Affiliation:
1Department of Pathophysiology, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0002-3606-5215
Affiliation:
2Faculty of Medicine, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0003-2718-5191
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0001-7742-1346
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0001-8145-7931
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
Email: levkiv@tdmu.edu.ua
ORCID: https://orcid.org/0000-0001-7327-051X
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0003-2584-5942
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0002-6505-6086
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0001-6898-1149
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0002-9980-4556
Affiliation:
4Department of Dental Surgery, I. Horbachevsky Ternopil National Medical University, 46003 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0002-1247-2430
Explor Med. 2024;5:574–583 DOl: https://doi.org/10.37349/emed.2024.00241
Received: May 16, 2024 Accepted: July 10, 2024 Published: September 03, 2024
Academic Editor: Giuseppe Minervini, University of Campania, Italy
The article belongs to the special issue Oral Health Interconnections and Multidisciplinary Approaches
Aim: This study provides a comparative analysis of oral dysbiosis of patients with periodontal diseases: chronic catarrhal gingivitis and generalized periodontitis, associated with various systemic pathologies, using a combination of the enzymatic method and interval scale. Studying the differences in the oral microbiota of patients with periodontal diseases and systemic pathologies can help comprehend the underlying mechanisms and create successful treatments.
Methods: An enzymatic method was used to diagnose and monitor the degree of oral dysbiosis of patients with different systemic pathologies and periodontal diseases. We applied particular inclusion and exclusion criteria to include patients in a study. The level of microbial presence in the oral cavity can be measured by analyzing urease enzyme activity.
Results: The research established that oral dysbiosis is observed in all groups of patients with periodontal diseases and systemic pathology: chronic colitis, chronic pancreatitis, and primary hypothyroidism. The article discusses an express method of diagnosing the microbiota of the oral cavity in combination with an interval scale. This combination makes it possible to classify patients according to the level of oral dysbiosis and prescribe further recommendations for treatment.
Conclusions: The association of periodontitis and linked comorbidities is a complex interplay involving common risk factors, pathophysiology, and bidirectional causal relationships. The imbalance of microorganisms in the oral cavities of patients with systemic and periodontal diseases highlights the need for a personalized medical treatment approach. Correcting dysbiosis of the oral cavity should complement antimicrobial treatment for periodontal diseases and the normalization of metabolic processes in the periodontium. It has been confirmed that there is a correlation between patients’ microbial colonization of the oral cavity and the values obtained by the enzymatic method, suggesting that this approach can serve as a rapid assessment of the oral cavity’s microbiocenosis.
The etiological connection between systemic pathologies and periodontal diseases is an essential area of study in dentistry and medicine. Research has shown a bidirectional relationship between periodontal diseases and systemic conditions such as diabetes, cardiovascular disease, and respiratory diseases [1]. Understanding this connection is crucial for providing comprehensive healthcare for patients, as treating periodontal diseases can have a positive impact on systemic health, and managing systemic conditions can, in turn, benefit periodontal health. This interrelationship underscores the importance of interdisciplinary collaboration between dental and medical professionals in providing holistic patient care.
The human gut and oral microbiota are the largest microbiomes in the human body. They are connected because the oral cavity begins the digestive system. The oral cavity is a reservoir of resident microbial species that can ectopically colonize the gut and contribute to intestinal pathologies or exacerbate them. The interplay between gut and oral microbiota may contribute to the development of various diseases. This is likely due to the influx of bacteria and inflammatory cytokines from inflamed periodontal tissues into the systemic circulation. Additionally, studies have revealed that oral bacteria, especially those associated with periodontal diseases, play a role in causing imbalances in the gut microbiota, leading to pathology associated with systemic diseases. Although oral microbes cannot readily colonize a healthy gut, they become enriched in the gut microbiota (e.g., patients with inflammatory bowel disease). In total, factors that trigger gut dysbiosis (e.g., inflammation, antibiotics and unhealthy diets) promote the ability of oral pathobionts to colonize the gut. Conversely, disruptions in the gut microbiota have been shown to impact the development of periodontal diseases negatively [2].
Periodontal diseases such as chronic catarrhal gingivitis, and generalized periodontitis are significant issues in our society today. Their progressive condition affects the tissues surrounding the teeth. Damaged periodontal tissues may result in tooth loss and problems with the function of the maxillofacial system, which decreases patients’ quality of life [3]. Microbial aggression and their waste products are currently identified as the primary etiopathogenic factors of periodontal diseases [4, 5]. The harmfulness of bacteria in the oral cavity is not the only factor that determines the health of the gums. The modern understanding of the aetiology of periodontal diseases emphasizes the importance of the immune system’s condition and the periodontal tissues’ ability to resist bacterial invasion. The immune system plays a vital role in resisting and fighting microorganisms, which affects the gum’s health [6]. The growth of chronic inflammatory conditions affecting the periodontal tissues poses a significant difficulty for contemporary dental practice. This pathology is often caused by secondary immune deficiency [7]. Periodontal sites infected with herpesvirus are more prone to injury than sites free of the virus, and active herpesviral infection is linked to a higher risk of progressive periodontal diseases [8, 9]. Thus, the resistance of periodontal tissues to bacterial invasion and the state of the immune system are critical factors in the etiopathogenesis of periodontal diseases [10, 11]. Various significant general factors determine periodontal tissues’ resistance to pathogenic influences [12]. Most researchers point out that systemic pathology is a prevalent factor in the progression of periodontal diseases [13]. It has been established that systemic and periodontal pathology have a mutual influence and potentiating effect and can exacerbate each other.
Systemic diseases are considered risk factors for reducing immune protection mechanisms [14]. Secretory IgA antibodies play an important role in oral homeostasis. They are an indicator of the mouth’s adaptive immunity and influence oral pathology by interacting with oral microorganisms. The normal oral microbiota is crucial for maintaining the immune system’s function [15]. In addition, general disturbances in the level of immunoglobulins in the human body may manifest as pathological lesions in the periodontal tissues. Patients with periodontal diseases who have a chronic infection and intoxication in their oral cavity should not consider it a localized disease but rather a source of autoinfection and autointoxication for the entire body. Disturbances in the oral cavity’s microbiocenosis system may be caused by various factors, such as a decrease in the host’s level of antimicrobial protection, the use of antibiotics and antimicrobial drugs, disruptions in the nutrition and digestive system, and exposure to unfavourable physical factors [16]. When the microbiocenosis is disrupted, it is known as “dysbiosis.” Dysbiosis is a condition where the normal microbiome population structure is disturbed, generally due to extrinsic factors like medications, diet, and disease [17]. Oral dysbiosis occurs when the antimicrobial systems of the oral cavity and the whole organism interact in a way that disturbs the balance of microorganisms in the mouth.
The article discusses an express enzymatic method of diagnosing the oral cavity microbiota in combination with the interval scale. This combination allows doctors to classify patients according to their oral dysbiosis level and prescribe further treatment recommendations.
The degree of oral dysbiosis was assessed in a total of 247 patients who have periodontal diseases and systemic diseases including chronic colitis, chronic pancreatitis, and primary hypothyroidism. Among them: 62 have chronic colitis, 102 have chronic pancreatitis, 83 have primary hypothyroidism. The comparison group consisted of 30 patients with periodontal diseases without systemic pathology. We applied such inclusion and exclusion criteria to the patients included in the study (Table 1). The patient sample is the same as in the study [13]. In this paper, we study another parameter: dysbiotic changes in the oral cavity of patients with periodontal diseases and systemic pathologies.
Criteria for inclusion and exclusion were used to select patients who participated in this study
| Inclusion criteria | Exclusion criteria |
|---|---|
| A compensated form of a systemic disease, in its remission stage, no more than ten years. | Decompensated form of a systemic disease, patients with Crohn’s disease, more than ten years in combination with another systemic illness. |
| Patients who use pharmacotherapy as a treatment method for systemic illnesses. | Patients after surgical interventions of systemic pathology. |
| The patients’ ages range from 18 to 60 years. | The patients’ ages are over 60 years. |
| Patients with systemic diseases and the presence of teeth. | Patients with systemic diseases and loss of teeth. |
The study included patients receiving outpatient treatment and under dispensary observation in city hospitals. Dentists examined patients on an outpatient basis at the Department of Dental Therapy of “I. Horbachevsky Ternopil National Medical University.” The department’s doctors confirmed the presence of systemic diseases based on valid national and international protocols. The process involved examining clinical and instrumental data, followed by reference to the international classification of diseases. The Bioethics Committee of I. Horbachevsky Ternopil National Medical University Ministry of Health of Ukraine approved the study protocols (excerpt from protocol No. 73) on April 3, 2023.
Before participating in the study, all patients were given information about study purpose and signed a consent form indicating their agreement to participate. Dental clinical examination of patients was carried out using the generally accepted main and extra examination methods. Diagnostic parameters such as probing depths, bleeding on probing, clinical attachment level loss, plaque index, and radiographs assessing alveolar bone level are utilized to diagnose periodontal diseases.
The Gingival index [18], and Papilla bleeding index [19], were used to diagnose gingivitis [20].
To diagnose generalized periodontitis, dental professionals assessed the depth of periodontal pockets and reviewed X-ray images for signs of osteoporosis, cortical plate damage, and destruction of inter-alveolar septa.
An enzymatic method developed by Levytskyi AP et al. [21] was utilized to diagnose and track the severity of oral dysbiosis. The level of microbial presence in the oral cavity can be measured by analyzing the activity of the urease enzyme. This enzyme is not naturally produced by the human body’s cells but rather by plant cells and by many bacteria, including opportunistic or pathogenic ones. It is possible to evaluate the microbial colonization of the oral cavity by comparing the urease activity of the sample under study with a similar indicator found in healthy individuals. Through this comparison, one can determine whether there has been an increase or decrease in the microbial colonization of the oral cavity. The activity of lysozyme is closely linked to the condition of antimicrobial systems. It reliably indicates the host’s non-specific and specific antimicrobial factors [22].
The methodology involved several subsequent phases:
Centrifuge tubes were used to collect oral fluid for 5 min in the morning without stimulation, on an empty stomach, and before morning oral hygiene. Additionally, oral fluid was collected 3 min after rinsing the mouth with water. The volume of oral fluid was measured after centrifugation (2,500 g, 5 min, at 0 + 5℃).
Levytskyi AP et al. [21] describe a method for determining urease activity in a methodology recommendation. The process relies on urease’s capacity to divide urea and produce ammonia. A readymade solution containing 0.1 M urea and Nessler’s reagent was used as a substrate for the experiment.
Lysozyme activity was determined using the bacteriolytic method, modified by Levytskyi AP [21]. The method’s basis was lysozyme’s capacity to induce the lysis of various bacteria, including Micrococcus lysodeikticus (standard strain 2665) cells. The substrate was a phosphate buffer containing a suspension of acetone powder from these bacteria.
Calculations according to the formulas determined the relative activities of urease and lysozyme (U relative and L relative):
where, U examined group and L examined group—activity of urease and lysozyme in the examined group of patients; U control group and L control group–activity of urease and lysozyme in the control group of examined patients.
The calculation of the dysbiosis degree (DD) in the oral cavity was determined by using a specific formula:
where, DD—dysbiosis degree in the oral cavity (units without dimensions); U relative—relative urease activity; L relative—relative activity of lysozyme.
The DD value always equals ‘‘1.0’’ in individuals with good health. When there is a disturbance in the microbiocenosis of the oral cavity, the DD indicator becomes higher than 1.0. The DD value increases with the oral dysbiosis’s severity [21].
The oral cavity has three degrees of dysbiosis [21]:
The first degree is the compensated stage, with a range from 1.5 to 3.
The second degree is the sub-compensated stage, with a range from 3 to 9.
The third degree is the decompensated stage, with a range from 9 to 20.
The resulting findings were computed using the descriptive statistics [23–25].
According to the results of the research, it was established that dysbiotic changes in the microbiocenosis of the oral cavity are observed in all groups of patients with periodontal diseases and systemic pathology: chronic colitis, chronic pancreatitis, and primary hypothyroidism (Table 2).
The degree of oral dysbiosis of patients with periodontal diseases and chronic colitis, chronic pancreatitis, and primary hypothyroidism
| Examination groups | Degree of oral dysbiosis | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | Total number of patients | ||||
| n | % | n | % | n | % | ||
| Patients with periodontal diseases without systemic pathology (І group) | 18 | 60.00 | 12 | 40.00 | 0 | 0.00 | 30 |
| Patients with chronic colitis (ІІ group) | 13 | 20.97 | 31 | 50.00 | 18 | 29.03 | 62 |
| Patients with chronic pancreatitis (ІІІ group) | 37 | 36.27 | 53 | 51.96 | 12 | 11.76 | 102 |
| Patients with primary hypothyroidism (ІV group) | 57 | 68.67 | 21 | 25.30 | 5 | 6.02 | 83 |
The researchers detected oral dysbiosis of the I degree more often in patients of the IV group (68.67%) and in patients of the I group (60.00%), dysbiosis of the II degree was highest in patients of the III group (51.96%) and I group (40.00%). Only five patients (6.02% of cases) of the IV group had a III degree of oral dysbiotic changes, and we did not detect such dysbiotic disorders in the I group. Whereas in the examined patients of the II group, an oral dysbiosis of the III degree was found in 29.03% cases.
Patients who have chronic colitis, chronic pancreatitis and periodontal diseases showed the highest values of oral dysbiotic disorders upon comparison (Figure 1).
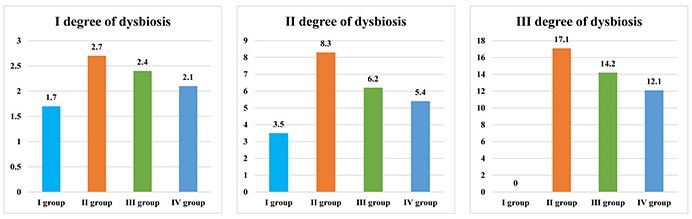
Numerical representation of I, II, and III degree oral dysbiosis in patients with periodontal diseases and systemic pathology
When the numerical values of oral dysbiotic disorders are compared with the values of all groups, it can be noted that they are the highest in individuals of the II group.
Thus, the I degree of oral dysbiosis, in the II group is 1.59 times higher than that in the I group, 1.13 times higher than that in the III group, and 1.29 times higher than that in the IV group of patients. The II degree of oral dysbiosis, is 2.37 times higher in the II group compared to the I group, 1.34 times higher compared to the III group, and 1.54 times higher compared to the IV group of patients. The III degree of oral dysbiosis, is 1.20 times higher in the II group than in the III group and 1.41 times higher than in the IV group.
The periodontal microbiota of patients with periodontal diseases and systemic pathology is disturbed in both number and species composition [13]. This suggests that systemic disorders impact the oral cavity’s microbiota, which can serve as a predictive factor for justifying preventive anti-relapse courses of antimicrobial pharmacotherapy, in particular, use of oral antiseptics.
Periodontal diseases are chronic infectious diseases that result from a response to a complex dental plaque microbiome containing various periodontopathic bacteria species. Periodontal diseases destroy the tooth-supporting tissues and lead to tooth loss. Epidemiological evidence suggests that periodontal infection is associated with an increased risk of a variety of diseases. Scientific research has proven a close relationship between systemic diseases, lesions of the hard tissues of the teeth and the state of the periodontal tissues [26, 27]. Inflammatory processes in the oral cavity can be the first clinical signs of systemic disorders in digestive, cardiovascular and other systems [28]. The presence of systemic diseases in the human body significantly affects the etiopathogenesis of periodontal diseases. Systemic diseases reduce the general immunity, involving the oral cavity’s local immunity. The level of the secretory IgA, which blocks microorganisms from attaching to mucosal epithelial cells, decreases. Similarly, the level of lysozyme, which is an intrinsic component of the oral immunity, decreases. These processes lead to oral dysbiosis. Dysbiosis is a condition marked by the imbalance of microbiocenosis and the proliferation of opportunistic microorganisms. It is commonly associated with periodontal diseases [29, 30]. Fungi, viruses, bacteria, and their exotoxins and endotoxins circulate systematically in the human body. Periodontopathogens’ virulence factors include the ability to invade and release toxins (leukotoxins of various strains of Actinobacillus actinomycetemcomitans and a specific lipopolysaccharide of Porphyromonas gingivalis), which activate inflammatory mediators, cytokines, B-lymphocytes, proteases, and other enzymes released upon contact with epithelial cells [31].
More research is required to determine methods for recovering and maintaining stability in the oral microbiota during periodontal diseases [32, 33]. Numerous researchers suggest incorporating nanotechnology in routine periodontal therapy to eradicate pathogenic bacteria, as bacterial colonization is among the initial factors that cause this condition [34, 35]. According to the American Academy of Periodontology recommendations, the correction of the body’s immune response during the development of periodontal diseases, along with the control of etiologically significant bacteria, should form the basis for the modern concept of periodontitis treatment [36]. It is known that treatment should be etiological, pathogenic, and symptomatic. This applies to the following groups of drugs: antibacterial agents, antiseptics, anti-inflammatory drugs, and stimulators of reparative processes. Similarly, it is necessary to include an immunomodulatory drug in the scheme of complex treatment. This will increase the level of humoral immunity of the oral cavity and contribute to a faster onset and a longer period of remission. Antimicrobial therapy is the priority during treatment of periodontal diseases [37] as it involves using antibiotics to target the pathogenic bacteria in the oral microbiome. However, the overuse of antibiotics can lead to the development of antibiotic-resistant strains of bacteria. The results of our research [13] indicate that antimicrobial drugs should be selected, considering the sensitivity of the oral cavity ecosystem. Moreover, with long-term and uncontrolled use, antibacterial therapy while treating periodontal diseases can even induce or exacerbate dysbiotic disorders. Numerous research studies validate alterations in the overall quantity of microorganisms or changes in the proportion of species, like a reduction in the count of beneficial bacteria and an upsurge in potential and even harmful microorganisms (i.e., Escherichia coli in the oral cavity) in periodontal diseases [38, 39].
Managing dysbiosis in the oral cavity involves using various strategies such as probiotics and prebiotics. Probiotics and prebiotics are emerging techniques for managing dysbiosis in the oral cavity [40]. Probiotics involve the use of live microbial organisms that confer health benefits to the host. Prebiotics are non-digestible food ingredients that promote the growth of beneficial bacteria in the gut. These strategies [41] have shown promising results in clinical trials, and further research is needed to establish their efficacy in managing dysbiosis in the oral cavity.
Understanding the systemic influence on oral health and utilizing enzymatic assessment can provide a comprehensive approach to managing periodontal diseases. Urease and lysozyme activity can be assessed using an enzymatic method, indicating their potential for diagnosis, planning treatment, and monitoring the progress of periodontal diseases. Periodontal diseases are primarily caused by microorganisms that can compromise the oral health when the body’s protective mechanisms weaken. Furthermore, the disturbed balance of the microbiocenosis plays a role in developing periodontal diseases. The enzymatic method evaluates both the state of microbiocenosis and the state of antimicrobial resistance of the body, aiding in determining dysbiosis of the oral cavity. The body resistance system includes lysozyme, an indicator of oral fluid’s antimicrobial protection. The oral biotope reflects the microbiocenosis of other biotopes of the human body to a certain extent. Therefore, oral dysbiosis can not be considered solely as a local syndrome. According to microbiological research data [13], there is a correlation between the microbial colonization of patients’ oral cavities and the values obtained by the enzymatic method. Thus, this method can be used as an express assessment of the microbiocenosis of the oral cavity.
The association of periodontitis and linked comorbidities is quite complex, involving both common risk factors and pathophysiology as well as bidirectional causal relationships. Achieving a holistic understanding of periodontitis-associated comorbidities may lead to new therapeutic options for treating periodontitis and associated comorbidities.
This paper considers an enzymatic method of diagnosing the oral cavity microbiota in combination with the interval scale. This combination allows the classification of patients with respect to the level of oral dysbiosis.
The results presented here suggest that periodontal diseases are prevalent in the examined group of patients, implying that the systemic disease directly affects the health of patients’ periodontal tissues. After comparing the severity of dysbiosis in the oral cavity among the patients involved in this study, we noticed that disorders were more prominent in individuals with colitis and periodontal diseases. Thus, a comprehensive approach to pharmacotherapy is necessary for the treatment of periodontal diseases in patients with chronic colitis, chronic pancreatitis, and primary hypothyroidism, taking into account the specific features of the pathogenesis of these diseases. Particular attention should be paid to choosing an antiseptic as an antimicrobial therapy for such a group of patients. Considering the degree of dysbiosis of the oral cavity, probiotics can be recommended in a complex treatment.
It has been confirmed that there is a correlation between patients’ microbial colonization of the oral cavity and the values obtained by the enzymatic method. Thus, this approach can serve as a quick evaluation of the oral cavity’s microbiocenosis.
The authors are grateful to Professor Orest Kochan for his advice and support and to Tetiana Yakushevych for professional support in language editing. The work is prepared within an inter-department research project «Development and implementation of differentiated approaches of diagnosis, treatment and prevention of periodontal and oral mucosa diseases» (state registration number 0123U100071).
OD: Conceptualization, Writing—original draft, Writing—review & editing. SC: Conceptualization, Investigation, Writing—review & editing. SB: Investigation, Data curation, Writing—review & editing. NC and ML: Validation, Writing—review & editing, Supervision. LP and KP: Investigation, Data curation, Formal analysis, Writing—review & editing. NM: Conceptualization, Investigation, Writing—original draft. MZ: Writing—review & editing, Supervision. NT: Investigation, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The Bioethics Committee of I. Horbachevsky Ternopil National Medical University approved the study protocol (excerpt from protocol No. 73) on April 3, 2023. All the patient investigations conformed to the principles outlined in the Council of Europe Convention on Human Rights and Biomedicine (April 4, 1997), the World Health Association Helsinki Declaration on Ethical Principles for Scientific Research with Human Participation (1964–2000), and the Order of the Ministry of Health of Ukraine No. 281 of November 1, 2000; Declaration of Helsinki “World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects” (2001), Code of Scientist of Ukraine (2009). All patients’ examinations were done with informed consent.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets for this study will be available on reasonable request to the corresponding author.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Martina Costanzo ... Ilenia Campione
Zeina Darwich ... Chadi Azmeh
Francesca Gorassini ... Gabriele Cervino
Gerardo Pellegrino ... Giuseppe Lizio
Alberto Enrique Varela ... José E. Rodríguez
Aiswarya Polumatla ... Tejaswin Polepalle
Aya Dawoud Agha ... Moudar Bakkour
Alessia Pardo ... Massimo Albanese
