Affiliation:
1Department of Medicine, ESIC Postgraduate Institute Medical Sciences and Research, New Delhi 110015, India
Email: dramit_67@yahoo.com
ORCID: https://orcid.org/0000-0001-9348-7601
Affiliation:
1Department of Medicine, ESIC Postgraduate Institute Medical Sciences and Research, New Delhi 110015, India
ORCID: https://orcid.org/0000-0001-6570-8577
Affiliation:
1Department of Medicine, ESIC Postgraduate Institute Medical Sciences and Research, New Delhi 110015, India
Affiliation:
1Department of Medicine, ESIC Postgraduate Institute Medical Sciences and Research, New Delhi 110015, India
Explor Med. 2025;6:1001271 DOI: https://doi.org/10.37349/emed.2025.1001271
Received: October 03, 2024 Accepted: November 21, 2024 Published: January 10, 2025
Academic Editor: Hongzhou Lu, The Third People’s Hospital of Shenzhen, China, Fudan University, China
Ventilator-associated pneumonia (VAP) is a common and serious complication in critically ill patients, particularly those requiring prolonged mechanical ventilation. Extensively drug-resistant (XDR) Acinetobacter baumannii has emerged as a significant threat in VAP cases, complicating treatment due to its exceptionally high level of antibiotic resistance. We present a case of a 62-year-old male patient with severe acute ischemic stroke, who developed VAP during his stay in the intensive care unit (ICU). Cultures confirmed XDR Acinetobacter baumannii as the causative organism. The infection was further complicated by the development of a lung abscess, a rare but severe consequence of VAP. The patient was treated with a targeted antimicrobial regimen consisting of colistin, vancomycin, and tigecycline. Despite the pathogen’s resistance profile, the patient’s infection showed gradual improvement with sustained treatment. However, the recovery process was significantly prolonged due to the complexity of the infection, necessitating extended supportive care during hospitalization. This case highlights the challenges posed by XDR infections in critically ill patients and the importance of a tailored antimicrobial approach to effectively manage severe complications such as lung abscess.
Ventilator-associated pneumonia (VAP) is a significant complication in critically ill patients, particularly those requiring mechanical ventilation due to acute neurological conditions such as stroke [1]. The incidence of VAP can be as high as 30%, significantly impacting morbidity, mortality, and healthcare costs [2–4]. The pathogens responsible for VAP vary widely, often reflecting the local epidemiology of hospital-acquired infections. Among these, Acinetobacter baumannii has gained importance for its association with VAP [5, 6]. This organism is not only capable of causing severe pneumonia but has also evolved mechanisms to resist multiple classes of antibiotics, leading to the emergence of extensively drug-resistant (XDR) strains. XDR Acinetobacter baumannii is particularly concerning due to its resilience and the limited treatment options available, resulting in poor clinical outcomes for affected patients [7–10]. The occurrence of lung abscess as a complication of VAP is relatively rare but can complicate the clinical course of affected patients, leading to further deterioration and potential surgical interventions.
This report describes the case of a patient with severe acute ischemic stroke who developed a lung abscess as a complication of VAP caused by XDR Acinetobacter baumannii. It aims to highlight the clinical implications of such infections, the challenges in diagnosis and treatment, and the importance of vigilant monitoring and appropriate management strategies in critically ill patients.
A 62-year-old male diagnosed with acute ischemic stroke was transferred to the medical intensive care unit (ICU) in view of altered sensorium. He had a history of hypertension and type 2 diabetes mellitus, both of which were inadequately controlled. He had no history of previous stroke or transient ischemic attack. Additionally, he was a chronic smoker with a history of occasional alcohol consumption. On examination, the patient was unresponsive, with a Glasgow Coma Scale (GCS) score of 3. His vital signs were: blood pressure 180/100 mmHg, heart rate 82 beats per minute, respiratory rate 10 breaths per minute, and oxygen saturation 84% on room air. The computed tomography (CT) scan of the head revealed a large hypodense area in the right fronto-parietal lobes, right caudate nucleus, anterior limb of internal capsule and lentiform nucleus, suggestive of acute right middle cerebral artery (MCA) and MCA-anterior cerebral artery (MCA-ACA) watershed territory infarct. Due to his compromised respiratory status, he was immediately intubated and placed on mechanical ventilation to maintain adequate oxygenation. Routine laboratory investigations did not reveal any significant abnormalities, apart from elevated blood glucose levels (250 mg/dL) and an increased HbA1c (9.5%), indicating poorly controlled glycemic status. The electrocardiogram showed sinus rhythm with no evidence of acute ischemia or arrhythmias. The echocardiogram revealed normal left ventricular function with no evidence of cardiac thrombus or valvular abnormalities. Intravenous thrombolysis was not considered due to the delayed presentation. Antiplatelet therapy and statin medications were initiated to prevent further thromboembolic events and reduce the risk of recurrent stroke. Insulin was initiated to optimize glycemic control along with other supportive measures such as blood pressure control, and osmotic therapy to reduce intracranial pressure and cerebral edema. On day 5 of ICU admission, the patient developed fever (temperature 102°F), purulent sputum production, and increased respiratory secretions with worsening oxygenation and increased ventilator requirements, indicative of VAP. On examination, decreased breath sounds were noted on auscultation of the left lung. Chest X-ray revealed a completely white-out left lung. The CT scan of the chest demonstrated complete consolidation of the left lung, as shown in Figure 1.
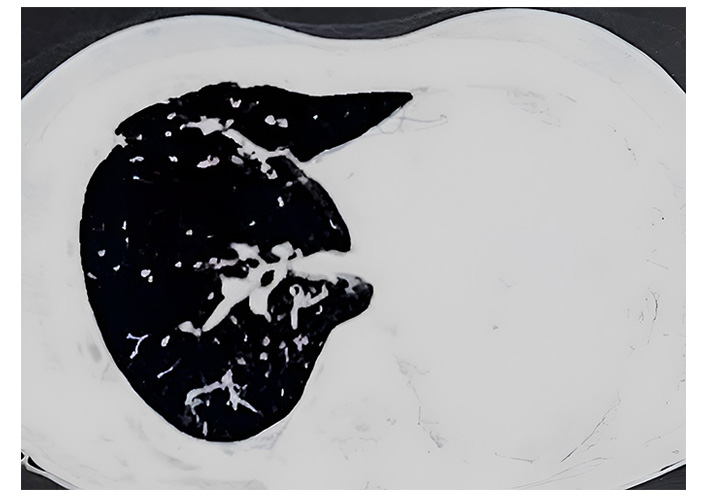
High-resolution computed tomography (HRCT) of the chest (axial view) showing complete consolidation of the left lung
The patient had already been receiving empiric broad-spectrum antibiotic therapy with intravenous piperacillin-tazobactam, which was escalated to a combination of meropenem and vancomycin. The endotracheal aspirate was sent for microbiological analysis which revealed gram-negative coccobacilli and culture growth of Acinetobacter baumannii. The isolated Acinetobacter baumannii strain was extensively drug-resistant, including resistance to carbapenems, and exhibited only intermediate sensitivity to colistin. Colistin was added to the ongoing antibiotic regimen of meropenem and vancomycin. Over the subsequent days, the patient showed gradual improvement in his neurological status. Repeated imaging of the head showed stabilization of the infarct without evidence of hemorrhagic transformation. Due to prolonged mechanical ventilation, a tracheostomy was performed to facilitate weaning from the ventilator and to provide long-term airway support. On day 15 of ICU admission, a new chest X-ray was performed due to increasingly purulent respiratory secretions and persistent fever, which revealed a cavitary lesion in the left upper lobe. A subsequent CT scan of the chest showed a thin-walled cavity (36 mm × 40 mm × 32 mm) with an air-fluid level in the anterior segment of the left upper lobe, consistent with a lung abscess (Figure 2).
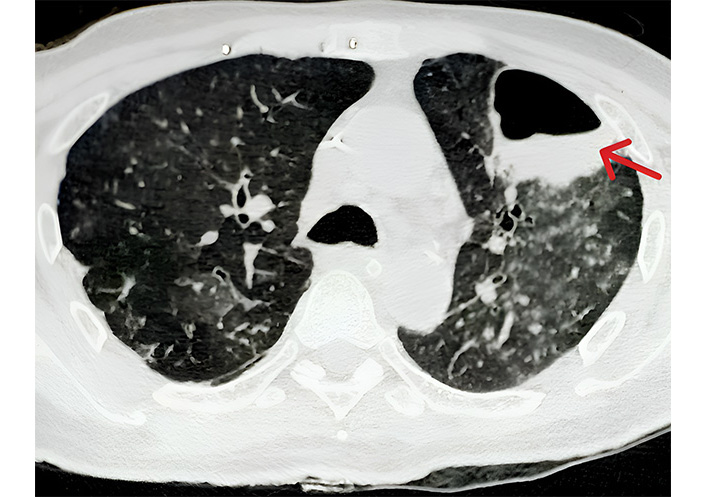
High-resolution computed tomography (HRCT) of the chest (axial view) showing a thin-walled cavity (red arrow) with air-fluid level in anterior segment of the left upper lobe with adjacent ground glass opacities
Acinetobacter baumannii was reisolated from the endotracheal aspirate, displaying the same high-level antibiotic resistance as previously detected. After careful deliberation, meropenem was discontinued and tigecycline was added to the antibiotic regimen including colistin and vancomycin. The patient responded well to the revised antibiotic regimen, with a gradual resolution of fever. Subsequently, he demonstrated signs of weaning readiness, including improved respiratory mechanics and tolerance of spontaneous breathing trials. After a period of rehabilitation and a gradual reduction in ventilatory support, the patient successfully underwent weaning trials and was eventually liberated from mechanical ventilation. He was transitioned to supplemental oxygen via a tracheostomy collar. He was eventually discharged from the ICU on day 38 to a step-down unit for further rehabilitation.
VAP is one of the most frequent ICU-acquired infections, with Acinetobacter baumannii being a leading pathogen responsible for such infections [6, 7]. However, the formation of lung abscess in association with Acinetobacter is an uncommon and challenging complication. Acinetobacter baumannii is notorious for its remarkable resilience in hospital settings, particularly in ICUs, and its alarming ability to acquire extensive drug resistance [8–10]. In this case, the pathogen demonstrated multidrug resistance, including resistance to carbapenems, which are often the first-line agents in treating severe gram-negative infections. The only antibiotic with intermediate sensitivity was colistin, a drug of last resort, which emphasizes the global challenge posed by multidrug-resistant (MDR) organisms in critical care settings.
Lung abscess formation, particularly cavitary lesions, is an atypical manifestation of VAP and is more frequently associated with organisms known to cause necrotizing infections, such as Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa [11–13]. In our patient, Acinetobacter seems to cause nosocomial pneumonia with the formation of a cavity. The cavitation likely resulted from the virulence of the Acinetobacter baumannii strain, leading to necrosis of lung tissue. The pathogenesis of such abscesses involves direct tissue destruction through the release of cytotoxic factors and the host’s inflammatory response, resulting in the formation of a cavity. The rarity of Acinetobacter-induced lung abscess adds complexity to the clinical management. Cavitary pneumonia is often more severe and requires prolonged treatment with a combination of antibiotics. In this case, the combination of colistin, vancomycin, and tigecycline was employed to target the XDR Acinetobacter strain after careful deliberation and a review of the literature [14–16]. The use of these antibiotics, particularly colistin and tigecycline reflects the limited therapeutic options available when dealing with such extensively drug-resistant pathogens [17–19].
In conclusion, this case emphasizes the importance of early recognition and aggressive management of VAP caused by XDR Acinetobacter baumannii, particularly when complicated by rare manifestations such as lung abscess. The development of cavitary lesions should prompt further imaging and microbiological analysis to guide appropriate therapeutic interventions. In the era of increasing antibiotic resistance, careful antimicrobial stewardship and timely escalation of therapy are essential to optimize outcomes in critically ill patients.
ACA: anterior cerebral artery
CT: computed tomography
ICU: intensive care unit
MCA: middle cerebral artery
MDR: multidrug-resistant
VAP: ventilator-associated pneumonia
XDR: extensively drug-resistant
AK: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. RC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. NR: Writing—original draft, Writing—review & editing. AD and ST: Validation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The Declaration of Helsinki was adequately addressed and the study was approved by the institutional ethics committee, ESIC PGIMSR (ESIC Postgraduate Institute Medical Sciences and Research), New Delhi, India (27/2024).
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from the participant.
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1973
Download: 27
Times Cited: 0
