Affiliation:
1Department of Ophthalmology, Cathay General Hospital, Taipei City 10633, Taiwan, China
Affiliation:
2Division of Cardiology, Department of Internal Medicine, Shuang Ho Hospital, Ministry of Health and Welfare, Taipei Medical University, New Taipei City 23561, Taiwan, China
3Division of Cardiology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei City 11002, Taiwan, China
ORCID: https://orcid.org/0000-0001-6615-3200
Affiliation:
4Institute of Biomedical Sciences, Academia Sinica, Taipei City 115201, Taiwan, China
5Division of Cardiology, Department of Internal Medicine and Graduate Institute of Clinical Medical Science, China Medical University, Taichung City 404328, Taiwan, China
Affiliation:
2Division of Cardiology, Department of Internal Medicine, Shuang Ho Hospital, Ministry of Health and Welfare, Taipei Medical University, New Taipei City 23561, Taiwan, China
3Division of Cardiology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei City 11002, Taiwan, China
Email: b8501043@tmu.edu.tw
ORCID: https://orcid.org/0000-0001-6067-1306
Affiliation:
6Department of Biochemistry, School of Medicine, College of Medicine, China Medical University, Taichung City 404328, Taiwan, China
Email: thcheng@mail.cmu.edu.tw
ORCID: https://orcid.org/0000-0002-9155-4169
Explor Med. 2024;5:732–749 DOI: https://doi.org/10.37349/emed.2024.00253
Received: August 13, 2024 Accepted: October 14, 2024 Published: October 31, 2024
Academic Editor: Leah A. Owen, University of Utah School of Medicine, USA
Hyperuricemia (HUA), defined by elevated serum uric acid levels, is well-established in its association with systemic conditions like gout and cardiovascular diseases. Recently, however, emerging research has revealed a potential connection between HUA and ocular disorders, particularly epiretinal pathologies. This review investigates the pathophysiological mechanisms linking HUA to epiretinal conditions, including epiretinal membrane formation, macular edema, and retinal vascular diseases. By thoroughly analyzing current literature, this review seeks to deepen the understanding of the relationship between HUA and epiretinal disorders, with the aim of informing new therapeutic strategies and enhancing patient outcomes.
Hyperuricemia (HUA), characterized by elevated serum uric acid levels, has traditionally been recognized as a significant risk factor for systemic conditions such as gout, cardiovascular disease, and metabolic syndrome [1]. Further evidence highlights the role of HUA in a wide range of systemic diseases, including hepatic steatosis, metabolic syndrome, diabetes, and thyroid dysfunction—all of which share common inflammatory pathways. In patients with metabolic syndrome, HUA is consistently observed and directly linked to chronic low-grade inflammation [2]. In this context, elevated uric acid levels correlate with inflammatory markers such as C-reactive protein (CRP) and pro-inflammatory cytokines, suggesting that uric acid may contribute to the chronic inflammation underlying metabolic syndrome. This establishes a mechanistic link between HUA and systemic inflammation. Alike, in diabetes, HUA is associated with insulin resistance, oxidative stress, and inflammation, all of which worsen glycemic control and accelerate the progression of diabetic complications [3]. Also, HUA has been implicated in the development of diabetic kidney disease, potentially accelerating the decline in renal function in affected patients [4]. The connection between HUA and inflammation is also evident in hepatic steatosis, where uric acid appears to contribute to the development of non-alcoholic fatty liver disease by inducing oxidative stress and activating pro-inflammatory pathways in hepatic cells [5]. This systemic link between uric acid and liver inflammation underscores the broad inflammatory role of HUA across various organ systems. Moreover, elevated uric acid levels are found in autoimmune conditions like thyroiditis, where HUA may exacerbate autoimmune inflammation by activating immune cells and promoting oxidative stress [6]. This relationship highlights uric acid’s broader role in disease pathology, extending beyond metabolic conditions to autoimmune diseases. HUA is also implicated in promoting insulin resistance and chronic inflammation, which may contribute to the development of new-onset diabetes [7]. As well, the link between HUA and inflammation extends to the eye. Recent research points to its potential role in ocular pathologies, particularly those affecting the vitreoretinal interface, collectively referred to as epiretinal pathologies [8]. These conditions encompass epiretinal membrane (ERM), macular edema, and vitreomacular traction (VMT), all of which can lead to significant visual impairment [9]. Retinal diseases primarily affect the retina, the light-sensitive tissue at the back of the eye responsible for capturing visual information. Common conditions include age-related macular degeneration (ARMD), diabetic retinopathy, and retinal detachment, all of which directly impair vision by altering the retina’s structure or function [10]. In contrast, epiretinal diseases involve the formation of membranes on the surface of the retina. Conditions such as ERMs and macular holes can lead to visual distortion and changes in visual acuity by disrupting the retina’s normal architecture [11]. Although both types of diseases may share underlying mechanisms, such as inflammation or vascular changes, the distinction lies in their specific anatomical locations and their effects on vision. Understanding these differences is essential for accurate diagnosis and treatment, as management strategies for retinal and epiretinal diseases can vary significantly [12]. Furthermore, epiretinal pathologies, while distinct from retinal diseases, exhibit overlapping mechanisms that may be influenced by conditions like HUA. Recent studies have highlighted the complex relationship between inflammation and eye diseases, indicating that immune responses to retinal injury can exacerbate both retinal and epiretinal conditions [12]. Moreover, the molecular characterization of idiopathic ERMs has identified two distinct clusters, suggesting different underlying mechanisms that may interact with systemic factors such as HUA [11]. The association between HUA and ocular diseases has gained increasing attention (Table 1). Studies have reported elevated serum uric acid levels in patients with a range of retinal disorders, such as diabetic macular edema [13], diabetic retinopathy [14], and impaired retinal microcirculation [15]. Research has identified multiple mechanisms through which uric acid may contribute to retinal damage, including oxidative stress, inflammation, and endothelial dysfunction [16]. Elevated levels of inflammation markers have been increasingly recognized as critical factors in the pathogenesis of epiretinal pathologies. For instance, Thounaojam et al. [16] demonstrated that monosodium urate significantly enhances retinal inflammation and promotes the progression of diabetic retinopathy. This finding underscores the potential of HUA to instigate inflammatory pathways that exacerbate retinal conditions. Moreover, a comprehensive review by Vishwakarma et al. [17] highlighted the intricate interplay between oxidative stress and inflammation in retinal diseases, suggesting that these processes may synergistically contribute to the deterioration of retinal health. They emphasized that oxidative stress can lead to cellular damage, further triggering inflammatory responses that may amplify retinal pathology. Despite these insights, the precise pathophysiological connections linking HUA and epiretinal pathologies remain partially understood, necessitating further exploration to elucidate these complex interactions (Figure 1).
Associations of serum uric acid levels with ocular conditions
| Authors | Years | Title | Results |
|---|---|---|---|
| Xiong et al. [18] | 2023 | Influence of Serum Uric Acid on Macular Choroidal Thickness and Ganglion Cell Inner Plexiform Layer Thickness | Higher uric acid levels are independently associated with macular choroid and ganglion cell inner plexiform layer thinning |
| Wei et al. [19] | 2023 | Serum Uric Acid Levels Are Associated With Macula Microvasculature Changes In Hypertensive White Matter Hyperintensity Patients | Serum uric acid levels are associated with macula microvascular changes in white matter hyperintensity |
| Geng et al. [20] | 2023 | Sex-specific association of serum uric acid trajectories with risk of incident retinal arteriosclerosis in Chinese population: A population-based longitudinal study | Higher serum uric acid levels are significantly associated with an increased risk of incident retinal arteriosclerosis |
| Qin et al. [13] | 2022 | Elevated level of uric acid, but not glucose, in aqueous humor as a risk factor for diabetic macular edema in patients with type 2 diabetes | Increased aqueous humor uric acid is an independent risk factor for diabetic macular edema, and intravitreal uric acid-lowering therapy could be beneficial |
| Yang et al. [21] | 2022 | Association of Serum Uric Acid With Retinal Capillary Plexus | Higher serum uric acid levels are significantly associated with lower vessel density in the retinal capillary plexus |
| Serra et al. [22] | 2021 | Detection of serum uric acid in primary open angle glaucoma: A pilot study | Serum uric acid levels may be decreased and correlated with worsening visual field and optical coherence tomography parameters |
| Li et al. [23] | 2019 | Association of serum uric acid levels with primary open-angle glaucoma: a 5-year case–control study | Decreased uric acid levels may be associated with an increased risk of primary open-angle glaucoma |
| Thounaojam et al. [16] | 2019 | Monosodium Urate Contributes to Retinal Inflammation and Progression of Diabetic Retinopathy | Uric acid contributes to leukostasis in the diabetic retina. Limiting uric acid production and accumulation in the diabetic retina may halt retinal inflammation and prevent hyperglycemia-induced breakdown of the blood-retinal barrier |
| Chen et al. [24] | 2017 | Serum uric acid concentration is associated with hypertensive retinopathy in hypertensive chinese adults | Serum uric acid concentration is significantly associated with hypertensive retinopathy |
This table summarizes various studies that explore the influence of serum uric acid levels on different ocular conditions and retinal changes. The studies listed provide insights into how elevated or decreased serum uric acid levels impact macular choroidal thickness, ganglion cell inner plexiform layer thickness, macula microvasculature, retinal arteriosclerosis, diabetic macular edema, retinal capillary plexus vessel density, primary open-angle glaucoma, diabetic retinopathy, and hypertensive retinopathy
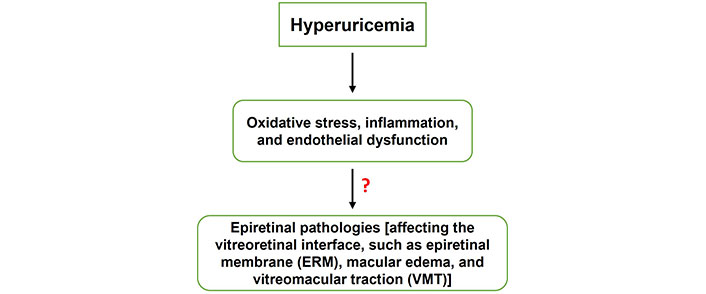
The exact mechanisms connecting hyperuricemia (HUA) to epiretinal pathologies remain incompletely understood. This figure outlines a proposed pathway whereby HUA leads to oxidative stress, inflammation, and endothelial dysfunction. These conditions can collectively contribute to various epiretinal pathologies affecting the vitreoretinal interface, such as ERM, macular edema, and VMT. Further research is needed to elucidate the detailed processes involved in these pathophysiological links
Understanding the pathophysiological links between HUA and epiretinal disorders holds significant clinical importance. Elucidating the mechanisms by which HUA contributes to the onset and progression of these conditions may facilitate the identification of new molecular targets for therapeutic intervention. Recognizing HUA as a modifiable risk factor has critical implications for patient management; early detection and treatment of elevated uric acid levels could reduce the risk of developing or exacerbating epiretinal conditions, ultimately preserving vision and improving long-term outcomes. Interventions aimed at lowering serum uric acid may complement existing treatments, providing a more integrated approach to care. This review offers a comprehensive overview of the current understanding of the relationship between HUA and epiretinal pathologies. It synthesizes the existing literature on the connections between HUA and conditions such as ERM, macular edema, and VMT. The underlying mechanisms, such as oxidative stress, inflammation, and endothelial dysfunction, are explored to explain this association. Moreover, the clinical implications of HUA in the diagnosis, prognosis, and treatment of epiretinal disorders are examined, along with potential therapeutic targets for future intervention. By addressing these objectives, this review contributes to the growing body of knowledge on the intersection of HUA and ocular health, offering valuable insights for clinical practice and guiding future research.
HUA is characterized by abnormally elevated serum uric acid levels, typically exceeding 7 mg/dL in men and 6 mg/dL in women [25]. Uric acid is the end-product of purine metabolism in humans, primarily synthesized in the liver via the enzymatic conversion of purine nucleotides to uric acid by xanthine oxidase. HUA can result from increased uric acid production, decreased renal excretion, or a combination of both [26]. Various factors contribute to its development, including dietary habits, genetic predisposition, renal dysfunction, and certain medical conditions [27]. Luo et al. [28] highlighted the relationship between dietary patterns and chronic kidney disease in HUA, while Tero-Vescan et al. [29] explored the role of fructose-induced HUA and its potential to promote oxidative stress. As well, Sun et al. [30] investigated the use of probiotics, bioactive compounds, and dietary strategies in HUA management, providing insights into holistic approaches to mitigate the condition. Genetic predisposition plays a crucial role in the development of HUA, particularly through polymorphisms in genes that regulate uric acid transport and purine metabolism. Qi et al. [31] explored the distinct phenotypic and genetic profiles of HUA, classifying gout patients based on their renal uric acid handling. This study identified subtypes of HUA, which have distinct clinical and genetic characteristics, helping to clarify how variations in renal uric acid excretion can lead to different manifestations of gout and associated comorbidities. Cho et al. [32] conducted an extensive cross-ancestry genome-wide meta-analysis, which provides a comprehensive overview of the genetic factors influencing serum uric acid levels across various populations. This large-scale study identified novel loci associated with serum urate levels and emphasized the importance of population diversity in understanding the genetic basis of HUA. These findings contribute to refining the genetic landscape of HUA and identifying potential therapeutic targets for managing elevated uric acid levels. Further advancing the understanding of HUA’s genetic underpinnings, Wu et al. [33] highlighted the role of the solute carrier family 2, member 9 (SLC2A9) gene variant (rs16890979) in uric acid transport. Using kidney organoids, the study demonstrated that this genetic variant significantly reduces uric acid absorption, thus providing a mechanistic explanation for its protective effect against HUA and gout. This insight underscores the importance of kidney function and uric acid handling in the pathophysiology of HUA. Also, Vávra et al. [34] focused on rare allelic variants in urate transporter (URAT) genes (SLC22A11, SLC22A13, and SLC17A1). Their research delved into the functional consequences of these variants and their association with HUA and gout. The study enriched our understanding of how rare genetic mutations can disrupt uric acid homeostasis, contributing to the development of gout in certain populations.
The regulation of uric acid levels is a highly orchestrated process, primarily influenced by the metabolism of purine nucleotides, which are derived either from dietary intake or through endogenous synthesis. A key enzyme in this metabolic pathway is xanthine oxidase, which catalyzes the sequential oxidation of hypoxanthine to xanthine, and then to uric acid. This process is a critical point in purine catabolism, as uric acid represents the final product in humans, unlike other mammals that further degrade uric acid [35]. Once produced, most uric acid is excreted through the kidneys, where renal mechanisms tightly regulate its reabsorption and secretion. URATs in the proximal tubules play a crucial role in maintaining serum uric acid levels by balancing these processes [36]. Exclusively, inhibiting xanthine oxidase has been a therapeutic target, and various dietary-based bioactive compounds are being explored for their potential to modulate this enzyme, providing alternative treatments for HUA [37]. Thus, maintaining serum uric acid levels within a physiological range involves a complex interplay of factors, including dietary intake [38], renal excretion [39], and enzymatic activity [40]. Renal clearance is primarily mediated by specific transporters in the proximal tubules, such as URAT1 and glucose transporter 9 (GLUT9) [41]. Dysfunction of these transporters can impair uric acid excretion, leading to HUA. Alterations in xanthine oxidase activity can also affect uric acid production, further contributing to dysregulation [42].
HUA is associated with a range of clinical manifestations and health consequences. Saag et al. [43] reported a link between HUA, gout, and cardiovascular mortality, especially in patients treated with febuxostat or allopurinol. Kim and Jun [44] noted that altered serum uric acid levels are implicated in kidney disorders, suggesting a role in renal pathophysiology. Dos Santos et al. [45] explored the connection between uric acid and kidney damage in systemic lupus erythematosus, while Lu et al. [46] demonstrated that HUA predisposes individuals to diabetes onset by inducing pancreatic β-cell death. Hamid et al. [47] observed an increased prevalence of gout in patients with inflammatory bowel disease, further indicating systemic implications of HUA. Emerging evidence also suggests a potential impact on ocular health. Biggerstaff et al. [48] found a link between gout and an increased risk of open-angle glaucoma in veterans, while Wu et al. [49] revealed an association between uric acid and interleukin-1β (IL-1β) levels in the tear fluid of HUA and gout patients. Posa et al. [50] recommended historical profiling of dry eye patients to identify possible triggers and comorbidities. These findings underscore the complex nature of HUA and its widespread effects, necessitating comprehensive management strategies tailored to individual clinical contexts.
Epiretinal pathologies encompass a range of disorders that affect the vitreoretinal interface, the delicate junction between the vitreous gel and the retina. This interface plays a critical role in ocular structure and function. Phillips et al. [51] emphasized its significance in the development of various retinal pathologies. Abnormal epiretinal tissue, in particular, has been implicated in the formation of macular holes, as described by Hwang and Kang [52]. Tetsumura et al. [53] identified short-term changes in mound-like epiretinal material, suggesting a link to posterior vitreous detachment. A clear understanding of these complexities is essential for accurate diagnosis and effective management of epiretinal disorders. ERM and other epiretinal pathologies, such as macular edema and VMT, significantly affect retinal structure and function. Each condition manifests through unique clinical presentations and mechanisms. Research has illuminated the compositional diversity and histological features of ERM, which is particularly relevant for understanding its impact on retinal health. Ożóg et al. [9] extensively reviewed ERM’s pathophysiology, demonstrating how this condition distorts the retina, resulting in impaired visual acuity and metamorphopsia. ERM is characterized by fibrocellular proliferation on the retinal surface, leading to traction and distortion of the underlying retinal layers. This mechanical disruption affects the photoreceptor alignment and overall retinal integrity, contributing to reduced retinal function and visual performance. Further, the diverse cellular makeup of ERM has been a focal point of recent studies. Andjelic et al. [54] applied advanced spectroscopy techniques, revealing that epiretinal proliferations in ERM are highly heterogeneous, consisting of multiple cell types, including glial cells, fibroblasts, and immune cells. These findings underscore the complexity of ERM tissues, suggesting that the cellular environment plays a critical role in disease progression and clinical variability. Histologically, da Silva et al. [55] analyzed idiopathic ERMs, identifying key structural features such as dense cellular layers with interspersed collagen and extracellular matrix proteins. These histological characteristics are consistent with the mechanical forces exerted by ERM on the retina, leading to retinal layer displacement and thickening. The presence of myofibroblasts within the membrane suggests active contraction, further contributing to the pathogenesis of retinal deformation and functional impairment. Yang et al. [56] investigated the relationship between foveal microstructure and visual outcomes after pars plana vitrectomy in patients with various types of ERM foveoschisis, underscoring the need for tailored management approaches for different epiretinal conditions. This multifaceted composition of ERM highlights its dynamic nature and the importance of considering both cellular and extracellular components in managing the condition. Through these histological and compositional insights, it becomes evident that ERM exerts a substantial impact on retinal function by disrupting normal retinal architecture, causing both structural and functional changes that manifest clinically as visual disturbances. Besides, epiretinal pathologies arise from diverse and multifactorial causes. Central to the development of these conditions are structural changes at the vitreoretinal interface, which can be influenced by aging, trauma, inflammation, or metabolic disorders like diabetes. HUA has recently been linked to retinal pathologies, particularly through its contribution to oxidative stress and inflammation, which may exacerbate damage to retinal tissue [8, 13]. These changes are especially concerning in conditions like diabetic retinopathy and glaucoma, where compromised vascular health interacts with metabolic stressors, potentially accelerating retinal degeneration [10, 16]. Additionally, the immune response to ocular injury and chronic inflammation plays a significant role in the progression of these disorders [12]. Understanding the risk factors, such as aging, metabolic conditions like diabetes and HUA, and ocular comorbidities, enables clinicians to better stratify risk, allowing for early detection and targeted interventions that may help prevent vision loss [9]. As new insights into the molecular mechanisms behind epiretinal pathologies emerge, especially the role of inflammatory pathways and oxidative stress, these factors are increasingly seen as therapeutic targets [17, 21].
Recent studies have drawn attention to the association between HUA and epiretinal pathologies, suggesting complex interactions with significant clinical implications (Table 2). While early research like that by Subramani et al. [57] found no direct correlation between serum uric acid levels and ARMD, other studies have since highlighted the role of HUA in more specific vitreoretinal conditions. Karimi et al. [58] noted a link between serum uric acid levels and the outcomes of intravitreal bevacizumab treatment in diabetic macular edema, pointing to the relevance of HUA in diabetic retinopathy. Moreover, Qin et al. [13] demonstrated that elevated uric acid levels in aqueous humor significantly increase the risk of diabetic macular edema, highlighting the involvement of HUA in retinal complications associated with diabetes. Recent research has broadened this view, indicating that HUA not only affects the retina but may also play a role in epiretinal pathologies such as ERM, VMT, and macular edema. These conditions, which affect the vitreoretinal interface, can lead to significant vision impairment. Xiong et al. [18] and Wei et al. [19] have shown that HUA may influence macular choroidal thickness and microvascular changes in hypertensive patients, extending its impact beyond retinal health to epiretinal structures. Associations between serum uric acid concentration and conditions like hypertensive retinopathy [24] and primary open-angle glaucoma [23] point to the wider implications for retinal vascular health. Thounaojam et al. [16] linked monosodium urate crystals with retinal inflammation and the progression of diabetic retinopathy, further underscoring the inflammatory mechanisms by which HUA may contribute to both retinal and epiretinal disease processes. Other studies, such as those by Yang et al. [21] and Li et al. [59], explored how serum uric acid levels affect retinal capillary plexus and microvessel function, emphasizing the broader implications of HUA on microvascular health. In addition, sex-specific associations between serum uric acid levels and conditions like retinal arteriosclerosis have been reported [20]. This complexity, combined with findings on primary open-angle glaucoma [22], reinforces the broad impact of HUA on ocular health, particularly regarding the interplay between systemic metabolic dysregulation and localized ocular changes. These studies collectively highlight the need for further research to unravel the mechanistic pathways by which HUA contributes to epiretinal pathologies.
Key studies investigating the relationship between hyperuricemia (HUA) and epiretinal pathologies
| Authors | Year | Objective | Findings | Implications |
|---|---|---|---|---|
| Thounaojam et al. [16] | 2019 | To examine the contribution of monosodium urate to retinal inflammation in diabetic retinopathy | Monosodium urate crystals promoted retinal inflammation and progression of diabetic retinopathy | Points to the inflammatory role of urate crystals in retinal diseases, advocating for anti-inflammatory treatments in hyperuricemic patients |
| Pai et al. [8] | 2022 | To examine the effects of short-term HUA on retinal structure in mice | HUA led to structural retinal changes, which were reversible with uric acid-lowering agents | Indicates potential benefits of early intervention with uric acid-lowering therapy to prevent retinal damage |
| Qin et al. [13] | 2022 | To investigate the role of uric acid in diabetic macular edema | Elevated uric acid in aqueous humor was identified as a risk factor for diabetic macular edema in type 2 diabetes patients | Suggests monitoring and managing uric acid levels in diabetic patients to reduce the risk of macular edema |
| Lu et al. [15] | 2022 | To study the relationship between serum uric acid and retinal microvasculature using optical coherence tomography angiography | High serum uric acid was correlated with microvasculature deficits in the retina | Reinforces the importance of controlling uric acid levels to preserve retinal vascular health |
| Wei et al. [14] | 2023 | To evaluate biomarkers for diabetic retinopathy complicated with HUA | Serum VEGF, high-sensitivity CRP, and cystatin-C levels were useful in diagnosing diabetic retinopathy with HUA | Supports the use of these biomarkers in clinical practice to improve the diagnosis and management of retinopathy |
| Su et al. [25] | 2023 | To explore the sex differences in the relationship between obesity-related indices and HUA | HUA was linked to obesity-related indices, with differences observed between sexes | Highlights the need for sex-specific approaches in managing HUA to prevent related ocular complications |
This table synthesizes key findings from various studies, demonstrating the multifaceted relationship between HUA and epiretinal pathologies, and underscores the importance of managing uric acid levels to prevent and mitigate retinal diseases. VEGF: vascular endothelial growth factor; CRP: C-reactive protein
Several mechanisms have been proposed to explain how HUA may contribute to the development and progression of epiretinal pathologies (Figure 2). Pai et al. [8] demonstrated that short-term HUA in mice caused structural retinal changes, which were reversible with uric acid-lowering agents. Goharinia et al. [60] questioned whether allopurinol, by targeting uric acid, could mitigate the oxidative stress involved in diabetic retinopathy. de Almeida Torres et al. [61] explored the protective effects of carnosine supplementation against oxidative stress in a high-calorie diet rat model, suggesting potential benefits in HUA-induced retinal damage. Bian et al. [62] proposed that knocking out the retinol dehydrogenase 12 (RDH12) gene in mice induces a HUA phenotype, implicating genetic factors in disease progression. In addition, Thounaojam et al. [16] highlighted the role of monosodium urate in retinal inflammation, a key driver in diabetic retinopathy. Rozanowska et al. [63] noted urate’s potential antioxidant properties, particularly its ability to scavenge retinoid cation radicals, adding a layer of complexity to its role in retinal health. These findings suggest that HUA may promote epiretinal pathologies through oxidative stress and inflammation, pointing to therapeutic interventions targeting uric acid.
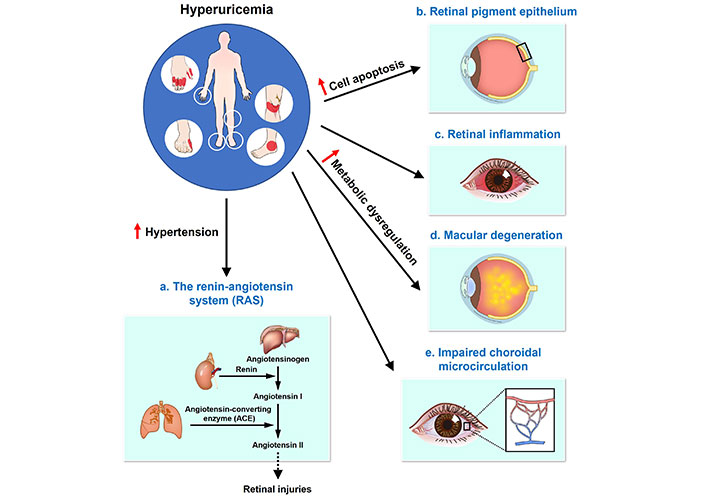
Proposed mechanisms linking hyperuricemia (HUA) to the development and progression of epiretinal pathologies. Five mechanisms (a–e) have been proposed to explain how HUA may contribute to the development and progression of epiretinal pathologies. (a) The RAS: HUA is associated with hypertension, which activates the RAS pathway. The sequence begins with angiotensinogen being converted to angiotensin I by renin, followed by the conversion of angiotensin I to angiotensin II by ACE. Angiotensin II can induce retinal injuries, contributing to epiretinal pathologies; (b) retinal pigment epithelium: HUA induces cell apoptosis in the retinal pigment epithelium, which can lead to retinal damage and epiretinal pathologies; (c) retinal inflammation: HUA is directly linked to increased retinal inflammation, which plays a critical role in the development of epiretinal pathologies; (d) macular degeneration: Metabolic dysregulation caused by HUA can accelerate macular degeneration, a key factor in the progression of epiretinal pathologies; (e) impaired choroidal microcirculation: HUA impairs choroidal microcirculation, leading to insufficient blood supply to the retina and contributing to the development of epiretinal pathologies. The figure illustrates these mechanisms, with labels indicating the roles of HUA in each proposed pathway. The arrow with a solid line indicates the direction of the effect, while the arrow with a dotted line represents the indirect effect across the membrane. The upward red arrows in the figure indicate an increase in various effects
The molecular pathways involved in the pathophysiological links between HUA and epiretinal pathologies are multifaceted (Figure 3). For instance, Zhu et al. [64] explored how uric acid exacerbates diabetic retinopathy by activating the Notch signaling pathway, contributing to vascular changes in the retina. Likewise, Qin et al. [13] reported elevated uric acid levels in the aqueous humor of patients with diabetic macular edema, further implicating HUA in retinal dysfunction. These studies highlight the role of uric acid in promoting oxidative stress, inflammation, and vascular abnormalities, which are key factors in retinal and epiretinal pathologies. More relevant to epiretinal conditions, Ożóg et al. [9] discussed the pathophysiology of the ERM, noting that inflammation and microglial activation play central roles in its development. Vishwakarma et al. [17] further supported this by showing that microglial activation, oxidative stress, and inflammation are critical in the progression of ERM. These findings underscore the potential involvement of HUA in exacerbating epiretinal diseases through similar mechanisms, especially in patients with systemic conditions like diabetes and hypertension. HUA is also associated with retinal microvascular changes, as evidenced by Lu et al. [15], who found that elevated serum uric acid correlated with reduced microvascular density and choroidal thickness. Such vascular changes are significant in the progression of epiretinal pathologies, as suggested by Thounaojam et al. [16], who demonstrated that monosodium urate crystals contribute to retinal inflammation and worsen diabetic retinopathy. Together, these findings point to HUA’s role in epiretinal diseases through a combination of metabolic dysregulation, inflammation, and vascular changes. Future therapeutic strategies may focus on targeting uric acid pathways, aiming to mitigate the risk and progression of epiretinal pathologies in individuals with HUA, particularly those with comorbid systemic diseases like diabetes and hypertension.
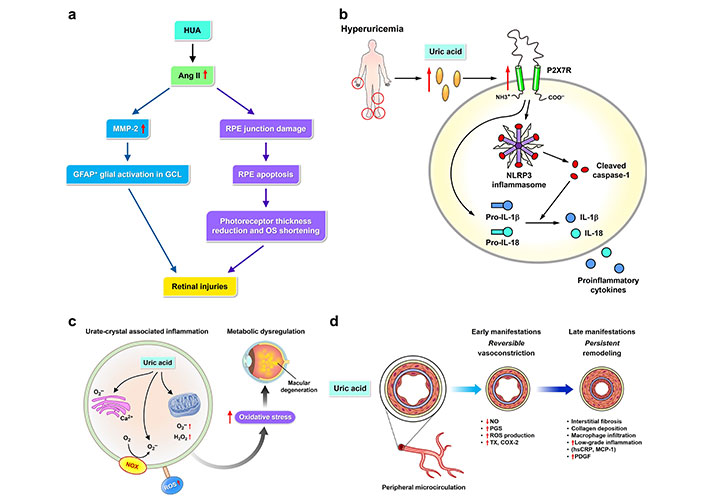
Molecular pathways of epiretinal pathologies promotion by uric acid. The pathophysiological mechanisms linking hyperuricemia (HUA) to the development and progression of epiretinal pathologies involve multiple molecular pathways (a–d). (a) HUA and angiotensin II (Ang II) (left): Uric acid increases Ang II levels, which in turn upregulates matrix metalloproteinase-2 (MMP-2). This leads to glial fibrillary acidic protein (GFAP)+ glial activation in the ganglion cell layer (GCL) and subsequent retinal injuries. Uric acid and retinal pigment epithelial (RPE) junction damage (right): Uric acid contributes to RPE junction damage and apoptosis. This results in a reduction of photoreceptor thickness and outer segment (OS) shortening, culminating in retinal injuries; (b) HUA and P2X7 receptor (P2X7R)/NOD-like receptor protein 3 (NLRP3) inflammasome: HUA leads to elevated uric acid levels, which activate the P2X7R and the NLRP3 inflammasome. This activation promotes retinal inflammation through the production of cleaved caspase-1, pro-interleukin-1β (IL-1β), pro-IL-8, IL-1β, and other proinflammatory cytokines; (c) urate-crystal associated inflammation: Uric acid crystals induce oxidative stress by generating reactive oxygen species (ROS) such as oxygen radicals and hydrogen peroxide. This leads to metabolic dysregulation and macular degeneration; (d) uric acid and impaired microcirculation: Uric acid affects peripheral microcirculation, causing early reversible vasoconstriction mediated by nitric oxide (NO) and prostaglandin synthase (PGS), and late persistent remodeling characterized by ROS production, interstitial fibrosis, collagen deposition, macrophage infiltration, low-grade inflammation, and elevated levels of high-sensitivity C-reactive protein (CRP, hsCRP), monocyte chemoattractant protein-1 (MCP-1), and platelet-derived growth factor (PDGF). The figure illustrates these pathways, highlighting the key molecular interactions that contribute to the development and progression of epiretinal pathologies. The red arrows in the figure indicate upregulation (upward arrows) or downregulation (downward arrows) of various molecular mediators. NOX: NADPH oxidase; TX: thromboxane; COX-2: cyclooxygenase-2
Considering the potential role of HUA in the development and progression of epiretinal pathologies, the screening and diagnosis of HUA in patients with retinal disorders are crucial. Screening typically involves measuring serum uric acid levels, a simple and widely available laboratory test. In patients with epiretinal pathologies, particularly those with risk factors such as advanced age, systemic diseases like diabetes or hypertension, or a history of ocular trauma or surgery, routine assessment of serum uric acid may help identify individuals at heightened risk of vision-threatening complications. Furthermore, a thorough ophthalmic evaluation, including retinal imaging (e.g., optical coherence tomography, fundus photography) and functional tests (e.g., visual acuity, visual field testing), should be conducted to assess retinal involvement and monitor disease progression [6]. Incorporating serum uric acid testing into routine ophthalmic evaluations could enable early detection of HUA-related retinal abnormalities, leading to timely and appropriate management. HUA has been linked to the pathogenesis of several epiretinal disorders and may affect both prognosis and management. Elevated serum uric acid levels have been associated with poorer visual outcomes and a higher risk of disease progression in patients with retinal pathologies. For example, Lu et al. [15] explored the relationship between retinal microvasculature, choriocapillaris flow, and serum uric acid levels, revealing significant correlations through swept-source optical coherence tomography angiography. HUA can complicate the management of epiretinal pathologies by exacerbating inflammation, vascular dysfunction, and oxidative stress in the retina. Patients with concurrent HUA and retinal disorders may require more aggressive treatment to control disease activity and prevent vision loss. Monitoring serum uric acid levels closely and intervening with urate-lowering therapies as needed could mitigate the adverse effects of HUA on retinal health, improving long-term visual outcomes. The treatment of HUA and its effects on epiretinal pathologies is an emerging area of research (Table 3). While direct clinical evidence linking HUA-lowering therapies with epiretinal pathologies remains limited, a broader understanding of uric acid’s systemic effects and its role in ocular diseases suggests that these therapies may hold potential. HUA is closely linked with several systemic diseases, including metabolic syndrome, hypertension, and kidney disease, which are all associated with microvascular damage that could affect the retina [1]. The management of HUA often includes pharmacological agents like xanthine oxidase inhibitors (e.g., allopurinol and febuxostat) and uricosuric agents, which aim to reduce serum uric acid levels. These therapies have proven effective in reducing HUA-related complications, particularly in conditions like gout. Although not yet specifically studied in epiretinal pathologies, these agents may indirectly benefit retinal health by reducing inflammation and oxidative stress—two mechanisms thought to contribute to retinal diseases [8, 35]. Pai et al. [8] demonstrated in a mouse model that short-term HUA can cause structural retinal changes, which were reversible upon the administration of uric acid-lowering agents. This finding suggests that reducing serum uric acid could potentially prevent or reverse retinal damage associated with HUA. Similarly, Li et al. [59] found a sex-specific association between serum uric acid levels and retinal microvessel alterations, implying that uric acid could be a modifiable factor in preserving retinal microvasculature. Moreover, uric acid has been implicated in the progression of retinal diseases through its pro-inflammatory effects. Thounaojam et al. [16] highlighted how monosodium urate contributes to retinal inflammation in diabetic retinopathy, further suggesting that HUA-lowering therapies could mitigate similar inflammatory processes in epiretinal diseases. Beyond pharmacological interventions, lifestyle modifications aimed at reducing uric acid levels, such as dietary changes, have also been explored. Certain bioactive compounds and dietary interventions that lower serum uric acid levels, such as reducing fructose intake, have shown promise in managing HUA [30]. These strategies could offer additional benefits for retinal health by targeting both metabolic and oxidative stress pathways. Future research should focus on clarifying the specific roles of uric acid-lowering treatments in preventing or reversing retinal damage, particularly in epiretinal pathologies. Given the systemic nature of HUA, its management could have far-reaching effects on retinal health, with potential implications for a range of retinal diseases, including ARMD and diabetic retinopathy [22, 65].
Potential therapeutic approaches targeting hyperuricemia (HUA) for the management of epiretinal pathologies
| Therapeutic approach | Mechanism of action | Potential benefits | Relevant studies |
|---|---|---|---|
| Xanthine oxidase inhibitors (e.g., allopurinol) | Reduces uric acid production by inhibiting xanthine oxidase | Decrease in serum uric acid levels, potential reduction in oxidative stress and inflammation associated with epiretinal pathologies | Xia et al. [66], 2020Silvani et al. [67], 2020 |
| Uric acid lowering agents (e.g., febuxostat) | Inhibits xanthine oxidase enzyme, leading to reduced uric acid synthesis | Lowering of serum uric acid levels, improvement in retinal microvascular health | Pai et al. [8], 2022Zhang et al. [68], 2023 |
| Antioxidant supplements (e.g., vitamin C, carnosine) | Neutralizes free radicals and reduces oxidative stress | Potential reduction in oxidative damage to retinal cells and tissues | Catalani et al. [69], 2021de Almeida Torres et al. [61], 2023 |
| Anti-inflammatory agents [e.g., NOD-like receptor protein 3 (NLRP3) inflammasome inhibitors] | Suppresses inflammatory pathways involving the NLRP3 inflammasome | Potential reduction in inflammation-related retinal damage | Lin et al. [70], 2024 |
| Nutritional interventions (e.g., low-purine diet) | Reduces dietary intake of purines, which are metabolized into uric acid | Lower serum uric acid levels, potentially less oxidative stress on retinal cells | Bian et al. [62], 2024Rivera-De-la-Parra et al. [65], 2024 |
| Angiotensin-converting enzyme (ACE) inhibitors | Lowers blood pressure and may have uric acid-lowering effects | Improvement in retinal blood flow and reduction in hypertensive damage to retinal vessels | Huang et al. [71], 2022Yang et al. [72], 2021 |
| Lifestyle modifications (e.g., increased physical activity) | Enhances uric acid excretion and improves overall cardiovascular health | Lower serum uric acid levels, improved retinal vascular health | Geng et al. [20], 2023Engin et al. [73], 2023 |
HUA, characterized by elevated levels of uric acid in the blood, has been implicated in various epiretinal pathologies such as diabetic retinopathy, age-related macular degeneration, and epiretinal membranes. Managing HUA could potentially mitigate these conditions by addressing the underlying metabolic and inflammatory processes. The table summarizes potential therapeutic approaches targeting HUA for the management of epiretinal pathologies, based on recent research findings. These approaches include pharmacological interventions, lifestyle modifications, and dietary supplements, each with specific mechanisms of action and potential benefits in ocular health
Despite growing evidence of a potential link between HUA and epiretinal pathologies, many key questions remain unanswered, warranting further investigation. Future research should focus on understanding the mechanisms driving this association and exploring therapeutic interventions to mitigate its adverse effects on retinal health. Mechanistic studies are needed to clarify how HUA contributes to the development and progression of epiretinal pathologies, particularly through oxidative stress, inflammation, endothelial dysfunction, and other relevant mechanisms. Large-scale epidemiological studies are also necessary to better define the characteristics of HUA-associated retinal disorders, identify at-risk subpopulations, and guide targeted screening and prevention efforts. Additionally, longitudinal studies are crucial to assess the progression of retinal changes in patients with HUA, evaluate the impact of serum uric acid levels on disease progression, and inform prognostic models. Investigating therapeutic targets and novel treatments for HUA-induced retinal pathologies involves exploring key molecular pathways involved in the disease. Nigam et al. [74] provided a systems biology perspective on the organic anion transporter family, highlighting its relevance to HUA-related ocular abnormalities. Wert et al. [75] emphasized the role of extracellular superoxide dismutase 3 (SOD3) in regulating oxidative stress at the vitreoretinal interface, suggesting it as a therapeutic target for HUA-induced retinal damage. Öhman et al. [76] identified the neurodegenerative nature of age-related vitreoretinal interface diseases, offering insights into therapeutic approaches targeting neurodegeneration in HUA-related retinal pathologies. Kaarniranta et al. [77] explored mitochondrial dysfunction in ARMD and suggested mitochondrial modulation as a therapeutic strategy. Wooff et al. [78] demonstrated that short-term photo-oxidative damage triggers molecular signals indicative of early retinal degeneration, underscoring the importance of interventions targeting oxidative stress pathways. Ai et al. [79] examined the inhibitory effects of Berberis dictyophylla F. on angiogenesis and apoptosis in diabetic retinopathy, proposing the hypoxia-inducible factor-1α (HIF-1α)/vascular endothelial growth factor (VEGF)/delta-like-ligand 4 (DLL-4)/Notch-1 pathway as a therapeutic target. Lewis Luján et al. [80] discussed nutraceuticals and drugs that promote mitophagy and mitochondrial biogenesis as interventions for mitochondrial dysfunction in dry ARMD. Engin et al. [73] investigated the link between oxidative damage and the antioxidant response in non-arteritis ischemic optic neuropathy, proposing antioxidant therapies for retinal pathologies associated with HUA. Thus, targeting key molecular pathways involved in HUA-induced retinal damage presents promising opportunities for novel therapeutic interventions, ultimately improving patient outcomes and quality of life. The emerging connection between HUA and epiretinal pathologies has significant implications for clinical practice and patient care. Clinicians should be aware of the potential ocular effects of HUA and consider screening for elevated serum uric acid levels in patients with retinal disorders, particularly those with systemic diseases or a history of HUA-related complications. Interdisciplinary collaboration among ophthalmologists, internists, and rheumatologists is essential for the comprehensive management of HUA-associated retinal disorders. A multidisciplinary approach that considers both systemic and ocular health may optimize treatment outcomes and improve long-term visual prognosis. Additionally, patient education and lifestyle changes are critical in the prevention and management of HUA-related retinal conditions. Encouraging healthy dietary habits, weight management, and adherence to prescribed medications can help lower serum uric acid levels and reduce the risk of vision-threatening complications. Overall, further research into the relationship between HUA and epiretinal pathologies, the development of novel therapeutic strategies, and the integration of evidence-based management practices are crucial steps toward enhancing care and outcomes for patients with HUA-related retinal disorders.
In conclusion, this review provides a thorough examination of the relationship between HUA and epiretinal pathologies, emphasizing the growing evidence that elevated serum uric acid levels contribute to the development and progression of retinal disorders. Clinical studies have established associations between HUA and various epiretinal abnormalities, while experimental research has begun to uncover the mechanisms behind HUA-induced retinal damage. The clinical implications of these findings are significant, as elevated serum uric acid levels affect disease prognosis and treatment outcomes. Screening for HUA in patients with retinal disorders may help identify those at higher risk for vision-threatening complications, allowing for more personalized management strategies. Also, targeting HUA as part of the treatment for retinal disorders could offer a novel approach to preventing and treating epiretinal pathologies. As we deepen our understanding of the connection between HUA and retinal health, it becomes clear that addressing HUA is essential in managing epiretinal pathologies. Recognizing HUA as a modifiable risk factor enables clinicians to take proactive steps in improving visual outcomes and patient care. Incorporating serum uric acid testing into routine ophthalmic evaluations could facilitate early detection of HUA-related retinal abnormalities, prompting timely intervention. Moreover, identifying new therapeutic targets for HUA-associated retinal disorders offers the potential for developing treatments that preserve retinal function and prevent vision loss. Future research should continue to explore the mechanisms linking HUA to epiretinal pathologies, investigate innovative therapeutic approaches, and translate these findings into clinical practice. Addressing HUA is a critical step toward enhancing the management and outcomes of patients with epiretinal disorders. By leveraging our knowledge of the pathophysiological connections between HUA and retinal disease, we can work to improve visual health and quality of life for those affected.
ARMD: age-related macular degeneration
ERM: epiretinal membrane
HUA: hyperuricemia
SLC2A9: solute carrier family 2, member 9
URAT: urate transporter
VMT: vitreomacular traction
The authors would like to acknowledge the assistance of OpenAI’s ChatGPT in refining the language and enhancing the clarity of this review article. The authors carefully reviewed and edited all content to ensure accuracy and integrity.
CYC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. JCL: Conceptualization, Investigation, Writing—review & editing. HYC: Data curation, Investigation, Writing—original draft. JJC: Validation, Supervision, Writing—review & editing. WRH: Conceptualization, Supervision, Writing—review & editing. THC: Conceptualization, Methodology, Supervision, Writing—review & editing. All authors have read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
