Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
3Molecular Oncology and Viral Pathology Group, Research Center of IPO Porto (CI-IPOP)/RISE@CI-IPOP (Health Research Network), Portuguese Oncology Institute of Porto (IPO Porto), Porto Comprehensive Cancer Center (Porto.CCC), 4200-072 Porto, Portugal
ORCID: https://orcid.org/0000-0002-3909-5903
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
4Department of Zootechnics, School of Sciences and Technology, University of Évora, 7000-812 Évora, Portugal
5Comprehensive Health Research Center (CHRC), University of Évora, 7000-812 Évora, Portugal
ORCID: https://orcid.org/0000-0001-5572-6317
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
ORCID: https://orcid.org/0000-0001-7698-1595
Affiliation:
6Centro de Química de Vila Real (CQVR), Chemistry Department, University of Trás-os-Montes and Alto Douro, 5000-801 Vila Real, Portugal
ORCID: https://orcid.org/0000-0003-0775-4457
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
7Department of Veterinary Sciences, University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
ORCID: https://orcid.org/0000-0003-1036-3326
Affiliation:
8Laboratory for Integrative and Translational Research in Population Health (ITR), Research Center in Physical Activity, Health and Leisure (CIAFEL), Faculty of Sports, University of Porto, 4200-450 Porto, Portugal
9UCIBIO-Applied Molecular Biosciences Unit, Translational Toxicology Research Laboratory, University Institute of Health Sciences (1H-TOXRUN, IUCS-CESPU), 4585-116 Gandra, Portugal
ORCID: https://orcid.org/0000-0002-1464-8700
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
10CERNAS-IPV Research Centre, Polytechnique Institute of Viseu, 3504-510 Viseu, Portugal
11Polytechnique Institute of Viseu, Agrarian School of Viseu, Campus Politécnico, 3504-510 Viseu, Portugal
ORCID: https://orcid.org/0000-0001-6829-4867
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
10CERNAS-IPV Research Centre, Polytechnique Institute of Viseu, 3504-510 Viseu, Portugal
11Polytechnique Institute of Viseu, Agrarian School of Viseu, Campus Politécnico, 3504-510 Viseu, Portugal
ORCID: https://orcid.org/0000-0003-3941-799X
Affiliation:
12The Mountain Research Center of the Polytechnic Institute of Bragança (CIMO), Campus Santa Apolónia, 5300-253 Bragança, Portugal
13Associate Laboratory for Sustainability and Technology in Mountain Regions (SusTEC) of the Polytechnic Institute of Bragança, Campus Santa Apolónia, 5300-253 Bragança, Portugal
ORCID: https://orcid.org/0000-0001-8744-7814
Affiliation:
12The Mountain Research Center of the Polytechnic Institute of Bragança (CIMO), Campus Santa Apolónia, 5300-253 Bragança, Portugal
13Associate Laboratory for Sustainability and Technology in Mountain Regions (SusTEC) of the Polytechnic Institute of Bragança, Campus Santa Apolónia, 5300-253 Bragança, Portugal
ORCID: https://orcid.org/0000-0002-9050-5189
Affiliation:
14Department of Engineering, University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
15Institute for Systems and Computer Engineering, Technology and Science (INESC-TEC), 4200-465 Porto, Portugal
ORCID: https://orcid.org/0000-0001-6573-7511
Affiliation:
16Department of Genetics and Biotechnology, University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
17Animal and Veterinary Research Centre (CECAV), University of Trás-os-Montes and Alto Douro (UTAD), 5000-801 Vila Real, Portugal
ORCID: https://orcid.org/0000-0003-4461-7021
Affiliation:
18Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE), Faculty of Engineering of the University of Porto (FEUP), 4200-465 Porto, Portugal
19Associate Laboratory in Chemical Engineering (ALiCE), Faculty of Engineering of the University of Porto (FEUP), 4200-465 Porto, Portugal
ORCID: https://orcid.org/0000-0002-8605-0054
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
ORCID: https://orcid.org/0000-0001-9870-6666
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
17Animal and Veterinary Research Centre (CECAV), University of Trás-os-Montes and Alto Douro (UTAD), 5000-801 Vila Real, Portugal
20Department of Animal Science, School of Agrarian and Veterinary Sciences (ECAV), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
ORCID: https://orcid.org/0000-0003-3880-3442
Affiliation:
3Molecular Oncology and Viral Pathology Group, Research Center of IPO Porto (CI-IPOP)/RISE@CI-IPOP (Health Research Network), Portuguese Oncology Institute of Porto (IPO Porto), Porto Comprehensive Cancer Center (Porto.CCC), 4200-072 Porto, Portugal
21Research Department of the Portuguese League against Cancer — Regional Nucleus of the North (LPCC-NRN), 4200-177 Porto, Portugal
22Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal
23Virology Service, Portuguese Institute of Oncology (IPO), 4200-072 Porto, Portugal
24Biomedical Research Center (CEBIMED), Faculty of Health Sciences of Fernando Pessoa University (UFP), 4249-004 Porto, Portugal
ORCID: https://orcid.org/0000-0003-3010-8373
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
3Molecular Oncology and Viral Pathology Group, Research Center of IPO Porto (CI-IPOP)/RISE@CI-IPOP (Health Research Network), Portuguese Oncology Institute of Porto (IPO Porto), Porto Comprehensive Cancer Center (Porto.CCC), 4200-072 Porto, Portugal
18Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE), Faculty of Engineering of the University of Porto (FEUP), 4200-465 Porto, Portugal
19Associate Laboratory in Chemical Engineering (ALiCE), Faculty of Engineering of the University of Porto (FEUP), 4200-465 Porto, Portugal
25Postgraduate Programme in Adult Health (PPGSAD), Department of Morphology, Federal University of Maranhão (UFMA), São Luís 65020-070, Brazil
ORCID: https://orcid.org/0000-0002-2151-2449
Affiliation:
1Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
7Department of Veterinary Sciences, University of Trás-os-Montes e Alto Douro (UTAD), 5000-801 Vila Real, Portugal
Email: pamo@utad.pt
ORCID: https://orcid.org/0000-0001-9519-4044
Explor Med. 2024;5:416–433 DOl: https://doi.org/10.37349/emed.2024.00228
Received: February 01, 2024 Accepted: April 26, 2024 Published: June 18, 2024
Academic Editor: Andrea Giannini, Sapienza University of Rome, Italy
The article belongs to the special issue Spotlight on Cervical Cancer: Prevention, Early-Diagnosis, and Treatments
Aim: Aloysia citrodora has a long history of traditional use in treating various ailments. This study evaluated the in vivo chemopreventive efficacy and systemic toxicity of an extract of A. citrodora in a transgenic mouse model of HPV16 (human papillomavirus type 16)-induced cancer.
Methods: The experiment involved six groups (n = 5): group 1 (G1, wild-type (WT), water), group 2 (G2, HPV, water), group 3 (G3, WT, 0.013 g/mL), group 4 (G4, HPV, 0.006 g/mL), group 5 (G5, HPV, 0.008 g/mL), and group 6 (G6, HPV, 0.013 g/mL). Throughout the assay, humane endpoints, body weight, food, and water consumption were recorded weekly. The internal organs and skin of the mice were collected for analysis after they were sacrificed. Toxicological parameters that were studied included hematological and biochemical blood markers, splenic and hepatic histology, and hepatic oxidative stress.
Results: A. citrodora extract seems to reduce the incidence of dysplastic and in situ carcinoma skin lesions induced by HPV16 in this model, suggesting that dietary supplementation with concentrations of 0.008 g/mL and 0.013 g/mL may have beneficial chemopreventive effects.
Conclusions: The extract did not induce any concentration-dependent toxicological effects on any of the parameters included in the study, indicating a favorable toxicological profile under these experimental conditions.
Aloysia citrodora, commonly known as lemon verbena, is a medicinal plant that is native to South America, North Africa, and Southern Europe. It is commonly used to treat various symptoms such as insomnia, diarrhea, rheumatism, and flatulence [1]. The essential oil extracted from the dried leaves of A. citrodora is effective in treating problems such as anxiety, stress, insomnia, some types of depression, nervous fatigue, multiple sclerosis, tachycardia, rheumatism, psoriasis, anorexia, dyspepsia, and asthma [2, 3]. The biological activities associated with this plant are diverse and related to its chemical composition. Therefore, differences in its applicability can be explained by variations in chemical composition [4]. It should be noted that the essential oil of A. citrodora has an antitumor effect, demonstrated by Salama et al. [5], who showed that it blocked the growth of melanoma cells in vitro and impaired the growth of primary tumor cells in vivo. Other studies revealed that A. citrodora possesses antiviral, antioxidant, anti-inflammatory and immunomodulatory properties [6, 7]. Given these positive results, an in vivo study would be useful to further advance the preclinical study of A. citrodora extracts as a potential cancer chemopreventive agent. Thus, this work aims to investigate the in vivo effects of an aqueous extract of A. citrodora in a transgenic mouse model of cancer induced by high-risk human papillomavirus type 16 (HPV16) [8]. HPV stands as the foremost cause of cervical dysplasia and cervical cancer. The enduring presence of HPV is recognized as a primary risk factor for the emergence of cervical lesions and their recurrence after treatment [9]. The study by Bogani et al. [10], states that persistence of HPV for up to one year increased the risk of recurrence of grade 2 invasive carcinoma, while persistence after the first year did not appear to be a risk factor. High viral load and persistence of HPV are significant factors in the development of primary and recurrent cervical dysplasia. Patients with HPV16/18 and other high-risk HPV types showed different rates of persistence at various time intervals, influencing the risk of recurrence. Risk factors such as tobacco use, and epigenetic interactions play a role in HPV persistence and patient outcomes [10]. It is important to highlight the importance of adopting vaccination against HPV [11]. A study carried out by Bogani et al. [12], reports that individuals who have been vaccinated have a slightly lower risk of recurrence than individuals who have not been vaccinated. The K14-HPV16 model has previously been used to investigate the effects of natural compounds such as Laurus nobilis (laurel) [13], vegetable toxins like ptaquiloside from Pteridium spp. (bracken) [14], and dietary polyphenols such as curcumin and rutin [15]. In previous studies, K14-HPV16 mice showed a strong tendency to develop excessive hepatic and splenic inflammation, rendering them a suitable model for studying the effects of A. citrodora extract. The present study aimed to evaluate the in vivo chemopreventive efficacy and systemic toxicity of an A. citrodora extract in an HPV16-transgenic mouse model of cancer. The extract exhibited a tendency to decrease the incidence of HPV16-induced lesions and displayed a favorable toxicological profile.
A. citrodora extract was obtained from a Portuguese company (Celeiro®, batch number 03LUC1099J211S) collected in May 2021 and prepared through infusion extraction. Three concentrations of the A. citrodora extract (0.006 g/mL, 0.008 g/mL, and 0.013 g/mL) were prepared in tap water heated to 90°C using a French press. The resulting mixture was filtered and allowed to cool at room temperature (20–25°C) for 20 min before being given to animals as drinking water every two days.
The LC-DAD-ESI/MSn method (Dionex UltiMate 3000 UHPLC, Thermo Scientific, San Jose, CA, USA) was used to determine the phenolic profile. The compounds were separated and identified following the procedure described by Bessada et al. [16]. The extracts obtained were redissolved in ethanol:water (80:20, v/v) mixture at a concentration of 50 mg/mL. A double online detection was performed using a DAD with preferred wavelengths of 280, 330, and 370 nm, and a mass spectrometer (MS). The MS detection was carried out in negative mode, using a Linear Ion Trap LTQ XL MS (Finnigan, Thermo Fisher Scientific, San Jose, CA, USA) equipped with an ESI source. Phenolic compounds were identified based on their chromatographic behaviour, and UV-vis and mass spectra, by comparison with standard compounds, when available, and data reported in the literature, resulting in tentative identification. The Xcalibur® data system (Finnigan, Thermo Fisher Scientific, San Jose, CA, USA) was used for data acquisition. For quantitative analysis, a calibration curve was constructed for each available phenolic standard based on the UV-vis signal: apigenin-7-O-glucoside (y = 10,683x – 45,794; R² = 0.996); p-coumaric acid (y = 301,950x + 6,966.7; R² = 0.9999); quercetin-3-O-glucoside (y = 34,843x – 160,173; R² = 0.9998); and verbascoside (y = 124,233x – 18,873; R² = 0.9999). For the identification of phenolic compounds without a commercial standard, quantification was performed using the calibration curve of the most similar available standard. The results were expressed as mg/g of extract.
Thirty female Mus musculus of the FVB/n strain were used in this study: twenty transgenic (HPV+) and ten wild-type (HPV–, WT) mice aged 16–24 weeks. This age group is known to develop dysplastic skin lesions that later progress into squamous cell carcinomas [8]. Female mice were selected due to their ease of maintenance, as males are known to be highly aggressive and territorial [17]. The mouse strain used in this study was generously donated by Doctors Jeffrey Arbeit and Douglas Hanahan from the University of California, through the National Cancer Institute Mouse Repository (USA). The animals were genotyped using a polymerase chain reaction technique, as previously described [18].
This experimental work was conducted at the animal facility of the University of Trás-os-Montes e Alto Douro (UTAD) with the authorization of the Ethics Committee (approval no. 852-e-CITAB-2020_A_1-e- 122 CITAB-2021) and the Portuguese Veterinary Directorate (approval no. 014139). During the experimental period, the animals were fed a standard diet (4RF21 GLP, Mucedola, Italy) ad libitum and drank tap water. The environmental temperature was maintained at 21–25°C, with a light-dark cycle of 12 hours of light and 12 hours of dark, and relative humidity of 40–60%. The experimental study involved six groups: group 1 (G1, WT, water, n = 5), group 2 (G2, HPV, water, n = 5), group 3 (G3, WT, 0.013 g/mL, n = 5), group 4 (G4, HPV, 0.006 g/mL, n = 5), group 5 (G5, HPV16, 0.008 g/mL, n = 5) and group 6 (G6, HPV16, 0.013 g/mL, n = 5). Following the guidelines of Russell and Burch regarding the use of animals for experimental purposes, particularly the 3Rs (reduce, refinement, and replacement), and aiming to minimize the number of animals used in this experiment without compromising its scientific quality, we administered the highest concentration to healthy animals [19]. We assumed that if toxicity were induced by A. citrodora, it would likely occur at the highest concentration. The humane endpoints were evaluated on a weekly basis, including body mass, hair appearance, eyes, ears and whiskers, mental status, and presence of papilloma, among other factors, as described by Silva-Reis et al., in 2021 [20]. Animals that reached a score of 4 or higher were euthanized before completing the experimental assay [21]. Throughout the experiment, the animals’ weight, water intake and food consumption were recorded on a weekly basis. At the end of the experimental trial, the animals were sacrificed using a 10:1 ratio of ketamine (100 mg/kg) and xylazine (10 mg/kg), followed by exsanguination via cardiac puncture, in accordance with the Federation of European Laboratory Animal Science Association guidelines [22]. Blood was collected for biochemical and hematological analysis, as well as for the comet assay. The heart, lungs, liver, spleen, and kidneys were collected for histological analysis and to assess oxidative stress.
Blood was collected into lithium heparin tubes and centrifuged at 20,000 rpm for 15 min. The plasma supernatant was then stored at –80°C. For microhematocrit evaluation, blood was centrifuged in capillary tubes at 12,000 rpm for 5 min. Biochemical parameters were studied using previously frozen plasma to determine concentrations of albumin, total protein, creatinine, urea, and alanine aminotransferase in an autoanalyzer (PRESTIGE 24I, PZ CORMAY). To measure blood glucose levels, the animals fasted for 12 hours before a blood sample was taken and analyzed using a GlucoMen Areo 2K glucometer with Glucomen Areo Sensor brand strips.
The alkaline comet assay (pH > 13) was performed using previously established methods [23, 24]. Normal melting point agarose (1%) was used to cover the slides. Four slides were prepared for each animal, with two slides used for the assay with the enzyme and the other two for the assay without the enzyme. To create a cell suspension, 20 µL of blood was diluted in 200 µL of ice-cold phosphate-buffered saline (1×) in a 0.5 mL microtube. Sixty µL of cell suspension were mixed with 600 µL of low melting point agarose (1%) and placed on four pre-coated slides (two replicates per slide), with 70 µL gels. The slides were then immersed in a lysis solution and rinsed in a washing buffer three times. To measure oxidatively damaged deoxyribonucleic acid (DNA) precisely, namely 8-oxoguanines and other altered purines, two slides per animal were incubated with formamidopyrimidine DNA glycosylase (FPG) for 30 min. FPG is a DNA lesion-specific enzyme that converts oxidized purines into DNA single-strand breaks. The enzyme was donated by Professor Andrew Collins from the University of Oslo, Norway. To allow DNA unwinding, slides with and without FPG treatment were gently immersed in a freshly prepared alkaline electrophoresis solution. The cells underwent electrophoresis in the same solution for 20 min at 25 V and a current of 300 mA. Subsequently, the cells were immersed in phosphate-buffered saline, followed by distilled water, dehydrated, and air-dried. To enable visual scoring, DNA was stained with a 1 µg/mL solution of 4,6-diamidino-2-phenylindole (Sigma-Aldrich Chemical Company, Spain) and observed using an Olympus BX41 fluorescence microscope at 400×. The nuclei were visually classified into five classes ranging from 0 (no tail) to 4 (almost all DNA in the tail) [25]. One hundred nuclei were observed per replicate (200 per case) and the genetic damage index (GDI) was expressed on an arbitrary scale of 0 to 400 (arbitrary units, AU) using the following formula:
GDI = (n class 0 × 0) + (n class 1 × 1) + (n class 2 × 2) + (n class 3 × 3) + (n class 4 × 4)
The tissue samples were fixed in 10% neutral buffered formalin and processed for histological analysis. They were then stained with hematoxylin and eosin. All slides were examined under light microscopy using a Zeiss Axioplan 2 microscope. Image processing was performed using the LAS Advanced Analysis Software Bundle (No: 12730448). Lesions found in the ear pavilion and chest skin were classified as epidermal hyperplasia, dysplasia, papilloma, or carcinoma, as previously described [15]. The lung lesions were classified as mononuclear inflammation, atelectasis, emphysema, bleeding, airway desquamation, congestion, hyalinization, and hemorrhage. The liver lesions were classified as vacuolar degeneration, inflammation, and congestion, while the spleen showed hyperplasia and congestion [13, 15]. The heart lesions were classified as myofiber hyalinization, congestion, hemorrhage, and vascular ectasia. The kidney lesions were classified as inflammatory infiltrate, hydronephrosis, congestion, hyperemia, and vascular ectasia, as previously described [15, 26–29].
Liver and kidney samples were collected and stored at –80°C. The samples were homogenized using Tissuelyser II QIAGEN in a cold buffer solution (0.32 mM sucrose, 20 mM HEPES, 1 mM MgCl2, and 0.5 mM phenylmethyl sulfonyl fluoride, pH 7.4). The homogenate was then centrifuged at 15,000 × g for 20 min at 4°C (Prism R, Labnet International, USA), and the resulting supernatants were collected for further analysis. Superoxide dismutase (Cu/Zn-SOD) activity was measured by reducing nitroblue tetrazolium generated by the xanthine/xanthine oxidase system at 560 nm [30]. A standard curve was created using SOD (Sigma, S7446) from bovine erythrocytes (0–600 U·mL-1). Catalase (CAT) activity was determined at 240 nm using a previously published method [31] and calculated using bovine CAT (Sigma, C40) as a standard (0–696 U·mL-1). Glutathione peroxidase (GPx) activity was determined at 340 nm [32] using an extinction coefficient of 6.22 mM-1·cm-1. Glutathione reductase (GR) activity was determined by measuring the increase in absorbance of NADPH at 340 nm [33] using a molar extinction coefficient of 6.22 mM-1·cm-1. Glutathione S-Transferase (GST) activity was determined by measuring the increase in absorbance at 340 nm resulting from the conjugation of the thiol group of glutathione to the 1-Chloro-2,4-dinitrobenzene (CDNB) substrate [34], using a molar extinction coefficient of 9.60 mM-1·cm-1. The levels of glutathione were determined by measuring its reduced (GSH) and oxidized states (GSSG) using the fluorochrome ortho-phthalaldehyde with excitation and emission wavelengths of 320 nm and 420 nm, respectively [35]. Concentrations were estimated based on standard curves of GSH (Sigma, G4251) and GSSG (Sigma, G4626) (0–1 mM). The oxidative stress index (OSI) was calculated as the ratio between GSH and GSSG. The synthesis of reactive oxygen species (ROS) was estimated using the fluorescent probe 2,7-dichlorofluorescein diacetate, according to a previously published method [36], with excitation and emission wavelengths of 485 nm and 530 nm, respectively. A standard curve was constructed using DCF (Sigma, 35848) (0–500 µM). The levels of malondialdehyde (MDA), an indicator of lipid peroxidation (LPO), were measured at 535 nm (MDA-thiobarbituric acid adducts) and 600 nm (nonspecific adducts) wavelengths using the thiobarbituric acid method described elsewhere [37]. MDA levels (Sigma, 31640) were estimated based on a standard curve (0–1,000 µM) of malonaldehyde bis(dimethyl acetal). Carbonyls (CO) were estimated at 450 nm using the methodology described by Mesquita et al. [38].
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 9. The normal distribution was analyzed considering the Shapiro-Wilks test and the analysis of variances considering the Levene test. For results with normal distribution, we performed an ANOVA followed by the Bonferroni test. For results without normal distribution, we use non-parametric tests such as the Kruskal-Wallis test. We considered values of P < 0.05 to be statistically significant. The parameters that apply this rule are body weight, food and drink consumption, organ mass, humane endpoints, hematologic, and oxidative stress parameters. A Chi-square test was performed to analyze the association between histological lesions and groups.
Humane endpoints = (sum score per animal) / (number of animals per cage)
Weight gain = (final weight – initial weight) / (initial weight) × 100 (%)
Average daily consumption (water/food) per animal per day = [(initial mass) – (final mass)] / (number of days between weighing’s) × (number of animals per cage) (g)
Relative weight of organs = (organ weight) / (body weight) (g)
The phenolic profile of A. citrodora extract was analyzed and several compounds were identified and quantified (Table 1). Most of the identified molecules were previously described in the literature, as mentioned in Table 1. Verbascoside was the main compound present, followed by luteolin 7-O-diglucuronide.
Retention time (Rt) (min), maximum absorption in the visible region (λmax) (nm), deprotonated ion ([M-H]) (m/z), mass fragmentation (MS2) (m/z), tentative identification, and quantification (mg/g extract) of the phenolic compounds found (mean ± standard deviation)
| Peak | Rt (min) | λmax (nm) | [M-H] (m/z) | MS2 (m/z) | Tentative identification | Quantification (mg/g extract) | Reference used for identification |
|---|---|---|---|---|---|---|---|
| 1 | 4.35 | 280 | 461 | 315(8), 135(28) | Verbasoside | 4.35 ± 0.27 | [29] |
| 2 | 5.02 | 328 | 487 | 179(100) | Cistanoside F | 3.13 ± 0.09 | [29] |
| 3 | 5.64 | 358 | 389 | 371(23), 345(100), 209(51), 179(10), 121(12) | Theveside | 2.43 ± 0.1 | [29] |
| 7 | 11.51 | 330 | 639 | 621(12), 459(23) | β-hydroxy-verbascoside/β-hydroxy-isoverbascoside | 3.17 ± 0.04 | [29] |
| 8 | 11.97 | 330 | 639 | 621(15), 459(22) | β-hydroxy-verbascoside/β-hydroxy-isoverbascoside | 3.58 ± 0.17 | [29] |
| 9 | 12.69 | 344 | 637 | 351(100), 285(73) | Luteolin 7-O-diglucuronide | 17.02 ± 0.06 | [30] |
| 10 | 14.99 | 314 | 163 | 119(100) | p-Coumaric acid | 2.69 ± 0.05 | [30] |
| 11 | 15.91 | 335 | 621 | 351(100), 269(24) | Apigenin 7-O-diglucuronide | 8.29 ± 0.11 | [30] |
| 12 | 16.66 | 331 | 623 | 461(21), 315(7) | Verbascoside | 115.07 ± 0.21 | [30] |
| 13 | 17.99 | 354 | 651 | 351(100), 299(4) | Chrysoeriol 7-O-diglucuronide | 3.53 ± 0.03 | [30] |
| 14 | 18.69 | 331 | 623 | 461(19), 315(14) | Isoverbascoside | 2.62 ± 0.01 | [30] |
| 15 | 19.23 | 331 | 623 | 461(16), 315(5) | Forsythoside | 8.23 ± 0.36 | [30] |
| 16 | 21.23 | 350 | 491 | 315(100), 300(24) | Isorhamnetin 3-O-glucuronide | 0.76 ± 0.01 | [30] |
| 17 | 22.56 | 332 | 637 | 491(6), 461(63), 315(14) | Eukovoside | 2.06 ± 0.01 | [30] |
| 18 | 22.48 | 332 | 651 | 505(5), 475(27) | Martinoside | 2.36 ± 0.06 | [30] |
| Total caffeoyl phenylethanoid glycoside (mg/g extract) | 147.00 ± 0.15 | ||||||
| Total phenolic acids (mg/g extract) | 2.69 ± 0.05 | ||||||
| Total flavonoids (mg/g extract) | 29.6 ± 0.15 | ||||||
| Total phenolic compounds (mg/g extract) | 179.29 ± 0.35 | ||||||
Rt: retention time; λmax: maximum absorption in the visible region; [M-H]: deprotonated ion; MS2: mass fragmentation
No behavioral or phenotypic changes were observed throughout the trial. Additionally, no mortality occurred during the experimental period. Regarding humane endpoints, statistically significant differences were found between group 2 (HPV, water) and group 4 (HPV, 0.006 g/mL), as well as between group 4 (HPV, 0.006 g/mL) and group 5 (HPV, 0.008 g/mL) (P < 0.05) (Table 2). All animals gained weight during the experiment. No animal reached the threshold for euthanasia (score 4). The body weight increased throughout the experimental assay, but there were no statistically significant differences between groups. In terms of weight gain, there were also no statistically significant differences between groups (Table 2). Regarding to food and water consumption per animal, HPV16 animals exhibited higher consumption levels (Table 2). Finally, although there were no statistically significant differences between groups, the weight of all organs was higher in the HPV16 groups compared to the WT groups (Table 3).
Humane endpoints, body weight (g) at the beginning and at the end of the study, weight gain (%), average daily consumption of the food (g) and drink (mL) during the experimental trial (mean ± standard deviation)
| Group | Humane endpoints | Body weight | Weight gain (%) | Average daily consumption | ||
|---|---|---|---|---|---|---|
| Initial (g) | Final (g) | Food (g) | Drink (mL) | |||
| G1 WT water | 0.08 ± 0.18 | 29.65 ± 1.61 | 31.54 ± 3.29 | 6.24 ± 7.18 | 4.02 | 5.07 |
| G2 HPV water | 0.36 ± 0.411 | 27.91 ± 3.19 | 28.69 ± 3.84 | 2.60 ± 3.65 | 4.24 | 6.46 |
| G3 WT 0.013 g/mL | 0.24 ± 0.22 | 25.24 ± 1.27 | 26.72 ± 0.95 | 5.92 ± 2.19 | 4.26 | 5.18 |
| G4 HPV 0.006 g/mL | 1.04 ± 0.092 | 23.83 ± 1.98 | 25.07 ± 2.00 | 3.12 ± 4.72 | 3.50 | 6.60 |
| G5 HPV 0.008 g/mL | 0.36 ± 0.26 | 28.21 ± 4.73 | 28.99 ± 4.24 | 5.38 ± 6.74 | 4.29 | 8.01 |
| G6 HPV 0.013 g/mL | 0.80 ± 0.40 | 24.44 ± 3.62 | 26.68 ± 3.89 | 9.24 ± 3.28 | 4.07 | 7.69 |
1 Statistically different from G4 (P < 0.05). 2 Statistically different from G5 (P < 0.05). G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL
Relative organs’ weight in milligrams (mean ± standard deviation)
| Organs | G1 WT water | G2 HPV water | G3 WT 0.013 g/mL | G4 HPV 0.006 g/mL | G5 HPV 0.008 g/mL | G6 HPV 0.013 g/mL |
|---|---|---|---|---|---|---|
| Heart | 3.8 ± 0.3 | 4.2 ± 0.5 | 4.0 ± 0.5 | 4.7 ± 0.3 | 4.3 ± 0.2 | 4.3 ± 0.7 |
| Lung | 6.0 ± 0.3 | 6.2 ± 1.3 | 6.0 ± 0.5 | 6.7 ± 0.5 | 6.5 ± 0.6 | 6.5 ± 0.6 |
| Spleen | 3.4 ± 1.3 | 4.5 ± 1.0 | 4.2 ± 1.0 | 5.2 ± 0.5 | 4.5 ± 1.0 | 5.0 ± 1.6 |
| Liver | 41.6 ± 2.8 | 47.1 ± 2.3 | 42.3 ± 3.0 | 44.1 ± 2.9 | 42.7 ± 5.0 | 44.6 ± 5.5 |
| Right kidney | 5.4 ± 0.5 | 6.1 ± 0.4 | 5.5 ± 0.9 | 6.1 ± 0.5 | 6.2 ± 0.6 | 5.9 ± 0.6 |
| Left kidney | 4.9 ± 0.5 | 5.8 ± 0.4 | 5.5 ± 0.5 | 5.9 ± 0.5 | 6.0 ± 0.5 | 5.6 ± 0.7 |
G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL
No concentration-dependent changes were observed for any parameters (Table 4). However, statistically significant differences were found between groups in terms of glucose levels: G2 (HPV, water) showed a larger increase compared to G4 (HPV, 0.006 g/mL) and G6 (HPV, 0.013 g/mL). The same trend was observed for the parameter total protein, which showed a statistically significant difference between G3 (WT, 0.013 g/mL) and G6 (HPV, 0.013 g/mL), with G6 showing a higher value.
Microhematocrit, glucose, and biochemical parameters (mean ± standard deviation)
| Parameters | G1 WT water | G2 HPV water | G3 WT 0.013 g/mL | G4 HPV 0.006 g/mL | G5 HPV 0.008 g/mL | G6 HPV 0.013 g/mL |
|---|---|---|---|---|---|---|
| Microhematocrit (%) | 46.84 ± 2.30 | 47.70 ± 1.54 | 45.52 ± 1.32 | 46.62 ± 2.27 | 44.68 ±1.34 | 47.38 ± 2.10 |
| Glucose (mg/dL) | 152.60 ± 12.54 | 174.72 ± 20.191, 2 | 144.40 ± 30.88 | 109.40 ± 26.25 | 137.00 ± 38.61 | 94.20 ± 38.79 |
| Albumin (g/L) | 3.19 ± 0.87 | 2.92 ± 0.13 | 3.01 ± 0.51 | 3.16 ± 0.18 | 2.91 ± 0.18 | 3.37 ± 0.40 |
| Total proteins (g/L) | 44.64 ± 4.36 | 44.79 ± 4.85 | 41.13 ± 6.451 | 49.48 ± 3.33 | 46.75 ± 2.98 | 52.89 ± 6.04 |
| Creatinine (mg/dL) | 0.28 ± 0.43 | 0.54 ± 0.41 | 0.25 ± 0.38 | 0.33 ± 0.67 | 0.46 ± 0.49 | 0.46 ± 0.90 |
| Urea (mg/dL) | 62.66 ± 8.02 | 63.58 ± 15.99 | 78.80 ± 4.13 | 66.18 ± 7.35 | 54.42 ± 10.72 | 59.18 ± 13.42 |
| ALT (U/L) | 65.36 ± 17.69 | 203.16 ± 281.33 | 88.70 ± 27.69 | 65.50 ± 22.37 | 78.63 ± 13.05 | 154.35 ± 136.12 |
1 Statistically different from G6 (P < 0.05). 2 Statistically different from G4 (P < 0.05). G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL. ALT: alanine aminotransferase
The comet assay did not reveal any significant genotoxic blood damage in association with the HPV16 transgenes. There was a tendency to increase the total GDI in HPV animals that ingested the extract when compared to their control. The same applies to oxidation damage revealed by GDIFpg (Figure 1), but these observations did not reach the level of statistical significance (P > 0.05).
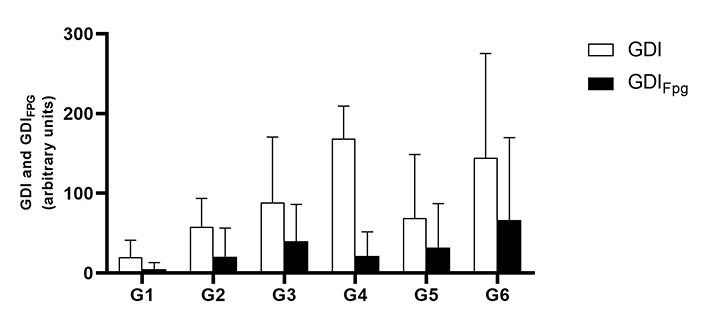
Mean values of the genetic damage index (GDI) in the blood, measured by the alkaline comet assay (mean ± standard deviation). Treatment without formamidopyrimidine DNA glycosylase (Fpg) treatment (GDI) is shown in white and treatment with Fpg (GDIFpg) is shown in black. G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL
Table 5 and Figure 2 summarize the results of the histology of the ear skin and chest skin. As expected, cutaneous lesions were exclusively observed in the HPV groups on the ear pavilion skin, while all animals in the two WT groups displayed normal (0%) auricular skin, indicating that the extract did not cause any harm to the ear skin. Lesions were recorded in all HPV groups. The number of animals with dysplasia was statistically lower in G1 (WT, water) than in G2 (HPV, water) (P < 0.05). Regarding the congestion parameter, G1 (WT, water) showed a statistically significant lower incidence than G2 (HPV, water) (P < 0.05), and G3 (WT, 0.013 g/mL) showed a statistically lower incidence than G6 (HPV, 0.013 g/mL) (P < 0.05). The number of animals with carcinoma in situ was statistically lower in G1 (WT, water) than in G2 (HPV, water) (P < 0.05). Regarding chest skin histology, the WT animals [G1 (WT, water) and G3 (WT, 0.013 g/mL)] exhibited normal chest skin (100%). This confirms that the extract did not cause any histological skin lesions in WT animals. G3 (WT, 0.013 g/mL) showed lower frequencies of sebaceous and epidermal hyperplasia than G6 (HPV, 0.013 g/mL) (P < 0.05).
Number of animals (%) with histological cutaneous lesions in all experimental groups
| Histological cutaneous lesions | G1 WT water | G2 HPV water | G3 WT 0.013 g/mL | G4 HPV 0.006 g/mL | G5 HPV 0.008 g/mL | G6 HPV 0.013 g/mL | |
|---|---|---|---|---|---|---|---|
| Ear pavilion lesions | |||||||
| Dysplasia | 0(0%)1 | 5(100%) | 0(0%) | 5(100%) | 4(80%) | 4(80%) | |
| Papilloma | 0(0%) | 3(50%) | 0(0%) | 2(40%) | 0(0%) | 1(20%) | |
| Sebaceous hyperplasia | 0(0%) | 4(80%) | 0(0%) | 3(60%) | 4(80%) | 1(20%) | |
| Inflammatory infiltrate | 0(0%)1 | 5(100%) | 0(0%)2 | 5(100%) | 5(100%) | 5(100%) | |
| Congestion | 0(0%)1 | 5(100%) | 0(0%)2 | 0(0%) | 0(0%) | 1(20%) | |
| Hyperplasia | |||||||
| Simple | Focal | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 1(20%) |
| Diffuse | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 1(20%) | (0%) | |
| Papillary | Focal | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Diffuse | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Papillomatosis | Focal | 0(0%) | 2(40%) | 0(0%) | 3(60%) | 4(80%) | 2(40%) |
| Diffuse | 0(0%) | 2(40%) | 0(0%) | 1(20%) | 0(0%) | 0(0%) | |
| Carcinoma | |||||||
| Carcinoma in situ | 0(0%) | 1(20%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Small invasive carcinoma | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Invasive carcinoma | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Chest skin lesions | |||||||
| Dysplasia | 0(0%)1 | 5(100%) | 0(0%) | 5(100%) | 4(80%) | 4(80%) | |
| Sebaceous hyperplasia | 0(0%)1 | 5(100%) | 0(0%)2 | 5(100%) | 4(80%) | 5(100%) | |
| Inflammatory infiltrate | 0(0%) | 4(80%) | 0(0%) | 4(80%) | 3(50%) | 3(50%) | |
| Simple | Simple | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Diffuse | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 1(20%) | |
| Papillary | Diffuse | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Focal | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Papillomatosis | Diffuse | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Focal | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Papiloma | Inverted papiloma | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Exophytic papiloma | 0(0%) | 2(40%) | 0(0%) | 1(20%) | 1(20%) | 1(20%) | |
| Carcinoma | Carcinoma in situ | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Small invasive carcinoma | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Invasive carcinoma | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
1 Significantly different from the G2 (P < 0.05). 2 Significantly different from the G6 (P < 0.05). G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL
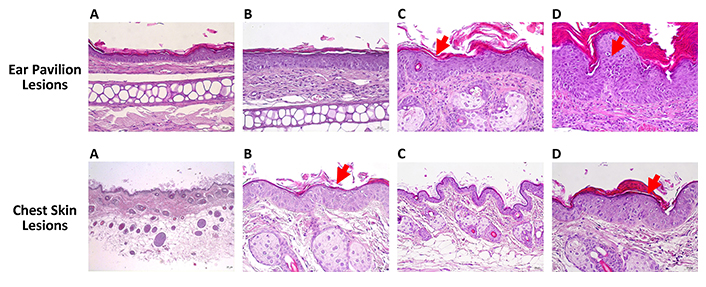
Histopathological changes induced by HPV16 oncogenes in wild-type (WT) mice. Hematoxylin and eosin stain. Ear pavilion lesions: (A) normal skin histology; (B) hyperplasia; (C) dysplasia (red arrow); (D) carcinoma in situ (red arrow). Chest skin lesions: (A) normal skin histology; (B) hyperplasia (red arrow); (C) dysplasia; (D) carcinoma in situ (red arrow). Scale bar = 25 μm. Magnification = 250×
The results of the histology of lungs, liver, spleen, and kidney are summarized in Table 6 and Figure 3. Statistically significant differences were observed in the incidence of atelectasis in the lungs, being higher in G2 (HPV, water) when compared with G1 (WT, water) (P < 0.05). No statistically significant differences were observed in the remaining selected organs.
Number of animals (%) with histological lesions in the lungs, liver, spleen, and kidney in all experimental groups
| Histological organs lesions | G1 WT water | G2 HPV water | G3 WT 0.013 g/mL | G4 HPV 0.006 g/mL | G5 HPV 0.008 g/mL | G6 HPV 0.013 g/mL | |
|---|---|---|---|---|---|---|---|
| Lungs | |||||||
| Mononucleated inflammation intensity | 0(0%) | 3(60%) | 0(0%) | 1(20%) | 1(20%) | 1(20%) | |
| Atelectasis | 0(0%)1 | 5(100%) | 2(40%) | 1(20%) | 1(20%) | 4(80%) | |
| Congestion | 3(60%) | 5(100%) | 0(0%) | 2(40%) | 0(0%) | 3(60%) | |
| Hyperaemia | 4(80%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Liver | |||||||
| Hydropic degeneration | 0(0%) | 1(20%) | 0(0%) | 0(0%) | 2(40%) | 0(0%) | |
| Inflammatory infiltrate | Periportal | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Centrilobular | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Midzonal | 0(0%) | 5(100%) | 3(60%) | 3(60%) | 3(60%) | 1(20%) | |
| Congestion | 5(100%) | 5(100%) | 1(20%) | 2(40%) | 3(60%) | 5(100%) | |
| Spleen | |||||||
| Diffuse white pulp: hyperplasia | 0(0%) | 0(0%) | 2(40%) | 1(20%) | 4(80%) | 1(20%) | |
| Expansion of the splenic red pulp: congestion | 0(0%) | 4(80%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
| Kidney | |||||||
| Inflammatory infiltrate | 1(20%) | 5(100%) | 0(0%) | 3(60%) | 3(60%) | 3(60%) | |
| Hydronephrosis | 1(20%) | 4(80%) | 1(20%) | 1(20%) | 1(20%) | 0(0%) | |
| Congestion | 5(100%) | 5(100%) | 5(100%) | 3(60%) | 5(100%) | 5(100%) | |
| Hyperaemia | 1(20%) | 3(60%) | 0(0%) | 0(0%) | 1(20%) | 0(0%) | |
| Vascular ectasia | 0(0%) | 1(20%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | |
1 Significantly different from the G2 (P < 0.05). G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL
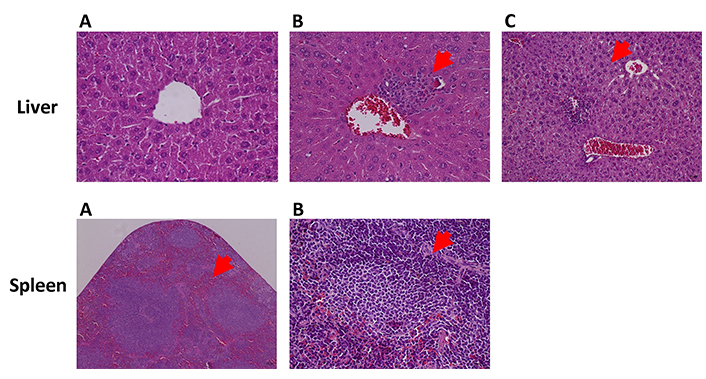
Hepatic and splenic changes in HPV16-transgenic mice, hematoxylin, and eosin. Liver: (A) congestion; (B) inflammatory infiltrate (red arrow); (C) hydropic degeneration (red arrow). Magnification = 400×. Spleen: (A) congestion (red arrow); (B) white pulp: hyperplasia (red arrow). Scale bar = 25 μm. Magnification = 100×
In terms of the analysis of hepatic oxidative stress, statistically higher levels of ROS were observed in G2 (HPV, water) when compared to G6 (HPV, 0.013 g/mL) (P < 0.05). Additionally, the ROS levels were significantly higher in G5 (HPV, 0.008 g/mL) than in G6 (HPV, 0.013 g/mL) (P < 0.05) (Figure 4). No statistically significant differences were found among groups for renal oxidative stress analysis (Figure 5). In the GST parameter there are statistically significant differences between G1 (WT, water) and G2 (HPV, water), and also between G2 (HPV, water) and G5 (HPV, 0.008 g/mL) (P < 0.05) (Figure 5).
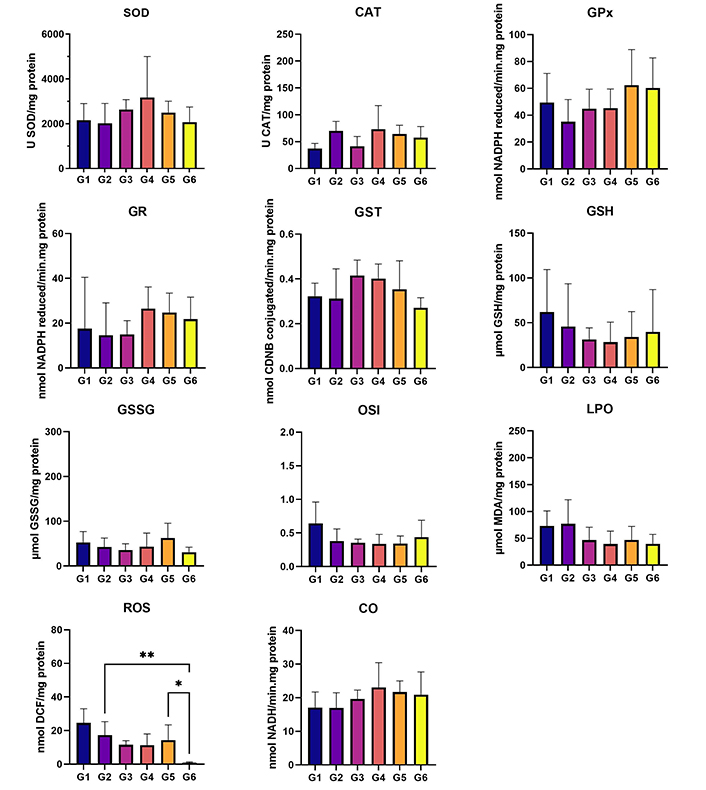
Liver oxidative stress. Cu/Zn-SOD (U SOD/mg protein); CAT (U CAT/mg protein); GPx (nmol NADPH reduced/min·mg protein); GR (nmol NADPH reduced/min·mg protein); GST (nmol CDNB conjugated/min·mg protein); GSH (µmol GSH/mg protein); GSSG (µmol GSSG/mg protein); OSI (GSH/GSSG); LPO (µmol MDA/mg protein); ROS (nmol DCF/mg protein); and CO (nmol NADH/min·mg protein). Statistically significant differences in the ROS marker between G2 (HPV, water) and G6 (HPV, 0.013 g/mL), and G5 (HPV, 0.008 g/mL). G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL
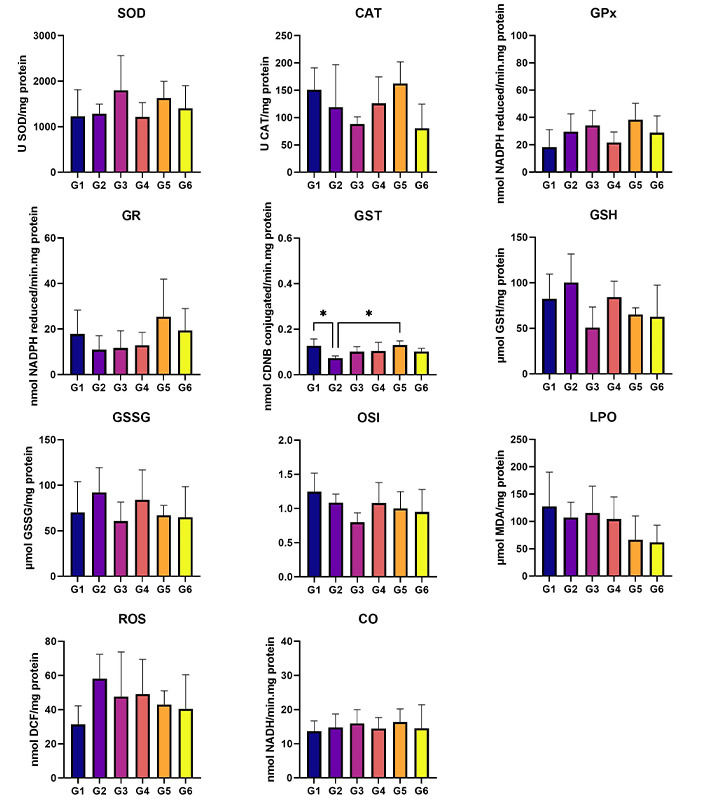
Kidney oxidative stress. Cu/Zn-SOD (U SOD/mg protein); CAT (U CAT/mg protein); GPx (nmol NADPH reduced/min·mg protein); GR (nmol NADPH reduced/min·mg protein); GST (nmol CDNB conjugated/min·mg protein); GSH (µmol GSH/mg protein); GSSG (µmol GSSG/mg protein); OSI (GSH/GSSG); LPO (µmol MDA/mg protein); ROS (nmol DCF/mg protein); and CO (nmol NADH/min·mg protein). Statistically significant differences in the GST marker between G1 (WT, water) and G2 (HPV, water). G1 are wild-type (WT) mice that drink water; G2 are mice with human papillomavirus (HPV) that drank water; G3 are WT that drank daily an aqueous extract of Aloysia citrodora at a concentration of 0.013 g/mL; G4 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.006 g/mL; G5 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.008 g/mL; G6 are mice with HPV that drank daily an aqueous extract of A. citrodora at a concentration of 0.013 g/mL
HPV is the most common sexually transmitted infection [39]. There are many different types of HPV, including non-oncogenic types (low-risk HPVs) that can cause warts [40]. Oncogenic types of HPV (high-risk HPVs), such as type 16 and 18, have the potential to initiate pre-malignant lesions that may progress into various types of cancer, including cervical, vaginal, vulvar, penile, anal, and oropharyngeal cancers. This progress occurs when the infection persists by evading immune surveillance [41–43]. Cell transformation is mediated by viral oncoproteins E6, E7 and E5, which interfere with cellular proliferation, differentiation, and survival [44]. The K14-HPV16 transgenic mouse model was developed, in which the human cytokeratin 14 (K14) gene promoter activates all early genes, including E6 and E7, specifically in the basal cells of the keratinized squamous epithelia [8]. Thus, this model shows potential for investigating the pathophysiological mechanisms underlying HPV16-induced carcinogenesis and assessing the effects of various natural compounds and different drugs on the progression of this disease [13, 14, 45, 46]. In this study, we evaluated the chemopreventive effects of A. citrodora extract for the first time in a K14-HPV16 transgenic mouse model. Tammar et al. [47] studied the phenolic compound profile of methanolic A. citrodora extract and identified nine phenolic compounds, including caffeoylshikimic acid, catechin-gallate, 3,4-Di-caffeoylquinic acid, acteoside, isoacteoside, martynoside, diosmetin, and apigenin. Caffeoylshikimic acid has been found to exhibit antiproliferative activity against various cancer cell lines and promote cancer cell apoptosis [48]. 3,4-Di-caffeoylquinic acid exhibited antioxidant activity [49]. Another antineoplastic agent that has been shown to have such activity was acteoside and isoacteoside [50]. Martynoside has demonstrated chemoprotective effects both in vivo and ex vivo [51]. Diosmetin is reported to exhibit anticancer, antimicrobial, antioxidant, oestrogenic and anti-inflammatory activities [52]. Apigenin has potential antioxidant, anti-inflammatory, and anticancer properties [53]. Our findings showed that there were no mortality or noteworthy alterations in terms of behavioral indicators (such as alertness, restlessness, irritability, and fearfulness), neurological symptoms (including bleeding, convulsions, gait abnormalities, and pain), respiratory function, or gastrointestinal effects (such as stomachache and diarrhea). The humane endpoints were not indicative of animals’ sacrifice before the end of the assay. These results indicate that, under the current experimental conditions, the A. citrodora extract does not interfere with the well-being of the animals, demonstrating its non-toxic properties. The body weight of WT and HPV mice treated with A. citrodora extract was significantly lower than that of the control groups. Negative changes in the animals’ body weight are commonly associated with toxicity [54]. Regarding the mean relative weight of internal organs, the HPV groups exhibited the highest weights. This may be related to the inflammatory infiltrate microscopically identified in these groups. A. citrodora extract is known to have a protective effect on glucose blood levels in diabetes [55] as evidenced by low levels in mice that consumed the extract. The parameters microhematocrit, albumin, and total proteins showed a tendency to increase in HPV animals that ingested the extract, these increments may be due to animal’s dehydration, caused by HPV skin lesions, as described in previous works [15]. A. citrodora extract did not significantly alter basal levels of genetic or oxidative damage. However, the concentration of 0.008 g/mL appears to be the least harmful and may have a greater protective effect. It is worth noting that the literature mentions the protective effects of A. triphylla, a plant from the same family of as A. citrodora, against genetic damage induced by cisplatin [56]. The histological examination of the collected organs in, WT mice did not reveal any lesion, while HPV mice exhibited hyperplastic lesions with dysplastic foci and, carcinoma in situ. The groups that ingested the A. citrodora extract showed a reduction in the most aggressive lesions, such as dysplasia and carcinoma. Lesions were solely identified in the chest from the HPV groups, the G2 (HPV, water) exhibited lesions such as hyperplasia and dysplasia. The severity of these lesions seems to decrease with the administration of A. citrodora extract. Although not statistically significant, there was decrease in the severity of liver injuries as the concentration of A. citrodora extract increased. A similar trend was observed in the spleen. The imbalance between antioxidants and oxidants leads to oxidative stress, which favors oxidative agents and results in the excessive production of free radicals, namely ROS, causing biological damage. The ROS are generated through normal metabolic pathways in the hepatic mitochondria and are regulated by antioxidant mechanisms to maintain low levels. Although necessary for normal cellular functions, elevated levels of free radicals can lead to oxidative stress [57]. Upon examining the WT and HPV control groups, it is evident that liver inflammation caused by HPV16 is associated with oxidative stress, similar results were previously observed [58]. To address this issue, it will be necessary to activate the antioxidant system by increasing the levels of enzymes SOD, CAT, and GR. However, even with the activated antioxidant defense system, it is insufficient to fully prevent oxidative stress. Upon examining the WT and HPV groups that consumed the A. citrodora extract, we observed a tendency towards decreased liver LPO and increased OSI, indicating a reduction in oxidative stress. This was associated with an increase in antioxidant enzymes SOD, CAT, GR. Notably, there was a significant decrease in ROS levels in HPV mice. At a concentration of 0.013 g/mL, the A. citrodora extract effectively reduced ROS levels. In the kidneys, oxidative stress leads to a decrease in OSI and LPO enzymes in the HPV and WT control groups, as well as a decrease in SOD and GR. However, in the groups exposed to A. citrodora extract, the decrease in oxidative stress was intensified. The parameters of renal functionality (creatinine) and hepatic functionality (ALT) did not differ between the groups. Therefore, combining these results with the results of oxidative stress, we can say that the consumption of A. citrodora does not have a negative impact on hepatic functionality. In this study, we evaluated the impact of an extract prepared through infusion of A. citrodora. In future research, it will be crucial to investigate the effects of the fresh plant. Additionally, we suggest isolating the various compounds identified in this plant and conducting individual assessments of their properties in the form of isolated extracts.
In this model, A. citrodora decrease the incidence of dysplastic skin lesions caused by HPV16. This suggests that dietary supplementation at concentrations of 0.008 g/mL and 0.013 g/mL has chemopreventive effects. Furthermore, these results show that the tested concentrations of A. citrodora were safe and did not induce toxicity under the current experimental conditions. Moving forward, it would be crucial to test concentrations higher than 0.013 g/mL to ascertain if we can achieve more promising outcomes against HPV16 lesions in this strain.
CAT: catalase
DNA: deoxyribonucleic acid
FPG: formamidopyrimidine DNA glycosylase
GDI: genetic damage index
GR: glutathione reductase
HPV: human papillomavirus
LPO: lipid peroxidation
MDA: malondialdehyde
MS: mass spectrometer
OSI: oxidative stress index
ROS: reactive oxygen species
SOD: superoxide dismutase
WT: wild-type
BMF: Conceptualization, Investigation, Writing—original draft. AIFR, MJP, MJN, IG, MMSMB, and CV: Writing—review & editing. HV, CVN, JS, MGS, LG, MID, LB, and LF: Investigation, Writing—review & editing. RM and RMGDC: Writing—review & editing, Supervision. PAO: Conceptualization, Writing—review & editing, Supervision.
The authors declare that they have no conflicts of interest.
The experimental work was approved by the animal facility of the University of Trás-os-Montes e Alto Douro (UTAD) with the authorization of the Ethics Committee (approval no. 852-e-CITAB-2020_A_1-e- 122 CITAB-2021) and the Portuguese Veterinary Directorate (approval no. 014139).
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Beatriz Medeiros-Fonseca has a Ph.D. grant with number [2020.07675.BD] (https://doi.org/10.54499/2020.07675.BD); Jéssica Silva has a Ph.D. grant with number [2020.07999.BD] (https://doi.org/10.54499/2020.07999.BD); Mónica G. Silva has a Ph.D. grant with number [UI/BD/151320/2021] (https://doi.org/10.54499/UI/BD/151320/2021) financed by FCT (Foundation for Science and Technology) through FSE (European Social Fund). This work is supported by National Funds by FCT-Portuguese under projects: CITAB [UID/04033/2020] (https://doi.org/10.54499/UIDB/04033/2020); CERNAS [UIDB/00681/2020] (https://doi.org/10.54499/UIDB/00681/2020); LEPABE [UIDB/00511/2020] (https://doi.org/10.54499/UIDB/00511/2020) and [UIDP/00511/2020] (https://doi.org/10.54499/UIDP/00511/2020); ALiCE [LA/P/0045/2020] (https://doi.org/10.54499/LA/P/0045/2020); CIMO [UIDB/00690/2020] (https://doi.org/10.54499/UIDB/00690/2020) and [UIDP/00690/2020] (https://doi.org/10.54499/UIDP/00690/2020); SusTEC, [LA/P/0007/2020] (https://doi.org/10.54499/LA/P/0007/2020); by national funds through the FCT/MCTES (PIDDAC). Furthermore, national funding by FCT/P.I., through the institutional scientific employment program contract for Lilian Barros, Maria I. Dias (https://doi.org/10.54499/CEECINST/00016/2018/CP1505/CT0004), Luís Félix (https://doi.org/10.54499/2021.00458.CEECIND/CP1690/CT0001) contract. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Kaoutar Anouar Tadlaoui ... Moulay Mustapha Ennaji
Praveen Kumar Chandra Sekar ... Ramakrishnan Veerabathiran
Racheal Ahuoyiza Ayeni ... Bonaventure Michael Ukoaka
