Affiliation:
1Department of Pathology, School of Medicine, Case Western Reserve University, Cleveland, OH 44106, USA
Email: wxd28@case.edu
Affiliation:
2Case Digestive Health Research Institute, School of Medicine, Case Western Reserve University, Cleveland OH 44106, USA
Affiliation:
3College of Food Science and Engineering, Northwest A&F University, Yangling 712100, Shaanxi, China
Explor Med. 2024;5:444–458 DOI: https://doi.org/10.37349/emed.2024.00230
Received: December 12, 2023 Accepted: April 13, 2024 Published: June 28, 2024
Academic Editor: Lee M. Wetzler, Boston University School of Medicine, USA
The article belongs to the special issue Gut Microbiota Derived Metabolites and Chronic Inflammatory Diseases
Postbiotics, representing the newest member of the family of biotics, are metabolites produced as a result of fermentation of lactic acid bacteria (LAB) in the De Man, Rogosa, and Sharpe (MRS) medium which includes proteins, sugars and minerals. The components of postbiotics includes exopolysaccharides (EPS), short-chain fatty acids (SCFAs), bacteriocins, antioxidants, and metabolizing enzymes. Several studies indicate that postbiotics have multiple properties such as antimicrobial, immunomodulatory, antioxidant, anti-inflammatory, anti-obesity, anti-diabetic, and anti-tumoral properties. Natural polysaccharides refer to the polysaccharides obtained from biological organisms including algae, plants, animals, and microorganisms. Polysaccharides are either branched or linear macromolecules and are composed of a few major and some minor monosaccharides, including glucose, fructose, mannose, arabinose, galactose, fucose, galacturonic acid, glucosamine, galactosamine or their derivatives. Similar to postbiotics, polysaccharides also exhibit anti-inflammatory, antibacterial, antitumor, antiviral, immunomodulatory, and antioxidant properties. Although polysaccharides cannot be directly digested by the human body due to the lack of specific enzymes, they can be digested by gut-residing bacteria including but not limited to LAB. Recent studies indicate that large non-starch polysaccharides such as alginate, fucoidan, chitosan, carrageenan, and guar gum can be degraded into low molecular weight oligosaccharides which in turn can provide health benefits to the human health. These new findings inspired us to propose a polysaccharides-based postbiotics, also called glycanbiotics, and their potential applications. We propose that polysaccharides can be fermented by probiotics, and subsequent removal of bacteria will increase the safety of their produced metabolites, including oligosaccharides, disaccharides, monosaccharides and their derivatives. These polysaccharides-based postbiotics may mimic metabolization of polysaccharides in vitro and consequently broaden the applications of postbiotics. Non-probiotics such as Akkermansia muciniphila and other bacteria can also be used for glycanbiotics production, thus providing novel applications for human health.
Postbiotics are microorganism-free metabolic byproducts produced as a result of fermentation of dietary fibers and other components by gut-residing microorganisms, mainly bacteria [1]. Mounting evidence demonstrates a critical contribution of postbiotics towards human health including anti-inflammatory [2], antibacterial [3], immunomodulatory [4], antioxidant [5] effects, as well as gut barrier repairing properties [6] in in vitro studies. Postbiotics affect health differently in comparison to probiotics and prebiotics: They do not include live bacteria, comprise a mix of small molecules, they can be easily absorbed and distributed to the whole body through the bloodstream. Unlike probiotics, the postbiotics do not include live bacteria, thus eliminating any potential risk of bacteremia, commonly found in weak/elderly people [7]. Moreover, bacteria-fermented postbiotics are composed of multiple small molecules easily absorbed by the digestive tract thus promoting health more directly, unlike the non-absorbable biological macromolecules prebiotics [8]. Postbiotics therefore represent a promising therapy for diseases. Current preclinical pilot studies are underway to test the efficacy and safety of postbiotics [NCT06230302], as well as possible applications in some patients, such as those affected by macular degeneration [NCT05056025] [9]. The potential applications of postbiotics include gastrointestinal diseases, inflammatory diseases, and chronic diseases including diabetes, obesity, immunological diseases, cancers, and others [10, 11].
Natural polysaccharides are biological macromolecules consisting of repeated units of monosaccharides or disaccharides and are widely present in foods, including vegetables, fruits, mushrooms, algae, and fungi [12]. The monosaccharides are mostly glucose, fructose, mannose, arabinose, galactose, galacturonic acid, or their derivatives, and the disaccharides are combinations of those monosaccharides. The linear or branched polysaccharides may consist of hundreds or up to thousands of repeated hexose units [12]. In this review, the natural polysaccharides are referred to as non-starch polysaccharides, such as cellulose, mushroom polysaccharides, carrageenan, fucoidan, pectin, alginate, guar gum, and chitosan.
Natural polysaccharides have demonstrated multiple lines of benefits to human health: anti-inflammatory [13], anti-oxidant [14], anti-cancer [15], hypoglycemic effects [16], cardiovascular protection [17], promotion of beneficial gut bacteria [18], intestinal epithelium repairing [19], neuroprotective effects [20], and cognitive health [21]. The low digestibility of natural polysaccharides leaves them undigested in the small intestine and renders them dietary fibers for the human microbiota in the large intestine, which produces the majority of enzymes required for the digestion of polysaccharides. The bacterial digestion of the natural polysaccharides leads to the production of multiple metabolites, including short-chain fatty acids (SCFAs), vitamins, and others [22]. Xiao et al. [23] recapitulated the results of multiple clinical trials performed for the treatment of type 2 diabetes mellitus using non-starch polysaccharides. The speculated mechanisms include regulation of insulin action, promotion of glucose metabolism, and regulation of postprandial blood glucose level and gut microbiota [23]. Mushroom-based polysaccharides have also been utilized in multiple anticancer clinical trials [24]. The results indicate that complementary use of mushroom polysaccharides combined with chemotherapy leads to increased disease-free survival of colorectal cancer patients and improved quality of life among lung cancer patients [25, 26]. Chitosan has also been employed in more than 100 clinical trials to investigate its safety/efficacy and its biomaterials/nanoparticles [27], its role in antimicrobial activities, lipid-lowering activities, diabetic foot ulcer treatment, chronic pain treatment, cancer therapy, and others [27].
Despite the fact that postbiotics have demonstrated a very promising potential in health intervention, they have shown some limitations. For instance, the current postbiotics are based on fermentation in a specific medium, De Man, Rogosa, and Sharpe (MRS), which includes peptone, beef extract, yeast extract, glucose, and minerals [28]. MRS mimics bacterial fermentation under only certain circumstances. Fermented foods, however, contain several fibers or polysaccharides that can be fermented by the gut microbiota in the intestine, representing a more complex situation compared to the current in vitro studies involving postbiotics. Hence, the published data indicate that further studies focused on postbiotics are needed. Moreover, the fermentation of a mix of polysaccharides by beneficial bacteria may boost the effect of postbiotics, based on the fact that the health benefits provided by polysaccharides are indirect and dependent on the availability of gut-residing bacteria since they are nonabsorbable by the human body. Herein we refer to specific polysaccharides-based postbiotics as glycanbiotics.
In this review, we focused on the current research progress in both postbiotics and polysaccharides and their potential combination. The postbiotics do not have clinical applications yet, therefore they need to be extensively studied. We propose that glycanbiotics represent a potential novel approach to health interventions.
In 2019, the International Scientific Association for Probiotics and Prebiotics (ISAPP) defined postbiotics as “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [29]. Effective postbiotics do not contain any live microbes, thus eliminating the potential harmfulness of the bacteria to humans, while still contributing to health benefits.
Postbiotics are a mixture of multiple metabolites and cell structures of bacteria, which includes bacterial exopolysaccharides (EPS), carbohydrates (such as teichoic acids and galactose-rich polysaccharides), proteins (p40, p75 molecule, lactocepin, bacteriocins, enzymes), lipids (butyrate, acetate, propionate, lactate, dimethyl acetyl-derived plasmalogen), vitamins (B-group vitamins), organic acids (3-phenyllactic acid and propionic), and other complex molecules (lipoteichoic acids, peptidoglycan-derived muropeptides) [30, 31]. They also include peptidoglycans, muramic acid or other complex molecules [31]. Those components render the outstanding beneficial contributions to human health.
The EPS, capsular polysaccharides (CPS), and cell wall polysaccharides (WPS) are all part of the bacterial WPS and all exhibit antimicrobial, immunomodulatory, and anti-inflammatory properties [32]. Among them, EPS are the most widely studied component. EPS are extracellular carbohydrate polymers with high molecular weight produced and secreted by microorganisms, mainly gram-positive lactic acid bacteria (LAB) [33]. EPS produced by Lactobacillus rhamnosus demonstrated substantial antibacterial activity against the pathogens of Salmonella enterica and Escherichia coli in an in vitro study [34]. EPS can enhance intestinal barrier function and protect against lipopolysaccharide (LPS)-induced barrier disruption [35]. Evidence also showed that EPS from Lactiplantibacillus plantarum subsp. promote intestinal barrier function by upregulating ZO-1 and occludin proteins and by inhibiting the expression of claudin-2 and pro-inflammatory cytokines, including IFN-γ, IL-6, and TNF-α in both inflammatory bowel disease mouse models and in vitro human Caco-2 cell lines [36].
SCFAs are also important metabolites identified in postbiotics [37]. SCFAs include acetate (C2), propionate (C3), and butyrate (C4), and are the most abundant fatty acids in the human intestine [37]. SCFAs can be produced when fermented by LAB such as Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus paracasei, and Lactobacillus brevis [38]. SCFAs are very important for maintaining a healthy intestinal epithelial layer and for protecting the intestinal barrier from the disruption of LPS [39]. Acetate is the most abundant SCFA, and it is mainly produced by enteric bacteria; propionate is a major substrate for gluconeogenesis in the liver, and is found in higher concentrations in circulation, due to its efficient transport across the gut epithelium [40]. Butyrate prevents nuclear factor-kappa B (NF-κB) from becoming activated by reducing the expression of pro-inflammatory cytokines, and also contributes to the regeneration of the intestinal epithelium. Butyrate also exerts antitumor effects via blocking histone deacetylases, which controls gene expression in cells [41].
LAB are capable of producing plenty of bacteriocins [42] which confer them antibacterial properties. Bacteriocins are small peptides that are released extracellularly once synthesized by bacteria. Bacteriocins are therefore used as food preservatives [43] for long and safe storage, as well as a method to control dysbiosis of the gut microbiota [42].
Antioxidant effect, represented by hydroxyl radical scavenging activity, helps to scavenge free radicals produced during oxidation processes [44]. Two strains of Lactobacillus fermentum were reported to contain a significant amount of glutathione peroxidase, which was later discovered to have strong in vitro antioxidant activities [45]. Kang et al. [46] conducted a comparative antioxidant activity assay among 17 strains isolated from Korean individuals and fermented foods, all of which displayed antioxidant activity at various levels.
During fermentation, the bacteria are too small to “swallow” the big nutrients, so they usually excrete multiple enzymes extracellularly to digest food. Based on their activities or functions, enzymes have been categorized into six groups: oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases [47]. The microbiota genome is much larger than the human genome so it encodes a lot more enzymes including carbohydrate-active enzymes (CAZymes) [48], which can metabolize multiple polysaccharides. Polysaccharides are normally indigestible by humans because humans, differently from bacteria, are unable to produce the appropriate enzymes to degrade the polysaccharides.
The currently studied postbiotics are produced by culturing the LAB in MRS broth [28], followed by an extraction step through centrifugation. Bacteria are then separated from the fermented products and heat-inactivated. MRS broth is composed of peptone, yeast extract, beef extract, glucose, and inorganic salts, and has been designed only for LAB. Studies have demonstrated that heat-inactivated Lactobacillus acidophilus can promote faster recovery from viral diarrhea than live Lactobacillus [49]. It was also demonstrated that non-viable Bifidobacterium bifidum MIMBb75 was able to significantly alleviate irritable bowel syndrome symptoms in comparison to the placebo [50].
Natural polysaccharides are naturally occurring macromolecular polymers which are obtained from natural sources such as algae, plants, and microorganisms such as fungi and bacteria [12]. They are the most abundant naturally occurring macromolecular polymers obtained from renewable sources such as algae, plants, animals, and microorganisms such as fungi and bacteria [12]. Interests in natural polysaccharides are booming due to their non-toxicity, safety, biodegradability, low prices, and availability. Natural polysaccharides originally either serve as energy storage or are used to form cell walls which provide structural support. To date, numerous polysaccharides have been shown to exhibit a wide range of biological activities, including anticoagulation, antiviral, antitumor, antioxidant, hypoglycemic, immunomodulatory, radioprotective, and other effects [12–17]. Traditional Chinese herbs also contain bioactive polysaccharides, depending on sources, which contribute to their therapeutic effects [51].
Natural polysaccharides are composed of many structurally diverse monosaccharide moieties joined together by glycosidic linkage, and are either linear or branched macromolecules, with positive charge (chitosan), negative charge (fucoidan, alginate, carrageenan), or neutral (pectin, inulin) [12, 52]. They are divided into four categories based on sources: plant polysaccharides (such as starch, cellulose, guar gum, inulin, and pectin), animal polysaccharides (such as chitosan, heparin, and chondroitin sulfate), microbial polysaccharides (such as mushroom polysaccharides, hyaluronic acid), and marine polysaccharides (such as alginate, carrageenan, chitosan and fucoidan polysaccharides) [12, 52]. They are categorized as glucans, mannans, arabinogalactans, galactans, fucoidan, fructan, polyxylose, and others, based on the composition of repeated subunits and linkage types [12, 52].
Due to lack of the digestive enzymes, humans are unable to digest and absorb large molecular polysaccharides in the small intestine. Therefore, most of them are utilized by symbiotic microorganisms in the large intestine. Polysaccharides can therefore reshape the microbiota community, rendering them prebiotics [52, 53]. The beneficial effects of polysaccharides on human health are, at least partly, obtained through metabolization of polysaccharides by the gut microbiota in the large intestine. CAZymes render the gut microbiota capable of degrading polysaccharides, with consequent potential benefits for the host [54]. Human gut bacteria produce hundreds of polysaccharides degrading enzymes, which account for up to 2.6% of the total enzymes encoded by the intestinal microbiota [55]. The Bacteroidetes encodes 137.1 CAZymes per genome on average, and Firmicutes encode 39.6 CAZymes per genome on average [56]. CAZymes are categorized as glycoside hydrolases, polysaccharide lyases, and carbohydrate esterases [48].
The metabolites of polysaccharides have demonstrated multiple lines of functions for the cells and human health. Polysaccharides can bind to invading pathogens, including viruses, bacteria, and other microorganisms, thus blocking their invasion into the human body. They can help repair damaged intestinal barrier, partly through SCFAs [57], and inhibit the invasion of pathogens deep in the human body. The use of polysaccharides can also help reshape the intestinal microbial community and help symbiotic microorganisms dominate the microbiota by suppressing potential pathogens [58, 59]. We presume that the metabolites of polysaccharides may include the precursors of biochemical pathways or even neurotransmitters, which directly or indirectly participate in multiple processes. Polysaccharides can also directly regulate gut functions by stimulating the secretion of interleukins and hormones, or by promoting microbial CAZyme expression, which can reduce the pathogenesis of gut diseases.
Multiple polysaccharides are commercially available and have shown wide applications. In this review, we discuss some of them, including carrageenan, fucoidan, chitosan, alginate, and guar gum. Those non-starch polysaccharides are mostly extracted from cell walls because they are rich in polysaccharides. Chitin, for example, is the main component of cell walls in plants, bacteria, and fungi [60]. Table 1 below summarizes the major and minor monosaccharides and their respective modifications and surface charges, which confer the polysaccharide’s important properties, such as antigen binding and solubility [61].
Natural polysaccharides and their components and charges
| Polysaccharides | Major monosaccharides | Minor monosaccharides | Charge | Reference(s) |
|---|---|---|---|---|
| Carrageenan | Sulfated D-galactose | Sulfated, negatively charged | [58, 62] | |
| Fucoidan | Fucose | Mannose, galactose, xylose, glucose, rhamnose, glucuronic acid, arabinose | Sulfated, negatively charged | [63, 64] |
| Chitosan | D-glucosamine | N-acetyl-D-glucosamine | Positively charged by amine groups | [65, 66] |
| Alginate | β-1,4-D-mannuronic acid (MnA) and α-1,4-L-guluronic acid (GlcA) | Negatively charged | [59, 67] | |
| Guar gum | Galactose, mannose | No charge | [68] |
Blank cells represent not applicable
Carrageenan is a high molecular weight sulfated polysaccharide derived from the cell wall of red algae (seaweed) [58]. There are three major types of carrageenan present in red algae: kappa (κ)-, iota (ι)-, and lambda (λ)-carrageenan, which contain repeated units of D-galactose and one, two, or three negatively charged organosulfates, respectively [69]. A representative carrageenan, for example, seaweed Kappaphycus alvarezii, has 50.8% carbohydrates, 3.3% proteins, 3.3% lipids, 15.6% ash, 12.4% sulfate groups, and 3.0% insoluble aromatics on average [70]. Commercially, carrageenan is utilized as gelling, thickening, water holding, and stabilizing agents, which is widely used as a common food additive. Carrageenan displayed potential antioxidant and immunomodulatory properties in both in vivo and in vitro studies [58, 62].
Fucoidan is a sulfated, L-fucose-rich polysaccharide found in the cell wall matrix of brown algae, with a range from 10 kDa to 10,000 kDa in molecular weight depending on the fucoidan source and extraction methods [71]. In addition to fucose, minor monosaccharides include glucose, xylose, galactose, and mannose [71]. With high sulfate content (more than 20%), fucoidan has drawn much attention due to its wide biological functions including antioxidant, antibacterial, anticoagulant, antidiabetic, antithrombotic, immunomodulatory, anticancer, and anti-proliferative effects [64, 71, 72], likely obtained by enhancing intestinal barrier functions [63]. Recent studies also reported that fucoidan exhibited strong anti-COVID-19 infection properties [73]. New potential fucoidan-based applications include its use as a drug delivery vehicle due to its non-cytotoxicity, biocompatibility, and biodegradability properties [74]. Fucoidan itself exhibited anti-cancer properties through arrest of cell cycle and apoptosis, control of angiogenesis, inhibition of metastasis, and immunomodulatory activities [75]; these effects are strongly dependent on the polymer molecular weight [76]. Fucoidan has also been extensively studied in regenerative medicines and wound healing, due to its vast biodiversity, cost-effectiveness, and simple procedures for extraction and purification [74].
Chitosan is a cationic polymer obtained by deacetylation of chitin, the second most abundant natural polymer in the world and a natural polysaccharide in fungi, insects, and the exoskeleton of crustaceans [77]. Chitosan is prepared by deacetylation of chitin through incubation in a strong base at high temperatures [78]. The product is a copolymer of D-glucosamine and N-acetyl-D-glucosamine (GlcNAc) connected with β-(1→4)-glycosidic bonds [78]. Chitosan is the only cationic polysaccharide in nature. Chitosan and its derivatives have been shown to be applicable in medicine, pharmaceuticals, food, cosmetics, agriculture, the textile and paper industries, the energy industry, and industrial sustainability due to their macromolecular structures and their unique biological and physiological properties, including solubility, biocompatibility, biodegradability, and reactivity [77]. Research has shown mucoadhesive, anti-inflammatory, antioxidant, antimicrobial, antifungal, anti-hyperglycemic, anti-tumor, and wound healing effects [79]. It has also been shown that chitosan can reduce tight junction permeability in the human intestinal Caco-2 cell line [65, 66].
Alginate is also derived from brown algae but with different monomer units. It is a linear polysaccharide consisting of β-D-mannuronic acid and α-L-guluronic acid with an anionic charge [80]. There are more than 200 types of alginate that are commercially available, and most of them are extracted from wild brown seaweed [81]. Due to its unique properties such as biocompatibility, low toxicity, and biodegradability, alginate-based biomaterials have been extensively used for the treatment of various diseases and the regeneration of diverse organs [82]. Alginate also exhibited multiple biological activities, including anti-hyperlipidemic, antimicrobial, anti-reflux, immunomodulatory, or anti-inflammatory activities [80–82].
Guar gum is a linear polysaccharide extracted from the endosperm of guar bean seeds [68]. It is mainly composed of galactomannans with no charge. The galactomannan has a gel-forming capability and has a mannose backbone and galactose units connected as side chains. Guar gum is widely used as an additive in food, pharmaceuticals, paper, textiles, explosives, oil well drilling, and the cosmetics industry. Studies demonstrated its potential application in weight reduction, lipidemia, and type 2 diabetes, as well as in drug delivery, tablet formulations, tissue engineering, and biosensing [83, 84].
Since humans are unable to utilize polysaccharides directly, their beneficial effects are obtained through polysaccharide metabolization by the gut microbiota. The actual effects on health are variable depending on the available enzymes of the microbiota community. After the metabolized monosaccharides enter the bloodstream, organs, and tissues can utilize them to synthesize composites such as cell surface polysaccharides or other polysaccharide conjugates.
The large intestine serves as a fermenter, in which the pass-through food nutrients, including proteins, carbohydrates, fiber/polysaccharides, and other unabsorbed materials, are digested and fermented by the gut microbiota with CAZymes [48]. While the bacteria are contained within the bowel by the intestinal epithelium, the metabolites can be easily absorbed into the blood and exert an impact on human health. The current definition of postbiotics is narrow and limited, and does not include all their properties. Hence, herein we suggest a new word, glycanbiotics, to describe the polysaccharides-based postbiotics which are different from the traditional postbiotics that use MRS medium for fermentation [28].
During fermentation of glycanbiotics, the biomacromolecules are degraded into low molecular weight oligosaccharides, monosaccharides or disaccharides, depending on enzyme availability. The oligosaccharides are defined by a degree of polymerization (DP) 2-10 [85]. Low DP oligosaccharides can be transported into the cell through membrane transporters and degraded into disaccharides and monosaccharides for further use [86]. The oligosaccharides have exhibited multiple biological effects including SCFAs production [85], intestinal pathogen elimination [85], reshape of the gut microbiota [85, 87], immune regulation [85, 87], improvement of intestinal epithelium integrity [85, 87], and intracellular polysaccharide synthesis [85] (Figure 1).
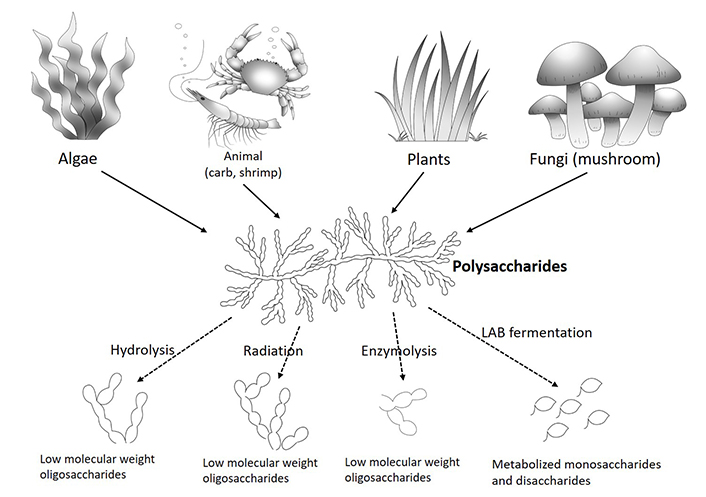
Oligosaccharides and glycanbiotics generation methods from polysaccharides and the possible mechanisms of action. LAB: lactic acid bacteria
In vitro, fucoidan oligosaccharides (FOS) can be obtained through hydrogen peroxide treatment. The hydroxyl radical scavenging activity of lower molecular weight fucoidan is markedly increased compared to the fucoidan polysaccharides [88]. FOS are also shown to have higher antiviral activity [89] and higher cytotoxicity than native fucoidan in cancer cells, such as AGS, MCF-7, and HepG-2 [90]. The chitosan-derived-oligosaccharide (COS) is superior to chitosan in terms of relieving the negative effects of excessive protein consumption [91]. The alginate oligosaccharides have shown immunomodulatory activities characterized by a better structure-activity relation compared to the one of polysaccharides, as indicated by both in vivo and in vitro studies [92]. Carrageenan oligosaccharides have a more pronounced anti-proliferative effect against human colon cancer cell lines (HT-29, HCT-116) than the original polysaccharides [93]. Moreover, the low-molecular-weight partially hydrolyzed guar gums are shown to maintain high levels of fermentability, reshape the colonic microbiota, and promote metabolite production compared to high-molecular-weight guar gums [94]. The studies listed here indicate that small molecules of oligosaccharides perform better than the large molecules of polysaccharides in relation to several functions. For a better treatment, polysaccharides should be metabolized by the bacteria into oligosaccharides and monosaccharides first, and then they can be metabolized into metabolite mixtures by enzymes secreted by bacteria.
Based on the findings shown here, we believe that breakdown of large molecules into small products of fermentation (glycanbiotics) can confer numerous health benefits: We presume that glycanbiotics can be used for chronic disease interventions such as gastrointestinal inflammation, chronic autoimmune disease, anti-ageing, anti-tumor, antibacterial treatments and more.
Unlike probiotics, postbiotics do not have live bacteria, thus eliminating the potential risk of harming weak/elderly individuals. Probiotics are commensal bacteria and are mostly safe; however, they still have potential risk for human health, especially for weak and immunodeficient individuals such as the elderly, children, cancer patients undergoing radiotherapy or chemotherapy, and HIV patients. Several publications reported bacteremia after oral administration of probiotics [95, 96].
Glycanbiotics can have a better shelf life than probiotics as they do not need to keep the bacteria alive. Probiotics tend to become non-viable over time, which shortens their shelf life. On the contrary, the inanimate nature of postbiotics means they are less sensitive to environmental conditions resulting in a longer shelf-life, thus enabling storage and transportation at ambient temperatures.
Since no safety concerns exist for glycanbiotics, more bacteria can also be studied as potential candidates for production and use of glycanbiotics, such as Akkermansia muciniphila (Akk), which is not recognized as probiotic yet. Akk has been shown to be beneficial to human health. In a randomized, double-blind, placebo-controlled clinical trial, Depommier et al. [97] showed that pasteurized Akk taken orally daily for 3 months significantly reduced insulin levels, insulin resistance, total plasma cholesterol, blood LPSs, slightly reduced body weight, fat mass, and hip circumference in overweight/obese individuals compared to the placebo group. In the placebo group, the overall benefit of live Akk supplementation was not significant. These benefits may be partly due to SCFAs [98]. Clearly, Akk has the potential to be employed for postbiotics production.
In regard to postbiotics/glycanbiotics, the risk of causing bacteremia is insignificant. Their use is therefore safer than probiotics; more importantly, postbiotic effects can be enhanced by testing multiple combinations of bacteria and fermentation diets. As shown in Figure 2, the currently known postbiotics are only obtained by the combination of LAB and MRS broth, while the glycanbiotics have the potential to produce different metabolites with consequent different impacts on health. Furthermore, their charges can help them to bind to microorganisms. Also, Akk and other potential probiotics can be used to generate postbiotics before they are approved as probiotics. The real intestinal environment is composed of thousands of bacteria species and strains. When food moves into the intestine, parts of the nutrients are digested and absorbed into the human body. The remaining parts, especially the fibers of fruits, vegetables, mushrooms, and seaweeds, are resistant to digestion from human digestive enzymes. These parts can be metabolized by the gut microbiota through fermentation process.
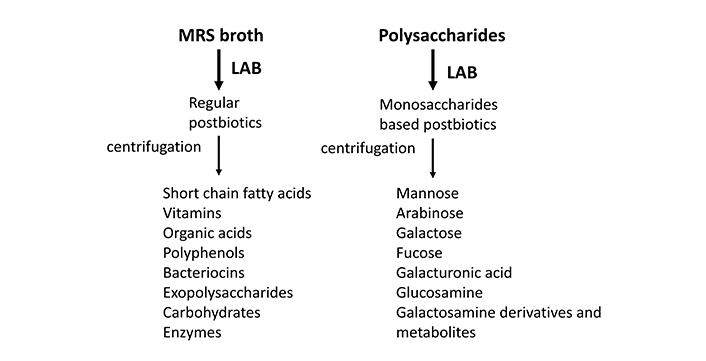
Metabolites of regular postbiotics and polysaccharide-based postbiotics (glycanbiotics). LAB: lactic acid bacteria; MRS: De Man, Rogosa, and Sharpe
The organized structure of polysaccharides exhibits huge variations and is very complex. The metabolites of the monosaccharides after fermentation are not well studied yet. The analysis of metabolites after fermentation is necessary to better understand potential health benefits. If the glycanbiotics production involves application of bacteria not recognized as probiotics yet, safety and regulatory considerations are necessary, as some bacteria may have endogenous toxins which may be harmful to human health. Mouse safety experiments can provide the answer. The optimization of commensal bacteria including probiotics, and food components, would be better to mimic human intestinal environments to ferment nature polysaccharides.
Also, the fermenting bacteria and polysaccharides may generate thousands of combinations which need plenty of research work. The actual effects depend on what bacteria and polysaccharides are employed. The starting experiments should focus on the single fermentations and then extend to more combinations to better understand their components and effects.
We expect the glycanbiotics to be applied with multiple combinations for each purpose, including food with long shelf storage, and health benefits.
Both natural polysaccharides and postbiotics have exhibited significant biological effects on health, including anti-inflammatory, antibacterial, immunomodulatory, antioxidant, gut barrier repairing and other effects. Natural polysaccharides are obtained from biological sources while postbiotics are produced by fermentation of LAB in the MRS medium. Research indicated that small molecular weight oligosaccharides originating from macromolecular polysaccharides provides better and more benefits to the human health. The oligosaccharides are so produced more often in the real world because of the presence of gut microbiota which can digest polysaccharides. Here we propose a new polysaccharide-based postbiotic, glycanbiotics, which can better describe what happens in the human gut thus providing novel applications for human health.
Akk: Akkermansia muciniphila
CAZymes: carbohydrate-active enzymes
EPS: exopolysaccharides
LAB: lactic acid bacteria
LPS: lipopolysaccharide
MRS: De Man, Rogosa, and Sharpe
SCFAs: short-chain fatty acids
WD: Conceptualization, Writing—original draft. LDM and JL: Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Olha Denefil ... Khrystyna Loza
Alejandra Vargas ... David A. Johnson
Shin Takasawa ... Maiko Takeda
Zeneng Wang ... Robert Koeth
