Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are integral to diabetes treatment, facilitating renal glucose excretion and offering benefits in cardiovascular risk reduction, kidney function preservation, and weight management. However, their integration into diabetes care presents challenges due to associated adverse effects. This review assesses the efficacy of SGLT2 inhibitors in diabetes management. A comprehensive literature search using PubMed and Google Scholar yielded pertinent studies. Clinical evidence demonstrates the effectiveness of SGLT2 inhibitors in glycemic control and in reducing cardiovascular risks in diabetes patients. Moreover, these agents exhibit positive effects on cardiovascular and renal functions. Despite their therapeutic promise, careful consideration is warranted when integrating SGLT2 inhibitors into diabetes care. Common adverse effects such as genitourinary infections, hypoglycemia, and sporadic diabetic ketoacidosis necessitate rigorous patient monitoring and education. Nonetheless, SGLT2 inhibitors offer a comprehensive approach to diabetes management, showing efficacy across multiple domains. In summary, SGLT2 inhibitors play a crucial role in diabetes care, offering benefits beyond glycemic control. However, their use requires careful patient selection, education, and monitoring to manage associated risks effectively. Awareness of potential adverse effects is essential for optimizing the therapeutic benefits of SGLT2 inhibitors in T2DM (type 2 diabetes mellitus) management.
Keywords
SGLT2 inhibitors, diabetes mellitus, SGLT2, canagliflozin, dapagliflozin, empagliflozin, ertugliflozinIntroduction
In the coming decades, diabetes will likely become a global health challenge of unprecedented proportions, driven by factors such as population growth, aging, and the increasing prevalence of overweight and obesity across the world. With diabetes imposing a significant burden on healthcare systems and individuals alike, it is imperative to note that the number of documented cases is expected to surge from 425 million in 2017 to 629 million by 2040. Notably, type 2 diabetes mellitus (T2DM) accounts for approximately 90% of these cases, making it the predominant form of diabetes. In 2021, an estimated 8.3% of adults aged 20 to 79 worldwide were diagnosed with T2DM, representing approximately 366 million adults [1, 2].
In contrast to its historical prevalence among elderly people, T2DM has emerged as a pressing global health issue in the 21st century, affecting a broader age spectrum of adults. The repercussions of diabetes extend beyond elevated blood sugar levels, encompassing a constellation of associated health conditions such as obesity, hypertension, cardiovascular diseases, and atherosclerosis, all of which collectively curtail lifespan and inflate healthcare costs [3–6].
In the quest for effective T2DM management, it has become crucial to explore innovative approaches. Although most current therapies for diabetes rely on insulin-dependent mechanisms, a new class of agents, sodium-glucose cotransporter 2 (SGLT2) inhibitors, has emerged. These inhibitors, unlike conventional methods, operate by enhancing the excretion of glucose through urine, reducing renal glucose reabsorption in the nephron. Their insulin-independent mechanism sets them apart, making them a viable option at any stage of T2DM treatment. Moreover, SGLT2 inhibitors display remarkable efficacy, particularly in cases related to insulin resistance and beta cell dysfunction, suggesting that SGLT2 inhibitors are a highly effective strategy for T2DM management [7].
SGLT2 inhibitors, categorized as oral medications, primarily work by inhibiting glucose reabsorption in the proximal tubule of the kidney, resulting in lowered glycated hemoglobin A1c (HbA1c) levels, weight loss, and positive effects on metabolic factors such as blood pressure, lipid profiles, and hyperuricemia. The discovery of SGLT2 inhibitors traces back to the discovery of phlorizin, a natural compound found in apple tree bark, which was identified in 1835 for its SGLT-inhibiting properties. Further advancements in understanding glucose transport mechanisms in the 1980s and 1990s culminated in the identification of SGLTs and a deeper grasp of their functions [8, 9].
SGLTs, integral members of the membrane protein family, facilitate the movement of substances such as glucose, amino acids, vitamins, and ions, primarily in the kidneys. Under normal circumstances, approximately 90% of glucose transported by SGLTs is reabsorbed in the kidneys. However, inhibiting these transporters disrupts glucose reabsorption, leading to glycosuria, and offers a promising avenue for effectively managing blood sugar levels in T2DM [7, 10, 11].
Notably, reports to the United States Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) have raised concerns about potential adverse effects associated with SGLT2 inhibitors, including diabetic ketoacidosis, acute kidney injury, serious urinary tract infections, and increased risk of venous thromboembolism. The FDA is also actively investigating reports of acute pancreatitis linked to SGLT2 inhibitor use [12, 13].
Furthermore, in a population-based cohort study of T2DM patients receiving standard care, Kutz et al. [14] 2023 found that starting an SGLT2 inhibitor or GLP-1RA reduced the risk of cardiovascular events and mortality compared to starting a dipeptidyl peptidase 4 (DPP-4) inhibitor, and this was true regardless of the patient’s frailty status. In contrast to DPP-4 inhibitors, SGLT2 and GLP-1 receptor agonists did not significantly raise the risk of serious side events such as genitourinary infections, amputations, bone fractures, diabetic ketoacidosis, or volume depletion/hypotension [14–16].
This review endeavors to present a comprehensive analysis of the existing evidence from significant clinical trials on SGLT2 inhibitors, shedding light on their potential benefits and associated adverse effects.
Literature search
Search strategy
Our literature search was conducted using two primary databases: PubMed and Google Scholar. The search was designed to identify studies and reviews related to the efficacy, safety, and mechanisms of action of SGLT2 inhibitors in the treatment of T2DM. The following search terms and Boolean operators were used:
Search terms: “SGLT2 inhibitors,” “canagliflozin”, “dapagliflozin”, “empagliflozin”, “ertugliflozin”, “type 2 diabetes,” “efficacy,” “safety,” “mechanisms of action,” “cardiovascular outcomes,” “renal protection,” “diabetic nephropathy,” “adverse effect.”
Boolean operators: AND, OR, NOT. For example, “SGLT2 inhibitors AND type 2 diabetes AND efficacy” and “SGLT2 inhibitors OR sodium-glucose co-transporter 2 inhibitors AND cardiovascular outcomes.”
Inclusion criteria
Study types: randomized controlled trials, observational studies, systematic reviews, and meta-analyses.
Population: adults with type 2 diabetes.
Interventions: use of SGLT2 inhibitors as a monotherapy or in combination with other antidiabetic agents.
Outcomes: studies reporting on efficacy (e.g., HbA1c reduction, glycemic control), safety (e.g., adverse events).
Language: articles published in English.
Publication date: articles published from January 2000 to the present.
Exclusion criteria
Non-human studies involving animal models without human data.
Non-original research like editorials, opinion pieces, and non-systematic reviews.
Irrelevant outcomes studies focusing on conditions other than type 2 diabetes or outcomes not related to the efficacy, safety, or mechanisms of SGLT2 inhibitors.
Mechanistic insights on SGLT2 effects
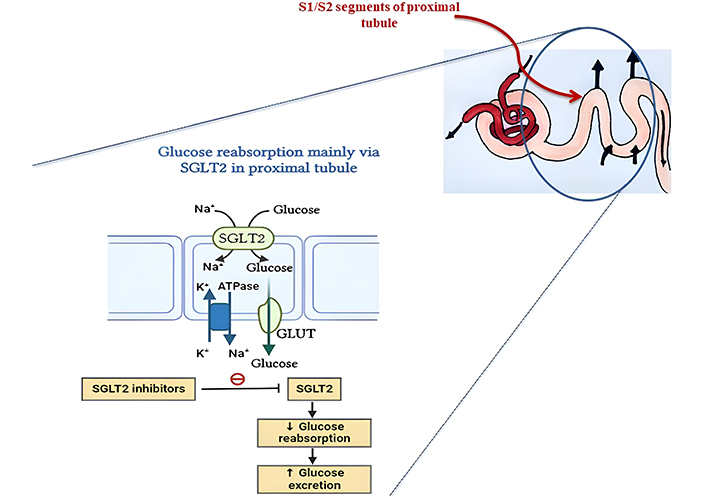
SGLT2 inhibition in the kidney’s proximal convoluted tubules (PCT) is the first step in a complex mechanism by which SGLT2 inhibitors achieve their therapeutic effects. These medications lower blood glucose levels by increasing glucose excretion in the urine and decreasing SGLT2 activity, which prevents the reabsorption of about 90% of the filtered glucose. Pancreatic beta-cell activity and insulin sensitivity are both preserved and improved as a result of this decrease in hyperglycemia, which aids in mitigating glucotoxicity. Natriuresis (sodium excretion) and osmotic diuresis (reduction of intravascular volume) caused by increased glucose excretion both lead to a substantial drop in blood pressure. Heart failure patients benefit from this diuretic action since it reduces both preload and afterload. The mechanism of action of SGLT2 inhibitors is illustrated in Figure 1.
Glucose is freely filtered from the blood through the glomerulus and is fully reabsorbed in the proximal tubule. SGLT2 in the S1 and S2 segments and SGLT1 in the S3 segment handle this reabsorption. SGLTs utilize the sodium gradient for glucose uptake, which is maintained by the Na+/K+ pump. Glucose then moves into the bloodstream via GLUT2 transporters. This mechanism is targeted by SGLT2 inhibitors to reduce glucose reabsorption and lower blood glucose levels.
Notable among SGLT2 inhibitors’ effects are their protective effects on the kidneys. These medications work by decreasing sodium reabsorption in the proximal tubule, which in turn increases sodium supply to the macula densa. This, in turn, causes afferent arteriolar vasoconstriction and a subsequent drop in intraglomerular pressure through tubuloglomerular feedback. This process lessens albuminuria, decreases the development of diabetic nephropathy, and aids in the preservation of renal function. Additionally, SGLT2 inhibitors improve metabolic health by enhancing lipid metabolism and encouraging caloric loss through glucosuria, which leads to mild weight loss.
The anti-inflammatory and antioxidative effects of SGLT2 inhibitors have also been noted. Their action of decreasing blood glucose levels decreases the activation of pathways like the NF-κB pathway and the production of reactive oxygen species (ROS), which are involved in chronic inflammation and oxidative stress. Cardiovascular health and metabolic stability are both improved by this decrease in inflammation and oxidative stress. Beyond diabetic control, SGLT2 inhibitors have extensive therapeutic promise due to these coupled mechanisms, which include cardiovascular, renal, and metabolic advantages [7, 8].
Marketed SGLT2 inhibitors and their significance in T2DM
The FDA has recently developed several SGLT2 inhibitors, including canagliflozin, empagliflozin, dapagliflozin, and ertugliflozin. For individuals with T2DM, it is advised that the utilization of these medications be coupled with an enhanced diet and physical activity. This combined approach aims to lower blood sugar levels effectively. These drugs can be employed either as a standalone treatment or in conjunction with other diabetes medications, such as metformin [7]. Different SGLT2 drugs available in the USA, Europe, and India are summarized in Table 1.
The different SGLT2 drugs available in the USA, Europe, and India
| Generic name | Brand name (India) | Brand name (USA) | Brand name (EU) | Approval | Typical dosage | Reference |
|---|---|---|---|---|---|---|
| Canagliflozin | Canagliflozin | Invokana | Invokana | U.S. FDA: 2013EMA: 2013CDSCO: 2020 | USA: 100 mg and 300 mgEU: 100 mg and 300 mgIndia: 100 mg and 300 mg | [17–19] |
| Dapagliflozin | Dapagliflozin | Farxiga | Forxiga | U.S. FDA: 2014EMA: 2012CDSCO: 2020 | USA: 5 mg and 10 mg EU: 5 mg and 10 mg India: 10 mg | [18–20] |
| Empagliflozin | Jardiance | Jardiance | Jardiance | U.S. FDA: 2014EMA: 2014CDSCO: 2022 | USA: 10 mg and 25 mgEU: 10 mg and 25 mgIndia: 10 mg and 25 mg | [20–23] |
| Ertugliflozin | - | Steglatro | Steglatro | U.S. FDA: 2017EMA: 2018 | USA: 5 mg and 15 mgEU: 5 mg and 15 mg | [24, 25] |
Canagliflozin
The initial SGLT2 inhibitor sanctioned by the FDA was canagliflozin, which received marketing authorization from Janssen, a subsidiary of Johnson & Johnson, in 2013. Canagliflozin is categorized under the gliflozin group, and its method of operation involves impeding the reabsorption of glucose within the PCT of the kidneys [7, 26].
Canagliflozin can influence two types of SGLT receptors, namely, SGLT1 and SGLT2. These SGLT2 receptors are found within the initial segments of the renal tubules and are primarily responsible for the majority of glucose reabsorption carried out by the kidneys. By obstructing SGLT2 receptors, canagliflozin leads to an increase in the amount of glucose excreted in the urine, ultimately resulting in a reduction in blood glucose levels [27]. The reabsorption of glucose through SGLT2 receptors is greater in individuals with T2DM than in those without T2DM. This fact underscores the potential of targeting SGLT2 as a therapeutic approach for T2DM. The inhibition of SGLT2 receptors presents a minimal risk of inducing hypoglycemia, as the excretion of glucose in the urine is elevated, leading to a decrease in blood glucose levels [28]. Unlike its affinity for the SGLT1 receptor, canagliflozin has weaker binding to the SGLT2 receptor. Conversely, other medications within the same class, such as dapagliflozin, empagliflozin, and tofogliflozin, exhibit a stronger affinity for the SGLT2 receptor [29].
Pharmacokinetics of canagliflozin
Canagliflozin is an orally administered, selective SGLT2 inhibitor that is swiftly reversible. The peak plasma concentrations of canagliflozin administered orally are reached within 1 to 2 hours [27]. Canagliflozin has an absolute oral bioavailability of 65%, which remains unaltered in the presence of food [25]. Uridine diphosphate-glucuronosyltransferase (UGT) 1A9 and UGT2B4 glucuronidate canagliflozin are two inactive metabolites, M7 and M5, respectively [29]. Multiple-dose experiments revealed that canagliflozin exhibited minimal accumulation over time, with a steady state achieved within a period of 4 days [30]. The elimination of dosages in the stool and urine accounted for 60% and 30%, respectively. In individuals with normal health conditions, the elimination half-life of canagliflozin following the administration of a 100 mg oral dose is approximately 10.6 hours, while for a 300 mg dose, it is approximately 13.1 hours [29]. There was no substantial variation in canagliflozin exposure based on age, race, sex, or body weight. The pharmacokinetics of canagliflozin are not altered in patients with mild to moderate hepatic impairment. Individuals with impaired renal function have increased systemic exposure to canagliflozin compared to those with normal renal function. However, their therapeutic efficacy is diminished due to a reduced filtered glucose load [27]. The concurrent administration of rifampin resulted in a slight decrease in canagliflozin plasma levels, necessitating the monitoring of glycemic control. Nonetheless, there were no observed medication interactions of clinical significance with metformin, glyburide, simvastatin, warfarin, hydrochlorothiazide, oral contraceptives, probenecid, or cyclosporine [27]. Canagliflozin effectively lowers plasma glucose and glycosylated hemoglobin levels by enhancing urine glucose excretion (UGE) and inhibiting the renal threshold for glucose (RTG) in a manner that is dependent on the dosage administered. The impact on the RTG was close to its maximum potential when it was administered at a dosage of 300 mg during the whole 24-hour dosing period. Conversely, when administered at a dosage of 100 mg, the effects on the RTG reach near-maximal levels for approximately 12 hours and experience a slight reduction over the nighttime period [27, 31].
Clinical efficacy of canagliflozin in patients with T2DM
In line with findings from the Canagliflozin Cardiovascular Assessment Study (CANVAS) program, also known as the CANVAS trial, canagliflozin can reduce the combined cardiovascular events of cardiovascular death, nonfatal heart attacks, and nonfatal strokes. Moreover, the CANVAS program also presented data supporting a 40% decrease in the estimated glomerular filtration rate (eGFR), the requirement for renal replacement therapy, or death due to renal issues when using canagliflozin [29, 30].
According to the Canagliflozin Treatment and Trial Analysis (CANTATA) research, when canagliflozin was administered either as a standalone treatment (CANTATA-M) or as a second-line treatment (CANTATA-SU, CANTATA-D) or even as a third-line intervention (CANTATA-D2, CANTATA-MSU), it led to a reduction in HbA1c levels. The outcomes of these studies played a pivotal role in gaining approval from the U.S. FDA for the medication. The findings of this investigation revealed highly significant outcomes with a p-value of less than 0.001. Comparing canagliflozin to a placebo, there was a reduction of -0.77% and 1.03% in HbA1c levels at the 26-week mark for doses of 100 mg and 200 mg, respectively. Furthermore, a substantial portion of patients achieved the target HbA1c level of less than 7% when canagliflozin was compared to the placebo [29, 31].
The clinical investigation carried out by Chakravorty and Patel in 2019 [32] aimed to assess the clinical efficacy of administering 100 mg of canagliflozin to individuals with T2DM within a real-world healthcare setting. This study also aimed to determine the effectiveness of canagliflozin on factors related to body fatness, such as body weight and body mass index (BMI). The outcomes of this retrospective cohort study demonstrated that the administration of 100 mg canagliflozin to a group of 50 T2DM patients over a span of 24 weeks resulted in a 1.6% reduction in HbA1c levels, a decrease in the fasting plasma glucose (FPG) level of 30.4 mg/dL, a reduction in the postprandial glucose (PPG) level to 76.1 mg/dL, a weight loss of 2.1 kg, and a decrease in BMI of 0.8 kg/m2. The real-world data derived from this research conclusively indicated that canagliflozin 100 mg led to significant enhancements in glycemic control, body fatness, and body weight in patients with T2DM, consistent with the outcomes observed in a clinical trial [32].
An additional research endeavor, referred to as the CREDENCE trial or the Clinical Evaluation of Canagliflozin and Renal Events in Diabetes with Established Nephropathy, conducted by Wada et al. in 2022 [33], aimed to assess the effectiveness and safety of canagliflozin within a specific subset of individuals from East and Southeast Asian countries who had a heightened susceptibility to renal complications. The outcomes of this investigation, which involved approximately 4,401 participants, revealed that canagliflozin played a role in diminishing the likelihood of experiencing renal and cardiovascular complications across a diverse array of participants diagnosed with T2DM and albuminuria. This included East Asian participants who exhibited a pronounced vulnerability to renal issues. Thus, this study conclusively demonstrated that canagliflozin effectively decreased the risk of renal and cardiovascular events among East Asian participants at elevated risk of renal complications within the broader CREDENCE study, all while avoiding any supplementary adverse effects [33].
Marfella et al. 2022 [34, 35] set out to determine how SGLT2 inhibitors (canagliflozin, dapagliflozin and empagliflozin) affected cardiac function in human diabetic hearts by analysing JunD expression in a separate clinical trial with 77 first-heart transplant patients with and without diabetes. The researchers found that diabetic patients who took SGLT2 inhibitors had a more gradual worsening of myocardial JunD/PPAR-γ pathway activity, myocardial insulin resistance, lipid buildup, and heart malfunction. Therefore, it appears that the negative effects of the diabetic environment on the JunD/PPAR-γ pathway in heart transplant patients could be effectively counteracted by taking SGLT2 inhibitors at the same time [34, 35]. Table 2 represents a comprehensive overview of the clinical trial data supporting canagliflozin’s outcome and its adverse drug reactions (ADRs).
Randomized clinical trial evaluating canagliflozin
| Study name | Sample | Duration | Outcome | Adverse drug events | Reference | |
|---|---|---|---|---|---|---|
| Common | Uncommon | |||||
| CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) | 4,200 participants | 5 to 5.5 years | Reduced likelihood of end-stage kidney disease, a two-fold increase in serum creatinine levels, or fatalities stemming from renal or cardiovascular origins within the canagliflozin group | Amputation, fracture, hyperkalemia, acute kidney injury | Renal cell carcinoma, breast cancer, bladder cancer, diabetic ketoacidosis, acute pancreatitis | [36] |
| CANVAS(Canagliflozin Cardiovascular Assessment Study) | 10,142 participants | 13.5 years | Decreased probability of hospitalization due to heart failure, advancement of albuminuria, and substantial decline in kidney function among individuals treated with canagliflozin | Amputations of toes, feet or legs, male or female genital infection, osmotic diuresis, volume depletion, urinary tract infections, fractures including low trauma fractures | Diabetic ketoacidosis, hyperkalemia, acute kidney injury, pancreatitis, malignancies | [37] |
| CANVAS(Canagliflozin Cardiovascular Assessment Study) | 215 participants | 1 year | The introduction of canagliflozin alongside existing sulfonylureas consistently led to enduring decreases in HbA1c levels and fasting plasma glucose, all the while avoiding an elevation in the occurrence of hypoglycemia and an increase in body weight | Male and female mycotic genital infection, renal impairment, osmotic diuresis, urinary tract infection, fracture, hypoglycemia | Hypokalemia, volume-related events | [38] |
Adverse events associated with canagliflozin
Several adverse events have been documented in connection with canagliflozin, such as urinary tract infections, fungal infections in the female genital area, adverse events related to osmotic diuresis, and adverse events related to reduced fluid volume [29]. Genital mycotic infections in women were 12.73% at 100 mg and 13.78% at 300 mg, compared to 2.9% for placebo after 52 weeks. Although lower than in women, men had a higher incidence of genital mycotic infections. The rates were 3.65% at 100 mg and 2.88% at 300 mg at 26 weeks, compared to 0.51% in placebo. Most genital mycotic infections were mild or moderate and rarely required canagliflozin cessation. CANVAS revealed a significant increase in genital mycotic infections in women (68.8 vs. 17.5 occurrences per 1,000 patient-years in canagliflozin vs. placebo; p < 0.001) [37]. Additionally, using information obtained from post-marketing surveillance, the U.S. FDA reported 19 instances of urosepsis or pyelonephritis stemming from urinary tract infections in individuals who were using canagliflozin [31].
With a median follow-up of 2.62 years, this double-blind, randomized trial included 4,401 people with T2DM and kidney disease. The most common results were renal or cardiovascular mortality, doubling of serum creatinine, or end-stage renal disease (ESDR). With occurrence rates of 43.2 and 61.2 per 1,000 patient-years, respectively, canagliflozin decreased primary outcome risk by 30%. Both end-stage kidney disease and the renal-specific composite of renal death, creatinine doubling, or both were 34% lower. Canagliflozin reduced the risk of heart failure-related hospitalizations, cardiovascular mortality, myocardial infarction, and stroke. The incidence of amputation and fracture did not change much [27].
Research conducted by Jonghe et al. in 2014 [30] suggested potential carcinogenic effects associated with canagliflozin. This finding was based on observations of increased occurrence of renal tubular tumors and Leydig cell tumors in rats exposed to canagliflozin. However, a systematic review of various randomized control trials revealed no compelling evidence to suggest a substantial increase in the risk of cancer among patients treated with canagliflozin [30].
Dapagliflozin
The subsequent medication within the gliflozin category is dapagliflozin, which is utilized for managing T2DM. Initially, in July 2011, the U.S. FDA declined the approval of dapagliflozin, citing the need for additional data. Subsequently, in January 2014, it was granted the U.S. FDA approval for effectively controlling blood sugar levels in T2DM patients when accompanied by appropriate dietary adjustments and physical activity. Moreover, in October 2014, the U.S. FDA also sanctioned a combined drug therapy containing dapagliflozin and metformin hydrochloride, recognized as Xigduo [7, 39].
Dapagliflozin functions selectively and reversibly to hinder the activity of the SGLT2 transporter. As previously noted, these SGLT2 proteins are found in the PCT of the kidneys and play a role in reclaiming glucose and sodium from the initial glomerular filtrate. In terms of the body’s normal processes, approximately 180 g of glucose are filtered daily and subsequently reabsorbed. The majority of this reabsorption primarily involves SGLT2, while the remaining portion is handled by SGLT1. In individuals in good health, no glucose is typically expelled in their urine. However, in cases of hyperglycemia, as observed in T2DM, SGLT2 receptors become more active, leading to increased glucose reabsorption and contributing to hyperglycemia. Eventually, this capacity becomes overwhelmed, resulting in the presence of glucose in the urine, a condition known as glycosuria. Blocking these SGLT2 receptors through the use of dapagliflozin prevents glucose reabsorption, causing glycosuria of approximately 80 g per day. This directly reduces glucose levels, independent of insulin. After ingestion, dapagliflozin reaches its highest concentration in the bloodstream within two hours. The drug is broken down by the kidneys and liver through the action of UGT1A9. The suppression of SGLT2 activity in the PCT by dapagliflozin triggers the excretion of glucose through urine, ultimately leading to a reduction in blood glucose levels [40, 41].
Pharmacokinetics of dapagliflozin
Dapagliflozin is a pharmacological agent that is administered orally and exhibits a high degree of selectivity in inhibiting SGLT2. This inhibition leads to an increase in the excretion of glucose through the kidneys, a process known as glucuresis. The primary objective of this mechanism is to improve the regulation of blood glucose levels in individuals diagnosed with T2DM [39]. When taken orally, dapagliflozin reaches peak plasma concentrations in approximately 2 hours. Dapagliflozin has been demonstrated to have an oral bioavailability of 78% throughout a wide dosing range (0.1–500 mg) with dose-proportional systemic exposure [40]. The extravascular distribution of dapagliflozin exhibits a substantial magnitude, with a mean volume of distribution estimated at 118 L [40, 41]. UGT1A9 is the primary enzyme involved in the hepatic and renal metabolism of dapagliflozin. The resultant metabolite, dapagliflozin 3-O-glucuronide, does not exhibit significant inhibitory effects on SGLT2 at levels that are relevant in clinical settings [42]. Merely a minute proportion, specifically less than 2% of the administered dose, of dapagliflozin is excreted in the urine without undergoing any chemical alteration. The primary route of elimination for dapagliflozin 3-O-glucuronide is through renal excretion, with approximately 61% of the administered dose of dapagliflozin being excreted as this metabolite in the urine. Oral administration of dapagliflozin at a dosage of 10 mg resulted in a half-life of 12.9 hours. Patients diagnosed with T2DM experienced the most significant elevations in UGE when T2DM was administered at dosages ≥ 20 mg per day [42, 43]. There were no statistically significant differences in dapagliflozin exposure across different demographic factors, such as age, race, ethnicity, sex, body weight, diet, or the prevalence of T2DM. A decrease in urinary glucose excretion was observed in healthy individuals compared to those with T2DM due to a reduction in the filtered load, which is calculated by multiplying plasma glucose levels with the glomerular filtration rate. Pharmacodynamic modifications are contingent upon the plasma glucose level and the functioning of the renal system [44]. Subjects with T2DM showed dose-related decreases in plasma glucose parameters and increased glucose excretion in the urine after several doses of dapagliflozin. Dapagliflozin exposure is increased in patients with significant renal or hepatic impairment. When dapagliflozin was studied in combination with other antidiabetic and cardiovascular medications, as well as pharmaceuticals that could alter dapagliflozin metabolism, no clinically meaningful drug interactions were discovered that would warrant dose adjustment [44]. The absorption rate of dapagliflozin was affected by the presence of food, resulting in a prolongation of the time to reach the maximum concentration (tmax) by approximately 1 hour and a decrease in the maximum concentration (Cmax) by 30–45% compared to the state of fasting. Nevertheless, the overall degree of absorption remains unaltered when concurrently delivered with food [45].
Clinical efficacy of dapagliflozin in patients with T2DM
A study was conducted by Oyama et al. in 2022 [45], to evaluate the effectiveness and safety of dapagliflozin concerning heart and kidney health, considering the concurrent usage of cardiovascular medications, in individuals with T2DM. The findings from this investigation indicated that dapagliflozin consistently lowered the likelihood of cardiovascular and kidney-related issues, regardless of whether patients were also taking different cardiovascular medications. Importantly, there were no notable interactions between the treatments and any significant safety events. The data obtained from this study underscored the clinical advantages and safety of dapagliflozin across a diverse group of T2DM patients, regardless of the medications they were concurrently using [45].
Another study, known as the DECLARE-TIMI 58 trial or the Trial Evaluating Cardiovascular Outcomes with Dapagliflozin—Thrombolysis in Myocardial Infarction 58 trial, involved a random assignment of individuals who had either T2DM or a history of atherosclerotic cardiovascular disease or risk factors for the disease into groups receiving either dapagliflozin or a placebo. The outcomes of this study revealed that dapagliflozin reduced the likelihood of hospitalization due to heart failure and improved renal outcomes, regardless of the initial systolic blood pressure (SBP). Additionally, there were no notable differences in adverse events of interest at different baseline SBP levels. The results of this study further confirmed that dapagliflozin delivers cardiovascular and renal benefits to patients with T2DM who are at a high risk of atherosclerotic cardiovascular disease, irrespective of their initial blood pressure [46, 47]. SGLT2 inhibitor therapy also possesses the potential to reduce the risk of major adverse cardiovascular events (MACEs) by approximately 65% at one year of follow-up. This reduction can be achieved through ameliorative effects on glucose homeostasis, as well as through the reduction of systemic inflammatory burden and local effects on atherosclerotic plaque inflammation, lipids’ deposit, and fibrous cap thickness (FCT) in patients with multivessel non-obstructive coronary stenosis (Mv-NOCS) who have T2DM [48]. In addition, it has been discovered that treatment with SGLT2 inhibitors in T2DM is associated with a lower incidence of intra-stent restenosis (ISR)-related events following acute myocardial infarction. This finding is irrespective of the degree to which glycemic control is achieved [49].
The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease trial, referred to as DAPA-CKD, aimed to investigate whether the effectiveness of dapagliflozin in individuals with T2DM was influenced by their prior glucose-lowering therapy (GLT). This study included the random assignment of 4,304 adults, among whom approximately 2,906 had T2DM. These participants had an initial eGFR between 25–75 mL/min/1.73 m2 and a urinary albumin-to-creatinine ratio ranging from 200–5,000 mg/g. They were administered either dapagliflozin at a 10 mg dose or a placebo once daily. The study’s findings revealed that dapagliflozin significantly reduced the incidence of kidney and cardiovascular events in patients with T2DM and chronic kidney disease (CKD), regardless of their baseline GLT regimen or combinations thereof [50]. Table 3 represents a comprehensive overview of the clinical trial data supporting dapagliflozin’s outcome and ADRs.
Randomized clinical trial evaluating dapagliflozin
| Study name | Sample | Duration | Outcome | Adverse drug events | Reference | |
|---|---|---|---|---|---|---|
| Common | Uncommon | |||||
| Dapagliflozin Effect on Cardiovascular Events (DECLARE-TIMI 58) Trial | 17,160 participants | 11 years | Reduced frequency of cardiovascular fatalities or hospital admissions due to heart failure and a preventive effect on cardiovascular incidents observed in the dapagliflozin group | Amputation, fracture, volume depletion, genital infections, urinary tract infection, diabetic ketoacidosis | Major hypoglycemia, acute kidney injury, breast cancer, bladder cancer, urosepsis | [51] |
| Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) Trial | 4,744 participants | 1.5 years | Decreased likelihood of heart failure exacerbation or mortality due to cardiovascular reasons within the dapagliflozin group | Fracture, amputation, volume depletion | Major hypoglycemia, renal adverse events | [52] |
| Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) Trial | 4,304 participants | 2.4 years | Continuous reduction in the estimated glomerular filtration rate by a minimum of 50%, end-stage kidney disease, or fatalities stemming from renal or cardiovascular origins in the dapagliflozin group | Fracture, volume depletion, renal related adverse events | Major hypoglycemia, amputation | [53] |
| Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) Trial | 6,263 participants | 2.3 years | Reduced rate of heart failure deterioration or cardiovascular-related fatalities among individuals in the dapagliflozin group | Lower limb amputation, volume depletion, renal related adverse events | Diabetic ketoacidosis, major hypoglycemia | [54] |
| Dapagliflozin versus Sitagliptin Treatment Efficacy on Prevention of Cardiovascular Risk Factors in Type 2 Diabetes Patients (DIVERSITY-CVR) Trial | 340 participants | 24 weeks | Dapagliflozin demonstrated superior efficacy in enhancing cardiometabolic risk factors and proved to be more effective in preventing cardiovascular events in individuals with early-stage type 2 diabetes that was not adequately controlled | - | Major hypoglycemia, fracture | [55] |
| NCT04120623 | 66 participants | 5 weeks | The combination of dapagliflozin and insulin represents a secure and efficient treatment option for ameliorating fluctuations in blood sugar levels, enhancing insulin sensitivity, and promoting weight reduction in individuals newly diagnosed with type 2 diabetes | - | Urinary tract infection | [56] |
| NCT01730534 | 17,160 participants | 4 years | Dapagliflozin exhibited clinical advantages and safety across a diverse spectrum of patients with type 2 diabetes, irrespective of their prior treatment. Consistently, dapagliflozin decreased the likelihood of cardiovascular and kidney-related consequences | - | Volume depletion, acute kidney injury, hyperkalemia, diabetic ketoacidosis, amputation | [57] |
Adverse events associated with dapagliflozin
In 2019, Wiviott et al. [51] did a randomized clinical trial to examine the cardiovascular effects of dapagliflozin in patients with T2DM. Of the 17,160 people surveyed, 10,186 did not have atherosclerotic cardiovascular disease; the median duration of follow-up was 4.2 years. While dapagliflozin did reduce cardiovascular death or heart failure hospitalization (4.9% vs. 5.8%), it did not significantly reduce major adverse cardiac events (8.8% in the dapagliflozin group and 9.4% in the placebo group). Renal incidents occurred in 6.2% of placebo patients compared to 4.3% of dapagliflozin patients. Mortality from any cause was 6.6% in the placebo group and 4.3% in the dapagliflozin group. Significant vaginal infections occurred in 0.9% of dapagliflozin patients compared to 0.1% in placebo, and diabetic ketoacidosis in 0.3% of patients. According to findings from this DECLARE-TIMI 58 trial, the dapagliflozin group exhibited fewer instances of serious adverse events, major hypoglycemia, acute kidney injury, and bladder cancer than did the placebo group. Moreover, the rates of amputation, fracture, volume depletion, and hypersensitivity were similar between the dapagliflozin group and the placebo group. Additionally, the trial revealed a greater incidence of diabetic ketoacidosis in the dapagliflozin group than in the placebo group. Furthermore, there were rare cases of serious genital infections reported more frequently in the dapagliflozin group than in the placebo group [51].
Empagliflozin
In 2014, Boehringer Ingelheim and Eli Lilly and Company collaborated to create this medication. It falls under the category of gliflozin, similar to other SGLT2 inhibitors. Empagliflozin, a member of this class, demonstrates significantly greater selectivity for the SGLT2 receptor, exceeding it by more than 25 × 102 fold, and it has minimal adverse effects on the gastrointestinal tract [7, 58].
Empagliflozin works by reducing the reabsorption of filtered glucose in the kidneys and lowering the threshold at which glucose is reabsorbed, resulting in increased excretion of glucose in the urine and decreased blood glucose levels. Importantly, this glucuretic effect is influenced by blood glucose concentration and the glomerular filtration rate, independent of insulin, making empagliflozin associated with a low inherent risk of hypoglycemia [59].
Pharmacokinetics of empagliflozin
Empagliflozin demonstrates fast absorption following oral treatment, with peak plasma concentrations being achieved within a median time of 1.5 hours. Following the attainment of the initial peak, plasma concentrations rapidly decrease, subsequently transitioning into a more gradual terminal phase. Systemic exposure to empagliflozin increases proportionally with dosage, and its pharmacokinetic characteristics remain consistent after a single dose and in a steady state, indicating linear pharmacokinetics throughout time [58]. The substance exhibits a half-life (T1/2) of 13 hours, possesses a substantial affinity for protein binding (86%), and is deemed suitable for once-daily dosing without significant safety concerns [59]. The primary metabolic pathway for this substance is predominantly glucuronidation. Empagliflozin is primarily excreted through renal elimination (55%) and fecal excretion (40%) without undergoing significant metabolic transformation [59, 60]. The area under the curve (AUC) for empagliflozin exhibited an increase of approximately 18% in patients with mild CKD, 66% in subjects with moderate CKD, 48% in subjects with severe CKD, and 48% in subjects with ESRD, likely due to a decrease in renal clearance. As CKD progresses, there is a concomitant decrease in the mean proportion of a drug’s dosage that is excreted through renal elimination. No adjustment of empagliflozin dosage is necessary for people with CKD due to the small increase in drug exposure [61]. Moreover, there is a lack of documented evidence about potential drug interactions between the prescription in question and other commonly prescribed drugs for the treatment of T2DM [60].
Clinical efficacy of empagliflozin in patients with T2DM
In the EMPA-REG OUTCOME clinical trial, researchers assessed the effectiveness of empagliflozin as an adjunct to standard treatment for T2DM patients, focusing on cardiovascular events and monitoring preexisting cardiovascular conditions. This study was designed as a randomized, double-blind, placebo-controlled, multinational trial with a noninferiority objective. A total of 7,020 eligible participants were included in the study, with 2,345 individuals receiving empagliflozin at a 10 mg/day dose, 2,342 receiving empagliflozin at a 25 mg/day dose, and 2,333 receiving a placebo. The treatment period lasted for a median duration of 2.6 years, and participants were followed for a median of 3.1 years. The results of this investigation indicated that empagliflozin exhibited protective effects on both the heart and kidneys in T2DM patients with cardiovascular issues, and these benefits were largely independent of glycemic control [59, 60].
The EMPA-CARD trial was conducted to investigate whether the inhibition of the SGLT2 receptor by empagliflozin could reduce inflammation. This trial focused on assessing inflammatory status by measuring decreases in inflammatory markers in patients diagnosed with both T2DM and coronary artery disease after a 26-week treatment period. This study aimed to provide insights into the mechanisms through which the inhibition of the SGLT2 receptor contributes to the favorable effects of this medication on clinical outcomes. The EMPA-CARD was a comprehensive, multicenter, triple-blind, randomized, and placebo-controlled study. In this research, a daily 10 mg dose of empagliflozin was compared to a placebo over 26 weeks. The primary outcome of interest was the alteration in plasma interleukin 6 levels after 26 weeks of treatment. Additionally, the secondary outcomes, which were designed to explore the primary objective of the study, were divided into four categories:
Changes in additional plasma inflammatory biomarkers, including high-sensitivity C-reactive protein (hs-CRP) and plasma interleukin 1-beta (IL-1b), were detected.
Alterations in oxidative stress parameters, such as lymphocytic ROS levels, plasma malondialdehyde (MDA) levels, carbonyl levels, (T-AOC), reduced glutathione (GSHr) levels, catalase (CAT) activity, and superoxide dismutase (SOD) activity, were detected.
Adjustments in platelet function, specifically CD62-P expression on the platelet surface.
Variations in glycemic status, including fasting blood glucose (FBS), HbA1c, and homeostatic model assessment for insulin resistance (HOMA-IR).
Additionally, transient global hypo-perfusion causes a brief loss of consciousness in vasovagal syncope (VVS), which has a rapid onset, a brief duration, and a full spontaneous recovery. In as many as 35% of instances, the VVS may return, resulting in a worse prognosis and a diminished quality of life. Recurrence of VVS is more common in patients with T2DM, and the presence of this disease is a strong predictor of future recurrences. Curiously, individuals with T2DM who also experience vagal tone excess and sympathetic tone withdrawal exhibit clinical manifestations of a severe autonomic nervous system malfunction. Resting tachycardia, exercise intolerance, irregular blood pressure regulation, orthostatic hypotension, and other serious changes to heart rate may result from this. Results from a prospective multicenter study by Sardu et al. in 2022 [62] indicated that the autonomic nervous system was different in 324 T2DM patients who used non-SGLT2 inhibitors compared to those who used SGLT2 inhibitors. The study found a higher rate of VVS recurrence at one year of follow-up in the non-SGLT2 inhibitor group. Recurrence of VVS was predicted by cardiac denervation indices and the probability of recurrence was reduced by the use of SGLT2 inhibitors [62]. Table 4 represents a comprehensive overview of the clinical trial data supporting empagliflozin’s outcome and its ADRs.
Randomized clinical trial evaluating empagliflozin
| Study name | Sample | Duration | Outcome | Adverse drug events | Reference | |
|---|---|---|---|---|---|---|
| Common | Uncommon | |||||
| Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) Trial | 7,020 participants | 3.1 years | Reduced incidence of cardiovascular-related fatalities, non-lethal heart attacks, or non-lethal strokes within the empagliflozin group | Urinary tract infection, genital infection | Hypoglycemia, acute renal failure, diabetic ketoacidosis, volume depletion, fracture | [60] |
| Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) Trial | 3,730 participants | 1.4 years | Fewer instances of heart failure hospitalizations and a decelerated decline in the estimated glomerular filtration rate were observed in the empagliflozin group | Volume depletion, hypotension, urinary tract infection, genital infection | Fracture, lower limb amputation, hypoglycemia, diabetic ketoacidosis | [63] |
| Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) Trial | 5,988 participants | 2.2 years | Empagliflozin lowered the combined likelihood of cardiovascular death or hospitalization due to heart failure in individuals with heart failure and a preserved ejection fraction, regardless of whether they had diabetes or not | Hypotension, symptomatic hypotension, urinary tract infection, genital infection, fracture | Lower limb amputation, ketoacidosis, hypoglycemia, acute renal failure | [64] |
| KCT0006157 | 152 participants | 24 weeks | Empagliflozin exhibited more pronounced reductions in HbA1c levels and greater weight loss in patients with type 2 diabetes who had insufficient glycaemic control despite being treated with metformin, sulfonylurea, and a dipeptidyl peptidase 4 inhibitor (DPP-4i) | - | - | [65] |
| NCT01177813 | 899 participants | 24 weeks | Empagliflozin offered a well-tolerated and effective approach to lowering HbA1c levels in patients with type 2 diabetes who had not been previously treated with medications | Urinary tract infection | Hypoglycemia, dyslipidemia, genital infection | [66] |
| NCT01167881 | 1,549 participants | 104 weeks | Empagliflozin could serve as an efficient and well-tolerated choice for second-line treatment in individuals with type 2 diabetes who have not attained satisfactory glycemic control with metformin alone | Urinary tract infection, genital infection | Fracture, hypoglycemia, dyslipidemia, hypertension | [67] |
Adverse events associated with empagliflozin
In the EMPA-REG OUTCOME experiment, a total of 7,020 patients received treatment. Among these patients, 55.5% were below the age of 65, 35.3% were between the ages of 65 and 75, and 9.3% were 75 years or older at the start of the trial. The trial revealed a notable incidence of genital infections among patients in the empagliflozin group. Nevertheless, the occurrence of adverse events, including hypoglycemia, acute renal failure, diabetic ketoacidosis, thromboembolic events, bone fractures, and events indicative of volume depletion, did not significantly differ between the empagliflozin and placebo groups [68, 69].
Moreover, it was noted that genital tract infections were more prevalent among patients in the empagliflozin group than among those in the placebo group. However, the rates of overall urinary tract infection, complicated urinary tract infection, and pyelonephritis were similar between the empagliflozin and placebo groups. Additionally, while urosepsis was reported infrequently, the proportion of patients experiencing this event was greater in the empagliflozin group than in the placebo group [70].
Ertugliflozin
In 2017, the U.S. FDA granted initial approval for ertugliflozin, followed by approval from the European Medicines Agency (EMA) in 2018. It was approved as a combination therapy with diet and exercise to effectively manage blood sugar levels in individuals with T2DM. The FDA’s recommended starting dose is 5 mg, which is taken once daily, with the option to increase to a 15 mg daily dose if additional blood sugar control is needed [71].
Ertugliflozin is a highly specific inhibitor of SGLT2 that effectively reduces the reabsorption of glucose in the kidneys, lowering the RTG and ultimately resulting in increased excretion of glucose in the urine. This action is independent of insulin secretion and sensitivity, as it operates through a different mechanism. Ertugliflozin can complement the effects of other oral medications, such as metformin, sitagliptin, and sulfonylureas, as well as insulin when used in combination. Ertugliflozin boasts excellent oral bioavailability, approximately 100%, and achieves its peak concentration within 1 hour when taken on an empty stomach or within 2 hours when consumed with a high-fat, high-calorie meal. With a half-life of 16.6 hours in patients with T2DM, it is suitable for once-daily dosing [72, 73].
Pharmacokinetics of ertugliflozin
Ertugliflozin has recently received approval from the U.S. FDA as an SGLT2 inhibitor. When administered orally, ertugliflozin reaches its Cmax within one hour under fasting conditions. The pharmacokinetics of ertugliflozin exhibits a dose-dependent increase ranging from 0.5 to 300 mg, and there is no observable difference in its administration when taken with or without food [73]. Ertugliflozin is categorized as a Class I medication according to the Biopharmaceutical Classification System, owing to its high absolute bioavailability of approximately 100% when administered under fasting conditions. The predominant metabolic pathway for the clearance of ertugliflozin involves the catalytic activity of the UGT family’s isoforms UGT1A9 and UGT2B7, which are responsible for glucuronidation, accounting for 86% of the process [74]. The percentage of the entire dose of the medicine that remains unchanged and is eliminated through urinary excretion is 1.5% [74–76]. According to research findings, individuals diagnosed with T2DM and varying degrees of renal impairment, ranging from mild to severe, are anticipated to exhibit an exposure to ertugliflozin that is up to 70% greater than that of individuals with normal renal function [77]. According to research findings, the pharmacokinetics of ertugliflozin are comparable between healthy individuals and patients with T2DM. Consequently, the administration of this drug is not contingent upon the patient’s dietary intake or specific racial background. Furthermore, adjustments to ertugliflozin dosage are unnecessary when coadministered with commonly prescribed medications or in the presence of renal impairment or mild-to-moderate hepatic impairment in patients [74, 76].
Clinical efficacy of ertugliflozin in patients with T2DM
In the Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial, commonly referred to as VERTIS CV, a multicenter, double-blind, randomized controlled trial involving individuals with T2DM and atherosclerotic cardiovascular disease, the findings indicated that ertugliflozin did not perform as well as the placebo in terms of the combined outcome, which included cardiovascular-related deaths, nonfatal myocardial infarctions, and nonfatal strokes [75, 76].
The effects of SGLT2 (and SGLT2 inhibitors) on inflammation and the amount of adipose tissue in obesity were also discovered. Sardu et al. 2023 [78] aimed to use over-inflammation and the down-regulation of sirtuins to determine the effect of SGLT2 breast expression on cardiovascular performance in premenopausal women with fatty vs. non-fatty breasts. They found that SGLT2/inflammatory cytokines were overexpressed in fatty breasts and downregulated in non-fatty breasts and that breast sirtuins were downregulated. Changes in myocardial performance index (ΔMPI) at one year of follow-up are associated with SGLT2/inflammatory cytokine expression, tissue sirtuin 3 (tSIRT3), and breast percentage density, in inverse order. At one year of follow-up, SIRT-3 raised the likelihood of normal cardiac performance (NCP), whereas fatty breast and SGLT2 inversely predicted NCP [78].
In a different study, researchers found that patients suffering from acute myocardial infarction who underwent coronary artery bypass grafting (CABG) had worse clinical outcomes due to increased inflammation, pericoronary fat expression of SGLT2, leptin, and SIRT6 [76].
In another phase III randomized, multicenter trial, the effectiveness and safety of ertugliflozin were evaluated as a standalone treatment for patients with poorly controlled T2DM. This study spanned 52 weeks, with the first 26 weeks constituting Phase A, a double-blind, placebo-controlled period. During this initial phase, 461 participants were randomly assigned to receive either a placebo, 5 mg ertugliflozin daily or 15 mg ertugliflozin daily. Following Phase A, an additional 26-week active-controlled period, known as Phase B, was conducted. In Phase B, participants in the placebo group who had not received additional GLT were given metformin in a blinded manner. The trial’s results revealed that over the course of 52 weeks, treatment with ertugliflozin led to improvements in glycemic control, along with reductions in body weight and SBP [76, 77]. Table 5 represents a comprehensive overview of the clinical trial data supporting ertugliflozin’s outcome and its ADRs.
Randomized clinical trial evaluating ertugliflozin
| Study name | Sample | Duration | Outcome | Adverse drug events | Reference | |
|---|---|---|---|---|---|---|
| Common | Uncommon | |||||
| Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial (VERTIS CV) | 8,246 participants | 3.5 years | Ertugliflozin was determined to be as effective as a placebo in relation to the combined outcome of cardiovascular death, non-fatal heart attack, or non-fatal stroke, within the established non-inferiority margin | Urinary tract infection, symptomatic hypoglycemia | Male and female mycotic infection of genitals, severe hypoglycemia, hypovolemia, acute kidney injury, pancreatitis, diabetic ketoacidosis, fracture, amputation | [79] |
| Evaluation of Ertugliflozin effIcacy and Safety (VERTIS FACTORIAL) | 1,233 participants | 52 weeks | When administered alongside metformin in patients with poorly managed type 2 diabetes, the combined use of ertugliflozin and sitagliptin resulted in superior glycaemic control over 52 weeks compared to each medication alone | Genital mycotic infections, urinary tract infection | hypovolemia, hypoglycemia | [80] |
Adverse events associated with ertugliflozin
According to the findings of the VERTIS CV trial, a greater occurrence of urinary tract infections and genital mycotic infections was observed in the group of patients treated with ertugliflozin compared to the group that received a placebo. However, there were no significant differences in the incidence of serious acute kidney injury, serious urinary tract infection, hypovolemia, fractures, or symptomatic or severe hypoglycemia between either the ertugliflozin-treated group and the placebo group. Furthermore, the data revealed that approximately 54 patients who received a 5 mg dose of ertugliflozin and approximately 57 patients who received a 15 mg dose of ertugliflozin underwent amputation, while only 45 patients in the placebo group underwent such procedures. Additionally, approximately 7 patients in the 5 mg ertugliflozin group and 12 patients in the 15 mg ertugliflozin group were diagnosed with diabetic ketoacidosis, while only 2 patients in the placebo group were diagnosed [79, 81–83].
Adverse events associated with SGLT2 inhibitors
SGLT2 inhibitors have shown substantial efficacy in improving glycemic control and providing cardiovascular benefits. Meta-analyses, such as those by Zelniker et al. in 2019 [83], have demonstrated significant reductions in HbA1c levels and MACEs in patients treated with SGLT2 inhibitors. Specifically, the reduction in MACEs (HR, 0.89; 95% CI, 0.83–0.96) and the mean reduction in HbA1c (0.77%; 95% CI, 0.70–0.84) highlight the positive impact of these drugs on diabetes management [83].
However, the use of SGLT2 inhibitors is associated with several adverse effects that require careful consideration.
Volume depletion and hypotension are also notable risks. Tang et al. 2016 [84] highlighted an increased risk of volume depletion events (OR, 1.44; 95% CI, 1.15–1.79), necessitating careful monitoring of fluid status in patients [85]. While the risk of fractures associated with SGLT2 inhibitors, particularly canagliflozin, was slightly elevated (HR, 1.23; 95% CI, 0.95–1.60), it was not statistically significant, according to Tang et al. (2016) [84].
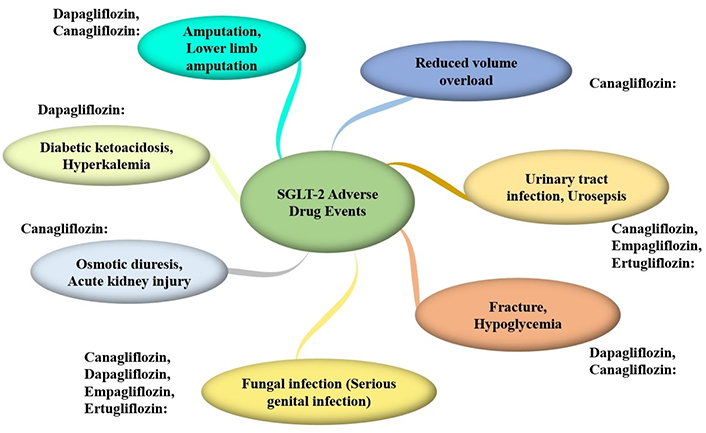
Furthermore, the CANVAS Program identified a concerning increase in lower limb amputations with canagliflozin (HR, 1.97; 95% CI, 1.41–2.75), emphasizing the need for vigilant monitoring in patients with preexisting peripheral vascular disease [37]. Figure 2 summarizes the adverse events associated with the use of SGLT2 inhibitors. SGLT2 inhibitors, including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, represent novel therapeutic approaches for treating T2DM.
Comparative evaluation of SGLT inhibitors vs. DPP-4 inhibitors
Despite the differences in the mechanisms of their action, both classes of medicines are extensively utilized in managing T2DM and provide unique advantages. SGLT2 inhibitors, including canagliflozin and empagliflozin, help patients with beta-cell dysfunction reduce their blood glucose levels through an insulin-independent mechanism that promotes glucose excretion in the urine and inhibits glucose reabsorption in the kidneys. Whereas DPP-4 inhibitors like saxagliptin and sitagliptin, enhance glucose-dependent insulin production and reduce glucagon release by preventing the breakdown of incretin hormones like GLP-1 [14].
Kutz et al. [14] recently performed a comparison analysis and found that primary cardiovascular effectiveness outcomes per 1,000 person-years for SGLT2 initiators were 34.3 and for DPP-4 initiators they were 47.7 (HR, 0.72; 95% CI, 0.69–0.75). Effectiveness outcomes associated with the initiation of SGLT2 include a decreased risk of acute myocardial infarction, hypertensive heart failure (HHF) all-cause mortality, and stroke, with a tendency towards a lower rate of stroke. Secondary safety outcomes, associated with SGLT2 initiators, include overall reduced rates of acute renal damage, hypoglycemia, severe urinary tract infection, and nonvertebral fracture when compared to DPP-4 initiators. In contrast to DPP-4 inhibitors, SGLT2 was linked with a greater overall incidence of diabetic ketoacidosis, vaginal infections, and lower-limb amputations. When comparing SGLT2 inhibitors to DPP-4 inhibitors, the results showed that the former reduced the need for hospitalization due to heart failure. The two groups’ efficacy outcomes were similar; however, genital infections and diabetic ketoacidosis were more common in the SGLT2 inhibitor group, and hospitalization for acute kidney injury was lower in the DPP-4 inhibitor group [14, 48].
On average, SGLT2 inhibitors lower HbA1c levels by 0.77%, body weight by 2.2 kg, and MACEs by 23%. In contrast, DPP-4 inhibitors lower HbA1c levels by 0.64%, have no effect on weight, and do not detectably increase MACEs [85]. While SGLT2 inhibitors are linked to an increased risk of genital and urinary tract infections [86], euglycemic diabetic ketoacidosis, and volume depletion, DPP-4 inhibitors are linked to an increased risk of pancreatitis, nasopharyngitis, and headaches, but they do not substantially raise the risk of hypoglycemia or weight gain [83]. Clinically, T2DM patients at high risk for cardiovascular disease or kidney disease may benefit most from SGLT2 inhibitors, while older adults and those with severe renal impairment are good candidates for DPP-4 inhibitors if they want effective glycemic control without weight gain or hypoglycemia. Therefore, physicians should weigh patient preferences, comorbidities, risk factors, and profiles when deciding between the two groups of drugs [87].
Conclusions
In summary, this updated literature review offers a comprehensive exploration of the role of SGLT2 inhibitors in the management of T2DM, along with an examination of potential adverse effects associated with their use. These inhibitors, which operate independently of insulin and target the kidneys, have revolutionized diabetes management. By reducing renal glucose reabsorption and enhancing glucose excretion in urine, they consistently lower plasma glucose levels in individuals with hyperglycemia.
Moreover, extensive clinical data demonstrate the remarkable efficacy of these SGLT2 inhibitors in not only achieving glycemic control but also mitigating the risk of cardiovascular events in diabetic patients. Their impact extends beyond glycemic management, encompassing beneficial effects on cardiovascular and renal functions.
However, the utilization of SGLT2 inhibitors is not without challenges. Common adverse effects, such as genitourinary infections, hypoglycemia, and the rare occurrence of diabetic ketoacidosis, necessitate careful patient monitoring and education to ensure their safe and effective use.
Initially, approved for treating T2DM, particularly in younger patients with obesity or metabolic syndrome, the application of SGLT2 inhibitors has expanded. They are increasingly utilized in patients with T2DM associated with cardiovascular or renal comorbidities and even in those with type 1 diabetes (T1D). Furthermore, with emerging evidence indicating potential benefits in improving cardiorenal functions in nondiabetic individuals, ongoing clinical trials are investigating their use in patients without diabetes who suffer from heart or kidney failure. Additionally, the potential preventive role of these inhibitors in the development of T2DM in individuals with impaired glucose tolerance (IGT) is being explored.
This review underscores the dynamic landscape of SGLT2 inhibitors, their multifaceted applications, and the need for vigilant clinical management to optimize their benefits while minimizing potential risks. Ongoing research holds promise for expanding the horizons of T2DM care and cardiorenal health.
Abbreviations
| ADRs: | adverse drug reactions |
| CANTATA: | Canagliflozin Treatment and Trial Analysis |
| CANVAS: | Canagliflozin Cardiovascular Assessment Study |
| CKD: | chronic kidney disease |
| CREDENCE: | Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation |
| DPP-4: | dipeptidyl peptidase 4 |
| EMA: | European Medicines Agency |
| FAERS: | FDA Adverse Event Reporting System |
| FDA: | Food and Drug Administration |
| HbA1c: | hemoglobin A1c |
| MACEs: | major adverse cardiovascular events |
| PCT: | proximal convoluted tubules |
| RTG: | renal threshold for glucose |
| SBP: | systolic blood pressure |
| SGLT2: | sodium-glucose cotransporter 2 |
| T2DM: | type 2 diabetes mellitus |
| UGT: | uridine diphosphate-glucuronosyltransferase |
| VVS: | vasovagal syncope |
Declarations
Author contributions
FH, HN: Conceptualization, Investigation, Writing—original draft. DS: Writing—original draft. M Arshad: Investigation, Writing—original draft. SZ: Investigation, Writing—review & editing. MKR: Investigation. MAH, M Akhtar: Writing—review & editing. AKN: Supervision. All authors reviewed and approved the final version of the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.