displayed a significant reduction in the expression of pro-inflammatory mediator TNF-α, and inducible nitric oxide (NO) synthase in skin biopsy specimens, when compared to the placebo. Further, a sustained reduction in skin redness after sunburn, enhancement of gene products involved in skin barrier repair, and increased skin expression of anti-inflammatory mediator arginase-1 were also evident with higher serum vitamin D3 levels [36]. UVB reduces the rate of DNA synthesis and calcipotriol, the synthetic analogue of 1,25(OH)2D, has protected cultured human keratinocytes against low doses of UVB (20 and 40 mJ/cm2) [37]. Camillo et al. [38] further investigated the efficacy of calcipotriol, in preventing UVB-induced photoaging in human dermal fibroblasts (HDFs). When isolated HDFs were stimulated with 100 nM calcipotriol for 24 h before exposing to UV, the cell proliferation improved comparative to the control. It also reduced UVB-induced ROS production, oxidative DNA damage, cellular senescence, and cell death by controlling the p53/p21 pathway, mitigating UVB-induced effects.
Role of vitamin D in preventing photoaging
Protection against DNA damage
UV radiation induces direct DNA damage via the formation of cyclobutane pyrimidine dimers (CPDs), and other mutations. UVR also indirectly damages the DNA through the actions of ROS and reactive nitrogen species [17]. Based on research conducted by Rybchyn et al. [39], unscheduled DNA synthesis was enhanced when UV irradiated human keratinocytes were treated with 1,25(OH)2D. Calcitriol increases the energy availability via enhanced glycolysis and activation of energy conserving processes such as mitophagy and autophagy, thus facilitating the repair of CPD. The treatment with over-irradiated metabolite derivatives 24-hydroxylumisterol3 [24(OH)L3] or 1,25(OH)2D at 1 × 10−10 M and higher concentrations have significantly reduced UV induced CPDs (P < 0.01) and 8-OHdG (P < 0.05) in UV-irradiated keratinocytes. These treatments have considerably increased (P < 0.05) ATP levels and extracellular acidification rate in the keratinocytes when compared with the UV vehicle [40]. Several other studies have also indicated the ability of 1,25(OH)2D to reduce UV-induced DNA damage in the skin [41–43].
Protection from oxidative stress
Vitamin D and its analogs carry the ability to reverse the UV-induced oxidative stress, which plays a major role in skin photoaging. In a study [44] conducted using human epidermal keratinocytes, vitamin D3, and lumisterol metabolites; 20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3, 1,20,23(OH)3D3, lumisterol, 20(OH)L3, 22(OH)L3, 20,22(OH)2L3, and 24(OH)L3 reversed UVB-mediated ROS production, in a dose-dependent manner. At 100 nM concentration, all tested derivatives significantly reduced oxidant formation in UVB-irradiated (50 mJ/cm2) human epidermal keratinocytes. Further, this study evidenced the involvement of protective mechanisms including the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) mediated antioxidant responses, p53-phosphorylation, and the induction of DNA repair for UV-induced cellular damage [44]. Nrf2 is a redox sensitive transcription factor that regulates cellular antioxidant defense responses against UV radiation and other environmental stresses [45]. Generally, the level of Nrf2 regulated antioxidant genes rapidly declines upon UV exposure in keratinocytes. The treatment with vitamin D3 and lumisterol derivatives strongly induced Nrf2 protein expression, mainly in the nucleus [44].
Vitamin D3 also protects the skin against photoaging by regulating p53 mediated pathways. The tumor suppressor protein p53 is activated upon exposure to UV radiation to control cellular responses to DNA damage. Activation of p53 enhances nucleotide excision repair and promotes G1 phase cell cycle arrest to repair damaged DNA before replication. Further, p53 also contributes to the complex regulation of cellular antioxidant defense mechanisms and apoptosis of cells with irreparable DNA damage [46, 47]. The nuclear p53 level significantly increased in 1,25(OH)2D treated keratinocytes after UVR exposure (P < 0.01), when compared to untreated keratinocytes [46].
The free radical NO is produced from L-arginine by the activation of NO synthase upon skin exposure to UV radiation. Higher concentrations of NO can cause DNA damage and lipid peroxidation by acting as a free radical or combining with superoxide to generate a cytotoxic oxidant, peroxynitrite (ONOO–). Peroxynitrite causes DNA base damage predominantly at G and 8-oxoG nucleobases, producing a variety of nitration and oxidation products [48]. Peroxynitrite also activates the nuclear nick sensor enzyme, poly(ADP-ribose) polymerase (PARP), reducing cellular NAD+ and ATP levels in the skin, leading to necrotic cell death [49]. Excess levels of NO can further inhibit nucleotide excision repair, activate mitochondrial apoptotic pathways, and function as a pro and anti-apoptotic modulator in a situation-based manner [47, 50]. Treatment of human keratinocytes with 1,25(OH)2D prior to UV irradiation reduces NO products, providing protection against antioxidant damage from UV [46].
Calcitriol further protects the skin cells against UV-mediated oxidative stress by inducing the radical scavenger metallothionein (MT). The photo-protective effect of MTs was validated by the observation that the skin of MT-null mice acquired a greater number of sunburns and apoptotic cells than normal mice, after UVB irradiation [51]. A study conducted by Karasawa et al. [52] demonstrated that MT mRNA was induced by 1,25(OH)2D in a dose-dependent manner, in cultured epidermal keratinocytes and in liver, kidney, and skin tissues in vivo. Lee and Youn [53] also confirmed the 1,25(OH)2D mediated induction in expression of MT, in the basal layer of mice skin.
Vitamin D also protects the cells against oxidative stress by activating antioxidative enzymes. The defense enzymes superoxide dismutase, catalase, and glutathione peroxidase scavenge excess ROS and decrease the intracellular levels of malondialdehyde, mitigating oxidative stress [54–56].
Protection from inflammation of the skin
The effect of vitamin D in modulating innate and adaptive immune systems is well documented. Since inflammation is a major contributor to UV-induced photo-damage, protection from vitamin D is immensely beneficial in combating photoaging. The skin progresses through three pathological phases after UV exposure. The early vasodilatory phase involves increased blood flow, edema, erythema, and pain sensitivity. In the second inflammatory phase, neutrophils, monocytes and T cells accumulate in the skin along with increased expression and release of pro-inflammatory cytokines. The third resolution phase contains many anti-inflammatory events to counteract the acute inflammation. Anti-inflammatory cytokines such as IL-4, IL-10, TGF-β are secreted from the skin at this stage [25]. It has been shown that UVR treatment increases the secretion of IL-10 and TGF-β cytokines in human keratinocytes [57, 58]. Calcitriol counteracts the inflammatory responses to UV photo-damage by suppressing inflammatory cytokines such as TNF, IL-1, interferon-gamma, and IL-2a while enhancing anti-inflammatory cytokines IL-4 and IL-10 [13, 59]. The proposed mechanisms through which vitamin D exerts its protective role against photoaging and skin diseases are depicted in Figure 3. The association between vitamin D and various dermatological conditions will be discussed in the next section.
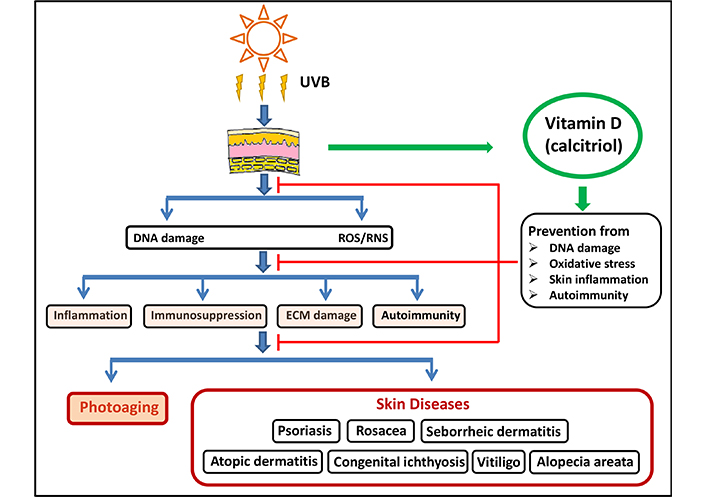
Protective mechanisms of vitamin D against photoaging and skin diseases. Vitamin D exerts its inhibitory power by preventing DNA damage, oxidative stress, skin inflammation, and autoimmunity, the factors that lead to photoaging and skin diseases. UVB: ultraviolet B; ROS/RNS: reactive oxygen species/reactive nitrogen species; ECM: extracellular matrix
Note. Adapted from “The impact of vitamin D on skin aging’’ by Bocheva G, Slominski RM, Slominski AT. Int J Mol Sci. 2021;22:9097 (https://www.mdpi.com/1422-0067/22/16/9097). CC BY.
Vitamin D and skin diseases
Recent studies have investigated the association between vitamin D deficiency with cutaneous disorders. The proven anti-inflammatory, immune-modulatory, and melanogenic properties of vitamin D have promoted clinicians to explore the therapeutic potential of topical and oral vitamin D analogs to treat various skin diseases. For this review, MEDLINE, PubMed, and Google Scholar databases were searched for articles including randomized controlled clinical trials (RTCs), other clinical observations, in vitro and in vivo research studies, and review articles related to vitamin D and skin diseases. The keywords for vitamin D metabolites and selected skin diseases were used in combination to extract the most relevant manuscripts. After duplicate removal and assessment of quality, the most relevant and recent articles for each disease were included for analysis. The diseases associated with a significant vitamin D deficiency are listed in Table 1 along with main references.
Skin diseases associated with vitamin D deficiency
| Skin diseases | Reference(s) |
|---|---|
| Psoriasis | [60, 61] |
| Rosacea | [62, 63] |
| Seborrheic dermatitis | [64, 65] |
| Atopic dermatitis | [66–68] |
| Congenital ichthyosis | [69, 70] |
| Vitiligo | [71–73] |
| Alopecia areata | [74–76] |
| Atopic eczema | [77] |
| Hidradenitis suppurativa | [78] |
Association of vitamin D with skin diseases
Psoriasis
Psoriasis is a chronic inflammatory autoimmune disease characterized by hyperproliferative epidermis with abnormal differentiation of keratinocytes. Erythematous skin plaques covered by hyperkeratotic scales are the main representations of the disease. Numerous epidemiological studies have suggested the possibility of an association between serum 25(OH)D levels and psoriasis [79–82].
In the meta-analysis conducted by Formisano et al. [79], psoriasis patients exhibited significantly lower serum 25(OH)D levels (21.0 ± 8.3 ng/dL) than controls (27.3 ± 9.8 ng/dL, P < 0.00001). In a case-controlled study with 120 psoriasis patients, Pokharel et al. [83] observed a statistically significant difference in the mean serum vitamin D levels in psoriatic patients than healthy controls (19.57 ± 6.85 ng/mL and 23.63 ± 6.40 ng/mL). Moreover, several review articles have also summarized topical vitamin D analogs as a successful treatment for psoriasis [84, 85]. In a systemic review, Zhao et al. [86] analyzed the success in psoriasis vulgaris treatment of vitamin D3 analog calcipotriol, and the combination of betamethasone dipropionate (500 µg/g) and calcipotriol (50 µg/g). Clinical observations suggested that the topical calcipotriol was well tolerated and efficacious for up to 20 weeks on a fixed-treatment regimen or up to 1 year when applied as needed. However, in the long-term application, calcipotriol/betamethasone dipropionate combination was more effective than calcipotriol alone [86]. Multiple research studies have also observed the success of oral vitamin D supplementation in treating psoriasis [87–90].
Rosacea
Rosacea is a chronic inflammatory skin disorder where patients experience redness, flushing, visible blood vessels, burning, and tingling sensations on the face. The association of rosacea and serum 25(OH)D level was previously investigated with mixed results. Some studies have indicated that the level of serum 25(OH)D was higher in rosacea patients [62], while others have reported the opposite with patients having low serum 25(OH)D levels [91]. A recent population-based cohort study utilizing 370,209 individuals from the United Kingdom indicated an inverse association between elevated serum 25(OH)D levels and risk of rosacea development, implicating the protective role of vitamin D against rosacea [63]. However, due to the mixed nature of observations, further investigations are required to determine the possible effect of vitamin D on rosacea.
Seborrheic dermatitis
Seborrheic dermatitis (SD) is another chronic inflammatory skin disease with a papulosquamous morphology, commonly affecting the scalp, face, and body folds. It is believed that an abnormal focal inflammatory immune response to metabolites of Malassezia yeast species, and enhanced sebaceous gland activity trigger SD. The common trend of SD is to increase the severity in winter and improve in summer with exposure to UV rays [64, 92]. Due to this periodic pattern, a correlation between vitamin D levels and SD was speculated and investigated in multiple studies. Numerous studies have shown the occurrence of vitamin D deficiency in SD patients [64, 65]. In a recent study, Akbaş et al. [64] demonstrated the association of earlier onset of SD with severe vitamin D deficiency (< 20 ng/mL), suggesting lower vitamin D levels as a risk factor for SD. The supplementation of vitamin D in treating SD has provided mixed results. In an early study, topical calcipotriol treatment failed to provide significant improvements for facial SD, allowing authors to conclude that it is ineffective against facial SD [92]. However, many subsequent studies have provided evidence for the protective effect of vitamin D supplements for SD. In a small scale clinical study conducted by Dimitrova et al. [93], supplementation of oral 1,600 IU cholecalciferol per day for 3 months reduced the recurrence of SD by 65.6%, in a patient group with low levels of serum 25(OH)D. The author observed that the patients who did not respond to the supplementation had higher baseline mean values of 25(OH)D, emphasizing that supplementation of cholecalciferol could be a viable option for patients with vitamin D deficiency [93]. In an early Japanese open trial, application of tacalcitol (1,24-dihydroxycholecalciferol) twice daily produced a 73% marked improvement in facial SD [94]. Basak and Ergin [95] divided sixty patients into two equal groups and treated with either calcipotriol (50 µg/mL) or betamethasone 17-valerate (1 mg/mL) solution twice daily for four weeks. Although both treatments displayed successful results, the betamethasone 17-valerate treatment was superior to that of calcipotriol treatment in all aspects.
In a retrospective study in Malaysia [96], 32 patients diagnosed with scalp SD were supplemented with twice-weekly doses of calcipotriol plus betamethasone dipropionate (CBD) gel (50 µg of calcipotriol plus 0.5 mg of betamethasone dipropionate per g of gel). After two weeks, more than 50% of the patients showed marked improvements and by the sixth week, 40.6% of the patients achieved complete clearance. After 10 weeks, 87.5% of the patients achieved complete clearance or marked improvements, proving the efficacy of the treatment.
Atopic dermatitis
Atopic dermatitis (AD) is a common chronic inflammatory skin disease affecting children and adults [97]. It is characterized by itchy, dry skin with eczematous plaques [98]. The risk factors associated with AD are genetic predisposition, epidermal dysfunction, and inflammation [99]. Multiple studies have examined the influence of vitamin D in the development of AD. Based on a study conducted in Brazil, 76.3% of the 152 AD patients indicated insufficient or deficient serum vitamin D levels [99]. In a Ukrainian study of 48 patients, vitamin D deficiency was observed among 62.5% of moderate and 90.9% of severe AD patients [100]. A study conducted in Türkiye [98] involving 96 AD patients and 90 healthy controls revealed that 58.3% of patients suffered from vitamin D deficiency. The level of vitamin D was statistically lower in severe AD patients than in mild or moderate patient groups. The authors also indicated that for each increment unit in serum vitamin D levels, the scoring atopic dermatitis (SCORAD) index decreased by 0.449 units emphasizing the protective role of vitamin D. In a meta-analysis conducted using 11 AD studies, Hattangdi-Haridas et al. [66]. identified a mean reduction in serum vitamin D concentration of 14 nmol/L in adults and 16 nmol/L in pediatric patients. When vitamin D level was measured in 41 pediatric and adult patients in Bangladesh, insufficiency and deficiency was detected in 75.6% of the AD patients [67]. Similar other studies conducted in Korea [101, 102], Canada [103], Poland [104] have also revealed an association between the severity of AD and lower vitamin D levels.
Furthermore, in a study conducted in Brazil, 116 AD patients received weekly oral vitamin D supplementation of 15,000 IU or 50,000 IU over 4 weeks, followed by a weekly 15,000 IU maintenance dose until three months. This raised their mean vitamin D level to 35.9 ng/mL compared to a baseline of 23.7 ng/mL (P < 0.001) while reducing the SCORAD index, and improving the categorization of AD in 82.7% of patients [99]. In a double blind, randomized placebo-controlled trial in Egypt, 92 pediatric patients were supplemented either with vitamin D3 1,600 IU/day or placebo plus baseline therapy of topical 1% hydrocortisone cream twice daily for 3 months. At the end of the treatment, serum vitamin D levels significantly improved in the treatment group along with a significant reduction in eczema area and severity index (EASI) score (20.42 ± 14.6 vs. 27.47 ± 10.11 P = 0.035) [105]. Based on a meta-analysis of five randomized controlled trials involving 304 patients, Park et al. [97] observed that the pediatric and adult patients’ SCORAD index and EASI scores significantly decreased in the vitamin D supplemented group when compared to the placebo. When supplemented with vitamin D, significant improvements could be observed in severe AD patients than that of individuals with moderate to mild AD. Moreover, high vitamin D doses such as > 2,000 IU/day accounted for favorable outcomes in the control of AD severity, whereas dosing of ≤ 2,000 IU/day failed to significantly control AD.
Congenital ichthyosis
Ichthyoses are a group of genetic disorders characterized by scaling, hyperkeratosis, dry skin, palmoplantar keratoderma, and erythroderma due to gene mutations associated with skin barrier formation. Inherited ichthyosis can be classified into ichthyosis syndromes and non-syndromic ichthyosis, where symptoms are visible only on the skin [106, 107]. A European study reported the serum 25(OH)D levels of 87 patients with inherited ichthyosis in which vitamin D deficiency was detected in harlequin ichthyosis (n = 2; median 25(OH)D = 7.0 ng/mL), rare syndromic subtypes (n = 3; 7.0 ng/mL), and keratinopathic ichthyosis (n = 17; 10.5 ng/mL). Reduced vitamin D levels were detected in transglutaminase-1 (TG1) proficient lamellar ichthyosis (n = 15; 8.9 ng/mL), Netherton syndrome (n = 7; 10.7 ng/mL), TG1-deficient lamellar ichthyosis (n = 12; 11.7 ng/mL), congenital ichthyosiform erythroderma (n = 13; 12.4 ng/mL), and X-linked ichthyosis (n = 8; 13.9 ng/mL). The median serum 25(OH)D level in ichthyosis vulgaris patients was 19.7 ng/mL (n = 10) indicating a deficiency, yet a relatively higher value compared to other types. Overall, the median 25(OH)D level of all the patients was 12.6 ng/mL, while 32 patients were deficient, 47 were insufficient, and 6 were normal with the serum vitamin D level [69]. A study conducted in France [108], the serum vitamin D level of 53 ichthyosis patients was determined. Of them, 88.7% of patients showed vitamin D levels below the optimum level of 30 ng/mL, 34% between 20–30 ng/mL, 26.4% between 10–20 ng/mL, and 28.3% < 10 ng/mL.
In a study conducted in India [109], seven severely vitamin D deficient children (< 4 ng/mL) with congenital ichthyosis (5 with autosomal recessive congenital ichthyosis; 2 with epidermolytic ichthyosis), were supplemented with a daily dose of 60,000 IU oral cholecalciferol for 10 days followed by a maintenance dose of 400 to 600 IU of cholecalciferol. During the vitamin D treatment, significant improvement in scaling was achieved by day 5, while further improvements were achieved by day 10 in 6 patients out of 7. The skin became near normal in all the cases of autosomal recessive congenital ichthyosis. This study emphasizes the remarkable clinical improvements of pediatric patients with ichthyosis by vitamin D supplementation, proving the clinical potential of vitamin D for pigmented skin types. However, few previous studies have indicated mixed results with vitamin D treatments in patients with ichthyosis. Topical calcipotriene 0.005% ointment was effective in treating one pediatric ichthyosis patient in Africa, displaying softening of the skin and desquamation after 6 weeks of the treatment. However, according to the same study, the skin condition was not improved in another patient when vitamin D3 is supplemented intramuscularly (600,000 IU) [110].
Vitiligo
Vitiligo is a common skin disorder characterized by depigmented patches on the skin due to destruction of melanocytes [71]. The etiology of the disease is multifactorial, with autoimmunity playing a significant role, along with the complex interactions of inflammatory, genetic, neural, and environmental factors [73]. Due to the immune protective and melanogenic effect of vitamin D, many studies have investigated its impact on controlling vitiligo.
In a cross-sectional case-control study involving 46 patients with vitiligo, significantly lower vitamin D levels were observed in patients when compared with healthy controls (P < 0.05) [111]. In a systematic review and meta-analysis, Upala and Sanguankeo [112] analyzed data from 1,200 patients from seven selected studies. Based on the analysis, the patient group’s pooled mean 25(OH)D level was significantly lower by 7.45 ng/mL, when compared to controls (P = 0.01) [112]. A meta-analysis that summarized data from 679 patients reported a positive association between serum 25(OH)D deficiency and the incidence of vitiligo [113]. However, no significant difference in the mean serum 25(OH)D level was detected between the vitiligo patients and the general population in Korea. When further analyzed, the authors observed a significant difference in the 25(OH)D level based on the disease duration and family history [114]. An Iranian study employing 98 patients and 98 age and gender-matched healthy individuals also failed to identify a significant difference in vitamin D levels between the patient and healthy groups [73]. In a systematic review which analyzed 27 randomized controlled trials (n = 1,198), calcipotriol was indicated as the most widely used (70%) topical vitamin D analogue for vitiligo, while tacalcitol and cholecalciferol accounted for 22% and 8%, respectively [115]. The effect of vitamin D in treating vitiligo has been investigated either as monotherapy or in combinations with phototherapy, including psoralen plus UVA (PUVA), narrow-band UVB (NB-UVB), or 308 nm excimer laser (EL). In a quasi-experimental study, 32 patients suffering from vitiligo were treated with systemic PUVA thrice weekly along with topical calcipotriol twice daily to only one side of the body. Based on the observations, the calcipotriol and PUVA treated-side displayed better results than PUVA alone, indicating the efficacy of mixed treatment [116].
Liu et al. [117] conducted a meta-analysis of fourteen studies (n = 642) and found that the combined effect of either calcipotriol or tacalcitol with NB-UVB provided superior outcomes to that of NB-UVB monotherapy alone. The authors also stated that when combined with NB-UVB treatment, tacalcitol exhibited better response to treatment than calcipotriol. However, none of the vitamin D analogs could enhance the efficacy of PUVA or EL treatments for vitiligo.
In another study, Kim et al. [118] compared the efficacy of vitamin D supplementation with an EL alone or as a combined therapy with vitamin D. The 26 patients employed in this study were randomly divided into two groups; the test group obtaining 308 nm EL with cholecalciferol injection, and the control group just with the laser treatment. After six months of treatment, 83.6% of patients in the test group displayed improvements in the vitiligo area scoring index (VASI) scores, whereas the control group showed a 54.7% improvement. They also concluded that significantly more patients in the combined group achieved VASI50 and VASI75 scores, indicating better improvements.
Alopecia areata
Alopecia areata (AA) is an autoimmune disorder characterized by sudden patchy hair loss in any hair-bearing area of the body. This condition usually begins in children and young adults, though can start at any age despite of the skin tone and gender. Currently, up to 2% of the global population is affected by AA [119] with a higher occurrence rate in females, especially in patients with late-onset disease as of age greater than 50 years [120].
Several studies have identified potential polymorphisms associated with AA [121]. Furthermore, several potential triggers have been identified including viral infections such as hepatitis B, C, and swine flu [122], vaccines for influenza, hepatitis, and coronavirus, [123, 124] and other factors such as stress and anxiety [125]. It has been showed that a lack of VDRs may adversely affect epidermal differentiation and hair follicles. Previous studies have also supported a correlation between lower levels of serum vitamin D, zinc, and folate levels in patients with AA when compared to controls [76, 126, 127].
Vitamin D has been employed to treat AA [128]. According to the early work of Xie et al. [129], a decrease in the expression of VDRs in hair follicles has led to active AA patches. In a recent research work by Dasankunju et al. [75] employing 30 AA patients and 30 age-and sex-matched controls, the mean serum vitamin D level detected was 30.2 ng/mL and 38.4 ng/mL, in cases and controls respectively. Vitamin D insufficiency/deficiency was found in 16 patients (53.3%) and a statistically significant relation was observed between serum vitamin D and AA. However, no significant relation was found between serum vitamin D levels and the gender, age, duration of the disease, occupation, number of alopecia lesions, or clinical types of AA [75].
Narang and co-workers [130] conducted a study using 22 adults with limited patchy AA. The observed total hair regrowth was 9% at 12 weeks, and ratio of severity of alopecia tool (SALT50) could be achieved in 46.2% (6/22). In another study by Çerman and co-workers [131], 48 patients with mild and moderate AA who failed topical steroids, displayed a total hair regrowth of 27.1% (13/36) at 12 weeks after undergoing calcipotriol monotherapy. Successful treatments have been reported combining calcipotriol with potent corticosteroids such as mometasone, and clobetasol compared to their monotherapeutic efforts [132, 133].
Treatment methods for AA also involve the use of intralesional vitamin D. In a recent study by Rashad and co-workers, injection of intralesional vitamin D (1 mL, 2.5 mg/mL) for 60 patients with patchy AA every 04 weeks for up to 03 sessions resulted in a hair regrowth score of 4 in 53% (n = 16). This was in comparison to 0% in patients treated with saline injection controls [134]. However, several side effects were reported including pinpoint bleeding, pain, and vasovagal attack in vitamin D treated patients than controls. Several studies summarized in Table 2 used vitamin D analogs as therapeutic agents against other dermatoses. Various vitamin D derivatives including calcitriol, calcipotriol, tacalcitol, maxacalcitol, and hexafluoro-1,25(OH)2D have been widely employed in treating skin diseases listed in Table 2.
Several dermatoses treated with vitamin D analogs
| Dermatosis | Treatment | Number of enrolled test patients | Response | Reference(s) |
|---|---|---|---|---|
| Epidermolytic acanthoma | Topical calcipotriol | 1 | Partial response. Some papules were present even after treatment. But those were lacking superficial keratotic material. | [135] |
| Clear cell acanthoma (CCA) | Topical calcipotriol | 1 | Complete and stable regression of CCA. Treatment has yielded complete regression after 2 months and no relapse after 1 year of treatment completion. | [136] |
| Bullous congenital ichthyosiform erythroderma (BCIE) | Topical maxacalcitol | 1 | Successful suppression of BCIE. A low dose (1.5 g/day) of topical maxacalcitol was sufficient, and there were no adverse effects on calcium metabolism. | [137] |
| Circumscribed plantar hypokeratosis | Topical calcipotriol | 1 | The lesion had been treated successfully and completely after 2 months. | [138] |
| Chronic kidney disease-associated pruritus | Calcipotriol solution | 13 | Partial response. Both validated modified pruritus assessment score (VMPAS) and visual analog scale (VAS) were significantly decreased (P < 0.05) after 2 and 4 weeks of calcipotriol treatment in comparison with a vehicle solution, respectively. | [139] |
| Recessive dystrophic epidermolysis bullosa (RDEB) | Vitamin D3 as a supplement/formula/enteral feed | 24 | All RDEB patients require a supplement or a formula or enteral/sip feed of vitamin D to maintain sufficient serum levels. The dose required to maintain sufficient serum levels increased with age. | [140] |
| Dystrophic epidermolysis bullosa (DEB) | Topical calcipotriol | 1 | Diminished itchiness and pain with complete wound closure within 14 days. | [141] |
| Calcipotriol | 12 | Partial response. Out of the 12 DEB patients, 6 had significantly healed wound area at the 14th day compared to placebo (88.4% vs. 65.5%, P < 0.05). | [142] | |
| Extramammary paget disease refractory | Calcipotriol | 3 | Partial response noted in 3/3 cases. Histopathological curing confirmed in 2/3 cases. | [143] |
| Sorafenib-associated hand-foot syndrome | Topical calcipotriol | 1 | Complete response. The lesions diminished during 14 days of treatment. | [144] |
| Linear atrophoderma of moulin | Topical calcipotriol | 1 | Partial response. After 3 months, lesion progression stopped but treated area partially improved. No further clinical improvement observed. | [145] |
| Oral leukoplakia | Calcipotriol gel | 20 | Partial response. Only 11 out of 20 patients had moderate to complete response. | [146] |
| Warts (facial verruca plana) | Topical calcipotriol | 1 | Complete response. Daily application completely resolved lesions after 8 weeks with no adverse reactions. | [147] |
| Disseminated superficial actinic porokeratosis (DSAP) | Calcipotriol and adapalene | 1 | Combination of calcipotriol and adapalene was successful in treating DSAP. | [148] |
| Axillary granular parakeratosis with osmidrosis | Topical maxacalcitol | 1 | Successful response. The pigmented lesion totally resolved within 5 months of treatment. | [149] |
Conclusions
UV-radiation exerts both beneficial and harmful effects on the skin. While UV-induced photoaging causes a detrimental impact on the skin, the UVB induced cutaneous synthesis of vitamin D protects the skin from various stresses. Upon UV exposure mechanisms involving DNA damage, oxidative stress, inflammation, apoptosis, ECM remodeling, collagen degradation, and immune suppression cause premature aging of the skin. Vitamin D can counteract the adverse effects of photo-damage by enhancing DNA repair systems, removal of ROS, anti-inflammation, and immunomodulation [150].
Despite the well documented role of vitamin D metabolites and analogs in treating psoriasis, emerging research supports the protective role of vitamin D against many common skin diseases. Vitamin D deficiency has been associated as a marker for certain dermatological conditions, emphasizing the importance of maintaining vitamin D adequacy for a healthy skin.
Abbreviations
| 1,25(OH)2D: | 1,25-dihydroxyvitamin D |
| 24(OH)L3: | 24-hydroxylumisterol3 |
| 25(OH)D: | 25-hydroxyvitamin D |
| AA: | alopecia areata |
| AD: | atopic dermatitis |
| cGAS: | cyclic GMP-AMP synthase |
| CPDs: | cyclobutane pyrimidine dimers |
| CSF: | colony-stimulating factor |
| ECM: | extracellular matrix |
| EL: | excimer laser |
| IL-6: | interleukin 6 |
| MMPs: | matrix metalloproteinases |
| MT: | metallothionein |
| NB-UVB: | narrow-band ultraviolet B |
| NO: | nitric oxide |
| Nrf2: | nuclear factor erythroid 2-related factor 2 |
| PUVA: | psoralen plus ultraviolet A |
| ROS: | reactive oxygen species |
| SCORAD: | scoring atopic dermatitis |
| SD: | seborrheic dermatitis |
| TGF-α: | transforming growth factor α |
| TLR9: | toll-like receptor 9 |
| TNF-α: | tumor necrosis factor-alpha |
| UV: | ultraviolet |
| VASI: | vitiligo area scoring index |
| VDR: | vitamin D receptor |
Declarations
Acknowledgments
The authors acknowledge the in-kind support provided by the Departments of Biosystems Technology, and Materials and Mechanical Technology at the Faculty of Technology, University of Sri Jayewardenepura, Homagama, Sri Lanka.
Author contributions
SSA: Conceptualization, Writing—original draft, Writing—review & editing. GAA: Writing—original draft, Writing—review & editing. RSD: Writing—review & editing.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.