Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is an emerging and rapidly growing health problem that currently affects more than one-third of the world general population and more than two-thirds of patients with obesity or type 2 diabetes. MASLD is associated with one or more cardio-metabolic risk factors (CMRFs) that determine the complexity of its natural history and management. Although the term MASLD encompasses a single disease, each CMRF has a different impact on MASLD, and the number of overlapping CMRFs results in a different rate of progression and outcomes of both liver and systemic disease. Its pathogenesis is characterized by insulin resistance, lipotoxicity and a complex cross-talk between liver, adipose tissue, muscle, intestine through the release of hepatokines, cytokines, myokines and inflammatory products. The stage of liver fibrosis is the best predictor of liver outcomes, such as liver failure and mortality, and also predicts the high risk of all-cause mortality associated with the disease. In many cases, the development of hepatocellular carcinoma (HCC) is associated with advanced fibrosis or cirrhosis, although it can occur at all stages of the disease, making prevention difficult. MASLD is characterized by increasing very low-density lipoprotein (VLDL) secretion and chronic low-grade systemic inflammation, which increase the risk of cardio-vascular, renal, and endocrine diseases and extrahepatic cancer. Thus, the management of MASLD requires a holistic approach and treatment of CMRFs through multispecialty collaboration. Currently, diet and physical activity are the effective first-line approaches. There are no approved drugs for the treatment of MASLD, apart from resmetirom, which in a percentage of cases improves metabolic dysfunction-associated steatohepatitis (MASH) and fibrosis. We summarize the wide and varied recent literature on the complex etiopathogenetic, clinical and therapeutic aspects of MASLD, connecting and interpreting it to facilitate clinical and management approaches.
Keywords
Steatosis, steatohepatitis, cardio-metabolic risk factors, cirrhosis, cardio-vascular disease, chronic kidney disease, endocrine diseaseIntroduction
Fatty liver disease is a growing global health problem, driven by increased use of alcohol, fat and sugar in the diet and a sedentary lifestyle. In a recent consensus [1], the general term steatotic liver disease (SLD) was chosen to classify individuals with hepatic steatosis (greater than 5% fat in the liver) due to various etiologies, and the former terms non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) were renamed to metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH), respectively. The new nomenclature emphasizes the etiopathogenetic role of metabolic factors, the inclusive metabolic diagnostic criterion, in contrast to the diagnosis of exclusion used for NAFLD, and eliminates two possible stigmas, i.e., alcoholic and fatty; it also emphasizes the concept of liver disease with multi-organ systemic involvement, e.g., cardio-vascular (CV), and renal and, redefines a holistic patient management, both at individual and population level [1].
MASLD is a systemic disease characterized by chronic liver damage, which can progress to cirrhosis and hepatocellular carcinoma (HCC) and, extrahepatic manifestations such as CV diseases (CVDs; atherosclerosis, heart disease, stroke), renal, endocrinological and extrahepatic cancers [2]. The diagnosis of MASLD is made on the basis of hepatic steatosis finding, detected either by imaging, blood biomarkers/scores or by liver histology, in the presence of at least one of the cardio-metabolic risk factors (CMRFs) listed in Table 1, and the absence of other causes of steatosis. The diagnosis of MASH, the form with the highest risk of disease progression, is made histologically on liver biopsy. The term cirrhosis-MASLD replaces that of cirrhosis-cryptogenic, if a metabolic criterion is present; moreover, MASLD-lean is not included in the diagnostic criteria, as the diagnosis is made on the basis of a metabolic criterion, so if steatosis and a metabolic factor are present, even in the lean subject, the diagnosis of MASLD must be made; as well as genetic factors are not enclosed since they do not determine MASLD, but can only be concomitant. If CMRFs are associated with moderate alcohol consumption, e.g., 20–50 g/day in women and 30–60 g/day in men, the condition is subclassified as metabolic alcoholic liver disease (MetALD). Other etiologies may be associated with MASLD and in this case, the diagnosis must be subcategorized (e.g., MASLD plus autoimmune, etc.).
CMRFs associated with MASLD†
| Metabolic factors | Reference value |
|---|---|
| Waist circumference | ≥ 102/83 cm in Caucasians or ≥ 90/80 cm in Asian |
| Blood pressure | ≥ 130/85 mmHg or drug treatment |
| Plasma triglycerides | ≥ 150 mg/dL or drug treatment |
| Plasma HDL | ≤ 40 mg/dL for men and ≤ 50 mg/dL for women or drug treatment |
| Fasting serum glucose | ≥ 100 mg/dL or 2-hours post-load glucose levels ≥ 140 mg/dL or HbA1c ≥ 5.7% or type 2 diabetes mellitus or drug treatment |
† Liver steatosis plus at least one out of 5 CMRFs for diagnosis of MASLD. CMRFs: cardio-metabolic risk factors; MASLD: metabolic dysfunction-associated steatotic liver disease; HDL: high-density lipoproteins
The metabolic risk factors for MASLD may be multiple and can influence disease progression in various ways. These factors include type 2 diabetes, dyslipidemia, visceral obesity, unhealthy diet, use of small amounts of alcohol, gut dysbiosis and others. At present, the natural history of MASLD is not completely known, although it has been demonstrated that it may have a bidirectional course, e.g., phases of disease progression and others of disease remission; moreover, in the majority of cases (around 80 per cent) the disease has a slow progression, while in other cases it has a rapid progression [3, 4]. It is likely that the steatosis burden and the various etiopathogenetic factors overlapping may play a role, as well as “multiple parallel hits” pathogenesis could explain the bidirectional course and the wide variability of disease progression among patients with MASLD. Thus, at present, in the single MASLD patient, prognosis assessment and consequently management appear to be complex.
This review summarizes the extensive recent literature, mostly from the last four years, on advances in knowledge of the complex etiopathogenetic, clinical and management aspects of MASLD with the aim of making it more understandable, linking and interpreting it, to facilitate the clinical approach, disease management and to guide future studies.
MASLD: prevalence and epidemiology
MASLD has universal distribution and is a rapidly growing disease, closely related to the increasing prevalence of obesity and type 2 diabetes worldwide. Currently, the reported prevalence of MASLD is from 32% to 38% in adults and 35.4% in pediatric age [5–7]. The highest MASLD prevalence has been found in Latin America, 44.37%; 31.2% in North America, and 25.1% in Western Europe [5]. A recent study predicts that the prevalence of MASLD will be 55% by 2040 [8]. The prevalence of MASLD rises in patients with type 2 diabetes to over 65% [9], and in overweight and obese patients to 69.9% [10]. In light of this high prevalence, MASLD can be metaphorically represented as an “elephant in a room”.
MASLD: pathophysiological mechanisms
The etiopathogenetic factors involved in the onset of MASLD include metabolic dysfunction and atherogenic dyslipidemia [11]. Insulin resistance in the liver, adipose tissue and muscle is present in almost all patients and is associated with increased free fat release from adipose tissue and free fatty acid load in the liver, which is worsened by increased de novo hepatic lipogenesis and reduced fatty acid oxidation [12]. Moreover, hepatic lipid overload enhanced the VLDL export from the liver to the blood, and this atherogenic dyslipidemia has an impact on CVD pathogenesis (Figure 1).
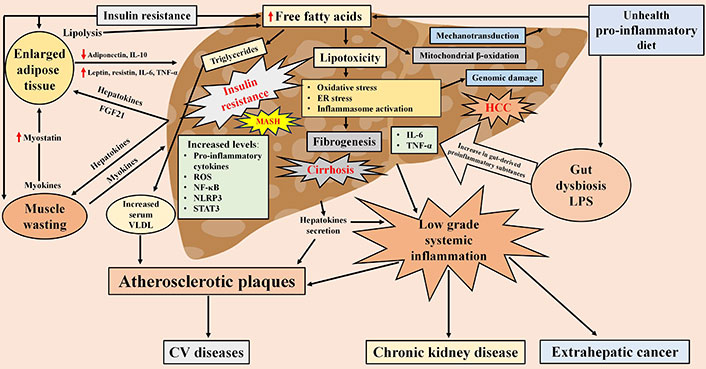
Pathophysiological mechanisms of MASLD characterized by ‘multiple parallel hits’, liver-adipose tissue-muscle-gut cross talk, and hepatic and extrahepatic clinical consequences. FGF21: fibroblast growth factor 21; VLDL: very low-density lipoprotein; ROS: reactive oxygen species; NF-κB: nuclear factor-κB; STAT3: signal transducer and activator of transcription 3; NLRP3: NOD-like receptor protein 3; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; CV: cardio-vascular; MASH: metabolic dysfunction-associated steatohepatitis; ER: endothelial reticulum; HCC: hepatocellular carcinoma; LPS: lipopolysaccharide. Red arrow pointing up means increase, red arrow pointing down means decrease
The pathogenesis of MASLD also involves a complex cross-talk between liver, adipose tissue, muscle, gut and other organs such as heart, kidney and brain [13]. As illustrated in Figure 1, this cross-talk occurs through the production of hepatokines, cytokines, myokines and inflammatory products released by these organs in connection with pathophysiological changes that occur mainly as a result of an excess of calorie intake, reduced physical activity and dysbiosis [14–16]. An average of 20–25% of individuals with MASLD have MASH, characterized by chronic liver inflammation; in addition, most of these patients have low-grade systemic inflammation contributing to extrahepatic manifestations (Figure 1) [17]. The etio-pathogenesis of inflammation is complex and multifactorial, defined as “multiple parallel hits” hypothesis, including insulin resistance, lipotoxicity, proinflammatory diets and gut dysbiosis, which contribute to the hepatic and systemic inflammation of MASLD [18]. These ‘multiple hits’ activate various pro-inflammatory cytokines and inflammasomes [NF-κB, ROS, endothelial reticulum (ER) stress, JNK, NOD-like receptor protein 3 (NLRP3), STAT3] that generate chronic inflammation, representing the key to the development of both liver fibrosis and extrahepatic complications (Figure 1) [19–21]. At present, however, in relation to the inflammation triggering, it is unclear which ‘hit’ acts first driving the disease progression and especially whether the various ‘hits’ have a different impact on the process.
Lipotoxicity reflects an important aspect of the pathogenesis of MASLD as specific lipids may exert proinflammatory effects promoting the release of various proinflammatory mediators, ER stress, the accumulation of inflammatory leukocytes in the liver and cell death [22–24]. Lipotoxicity-mediated inflammation further worsen pre-existing insulin resistance both directly and through upregulation of proinflammatory cytokines and NF-κB (Figure 1) [25, 26]. The accumulation of saturated fatty acids in the ER results in ER stress, which is characterized by the release of the transcription factor C/EBP homologous protein (CHOP). This, in turn, activates JNK and increases transcription of the BH3 protein, PUMA protein, and the BH3-only protein, Bim. The subsequent activation of the pro-apoptotic protein Bax is a consequence of this process. Bax results in mitochondrial dysfunction with cytochrome release and the activation of caspases, which induce apoptosis. Furthermore, mitochondrial dysfunction leads to an excess of ROS, which induce oxidative stress, thereby causing cell death [27]. Mitochondrial autophagy plays a crucial role in eliminating ROS, thereby maintaining mitochondrial homeostasis. Additionally, an excess of saturated fatty acids has been demonstrated to interfere with mitochondrial structure and function, leading to limitations in respiratory efficiency and resulting in damage to liver cells [27]. Thus, through altered regulation of mitochondrial function, lipotoxicity plays a central role in the pathogenesis of MASLD.
Type 2 diabetes is closely associated with hyperinsulinemia and insulin resistance. Insulin resistance, which is also mediated by other inflammatory conditions such as endoplasmic reticulum stress and obesity, is associated with low systemic inflammation, ER stress and lipotoxicity [28–30]. Visceral obesity has a significant impact on insulin resistance increasing the levels of inflammatory cytokines and altering adipokines secretion with high levels of leptin and low levels of adiponectin, promoting lipotoxic lipid deposition in the liver, thus contributing to disease progression [31, 32].
The intestinal dysbiosis in MASLD is another factor that causes inflammation [33]. Patients with MASLD show reduced diversity and changes in the gut microbiota, particularly those with advanced liver disease [34]. Moreover, decreased levels of Bacteroides and Prevotella, and a high presence of Clostridium coccoides in the feces of MASH patients have been reported [35, 36]. Intestinal dysbiosis increases levels of hepatic and systemic lipopolysaccharides (LPS) [37]. Dysbiosis and LPS are associated with an increase both in gut-derived proinflammatory substances such as lactate, ethanol, or trimethylamine oxide, and IL-6, TNF-α and hepatic lipogenesis that are able to activate stellate cells and hepatic fibrogenesis (Figure 1) [38]. However, caution should be exercised when interpreting data from studies investigating changes in the gut microbiota in MASLD, as other potential confounding and co-occurring factors may have influenced the results, including the mode of diagnosis of MASLD, stages of liver fibrosis, obesity, type 2 diabetes, type of diet, inflammation, and evolution of the microbiota over time. Therefore, despite current evidence, the pathogenic causal role of the microbiota in MASLD needs to be further evaluated with more standardized and reproducible methods, as well as the impact of gut microbiota manipulation via diet, probiotics, prebiotics and symbiotics, and fecal microbiota transplantation [39].
A relevant pathogenetic factor is pro-inflammatory diets [40]. It has been shown that an excess intake of specific dietary components particularly high saturated fat, but also processed and ultra-processed foods, high fructose foods, and sugar-sweetened beverages, can cause low-grade inflammation of the liver and intestine, promoting also the absorption of LPS [41]. An excess of fat, salt and milk derivatives in diet promotes bacterial growth able to module inflammation (Figure 1) [42].
Hepatic sinusoidal endothelial cell dysfunction represents a further crucial factor in the pathogenesis of MASLD and associated CV damage. This process is influenced by a multitude of mechanisms, including inflammatory responses, activation of stellate cells, increased vascular resistance, and alterations in microcirculation [43].
Recently, an emerging pathogenic aspect that needs to be taken into consideration includes mechanotransduction which is a complex process involving mechanosensors, located in the plasma membrane or within the liver cell, and mechanotransmission to the cell nucleus, which occur either by physical connection between mechanosensory and the nucleus or by mechanosignaling through biochemical pathways [44, 45]. Lipid droplets stored in the liver are composed of a neutral lipid core of triacylglycerols and esterified cholesterol bound externally by phospholipids. When accumulation of lipid droplets become excessive, they tend to merge, swelling the hepatocyte and distending the plasma membrane [46], altering the biomechanics of hepatocytes, microcirculation and liver as a whole organ [47–49], further worsen by disruptions induced by other ‘hits’, such as inflammation, endothelium and vaso-regulation dysfunctions, neoangiogenesis and fibrosis [50, 51]. In the early stage of MASLD, fat accumulation, hepatocellular ballooning and increased interstitial fluid cause compression of the hepatic capsule, altering viscoelasticity and increasing stress in the hepatic vascular space, which contributes to the progression, in the nonfibrotic stage, of the disease. Altered mechanical homeostasis in liver sinusoids during MASLD predisposes to a pro-angiogenic and pro-fibrotic environment [45]. Although the role of mechanotransduction in the development of fibrosis is well known, its role in the progression of MASLD is not yet fully understood [52–55], stressing the need for further studies that analyze all disease stages in order to optimize its management.
MASLD: risk factors and liver damage
Physical inactivity, obesity and overweight are the main risk factors for MASLD, particularly visceral obesity, in which MASLD can reach a prevalence of more than 80% [56]. In type 2 diabetes, the prevalence of MASLD is reported to be more than 65% and among these over 66% had MASH and significant liver fibrosis [9]. It is important to underline that type 2 diabetes plays a key role among the risk factors for MASLD. There is a bidirectional relationship between the two conditions since they show a causal mutuality and their association worsen and accelerates both disease progression [57]. In dyslipidemic patients, on the other hand, the prevalence of MASLD varies from 20% to 80% [58, 59]. It should be considered that often in the same patient there is more than one CMRF. It is reported that about 84% of patients with MASLD had two or more CMRFs [60]. As already discussed above, etiopathogenetic risk for MASLD is very heterogeneous and it is conceivable that each single risk factor takes part in developing both hepatic steatosis and fibrosis. Furthermore, the complex overlapping between the various CMRFs can generate different degrees of liver damage and variability in clinical outcome. Therefore, it is possible to speculate that MASLD, although the term encompasses a single disease, may be different in each patient with regard to disease burden, liver fibrosis, complications and outcomes. A recent study [61] evaluated the role of different CMRFs on MASLD progression, investigating the effects of different CMRFs combinations on the prevalence of hepatic steatosis and advanced liver fibrosis (Table 2). In subjects with a single CMRF, such as overweight/obesity, the lowest prevalence of MASLD and advanced fibrosis found to be 36% and 3.5%, respectively. In subjects with 2 CMRFs, such as overweight/obesity plus elevated glucose, the prevalence of MASLD and advanced fibrosis was 53.7% and 10.9%, respectively. While in subjects with 3 CMRFs, such as overweight/obesity plus high blood glucose plus low high-density lipoprotein cholesterol (HDLc) values, the prevalence of MASLD and advanced fibrosis was 72.3% and 14.5%, respectively. In subjects with 4 CMRFs, such as overweight/obesity plus high blood glucose plus low HDLc values plus hypertriglyceridemia, the prevalence of MASLD and advanced fibrosis was 85.2% and 21.7%, respectively, whereas, if hypertension replaced hypertriglyceridemia in the 4 CMRFs, the prevalence of MASLD and advanced fibrosis was 83.3% and 15.4%, respectively, underlining a possible greater fibrogenic role of hypertriglyceridemia. In subjects affected by all 5 CMRFs, high degree of MASLD and advanced fibrosis were observed, with a prevalence of 83.5% and 24.2%, respectively. Furthermore, a recent study [62], conducted on a large population for 2.8 years, evaluated the correlation between each single CMRF and the development of MASLD, showing that all CMRFs significantly increase (P < 0.001) the risk of MASLD, and in particular the HR (95% CI) was for overweight/obesity, 4.058 (3.728–4.417), for hypertension, 1.719 (1.579–1.871), for hyperglycemia, 1.778 (1.613–1.959), for high triglycerides, 3.113 (2.864–3.383), for low HDLc, 1.655 (1.506, 1.819). These data indicate a possible different pathogenetic role of CMRF in determining the degree of disease and its progression, so it is necessary to evaluate the individual and combined weight of CMRFs in determining the evolution of MASLD in order to establish a prognosis and optimize its management in the single patient.
Effect of the number of CMRFs involved in the development of MASLD on the prevalence of liver steatosis and the risk of advanced liver fibrosis [62] and the incident risk of CVD [63]
| Number of CMRFs | Prevalence of liver steatosis [62] | Prevalence of liver advanced fibrosis [62] | Incident risk of CVD, SHR (95% CI) [63] | P[63] |
|---|---|---|---|---|
| 0 | < 5% | 0% | 1.00 (reference) | |
| 1 | 36.0% (31.0%–41.4%) | 3.5% (0.2%–6.7%) | 1.41 (1.30–1.53) | < 0.001 |
| 2 | 53.7% (47.7%–59.6%) | 10.9% (6.2–15.6%) | 1.65 (1.53–1.79) | < 0.001 |
| 3 | 72.3% (65.3%–79.4%) | 14.5% (9.7%–19.2%) | 1.86 (1.72–2.02) | < 0.001 |
| 4 | 85.2% (81.8%–88.7%) | 21.7% (15.9%–27.4%) | 1.92 (1.76–2.09) | < 0.001 |
| 5 | 83.5% (79.8%–87.7%) | 24.2% (14.9%–33.5%) | 2.15 (1.95–2.37) | < 0.001 |
CMRFs: cardio-metabolic risk factors; CVD: cardio-vascular disease; MASLD: metabolic dysfunction-associated steatotic liver disease; SHR: subdistribution hazard ratio
It has recently been shown that first-degree relatives of MASLD patients with advanced fibrosis or cirrhosis have a 15% increased risk of developing MASLD with advanced fibrosis (F3–F4), suggesting that MASLD-related liver fibrosis may be inherited, in addition to common familial risk behaviors such as excessive alcohol consumption, weight gain, and a sedentary lifestyle. Therefore, these individuals should be considered at risk of advanced fibrosis and should be screened, as well as advised to induce appropriate lifestyle changes [64].
MASLD: natural history
The heterogeneity of CMRFs and their possible fluctuations over time lead to variability in the progression of MASLD and therefore, given its complexity, its natural history is not yet fully understood. Moreover, as it is not only a hepatic but also a systemic disease, its specific overall impact on all-cause mortality and extrahepatic disease progression is not easy to determine. To emphasize the variability of its course, studies and two meta-analyses conducted with the aim of assessing the course of liver disease, basing on paired liver biopsies, have shown that pathological changes in MASLD are dynamic and bidirectional in nature, and disease severity may remain stable, progress or regress over the time [3, 4, 65]. In general, the data suggest that MASLD progresses slowly in more than three quarters of cases and rapidly in less than a quarter, although the factors responsible for rapid progression are not well understood. Thus, further studies will be needed to fully elucidate the complex natural history of MASLD.
Overall mortality
A recent study conducted on a large group of patients with MASLD followed for a median of 26.9 years reported that the disease is associated with an increased risk of all-cause mortality [adjusted hazard ratio (aHR): 1.19, CI: 1.06–1.34] [66]. Similarly, another recent study involving a large group of MASLD patients reported an increase in all-cause mortality (aHR: 1.127, 95% CI: 1.056–1.201) during a follow-up of 23.7 ± 7.62 years [67]. A further study evaluated the impact of MASLD on all-cause mortality in a large population followed for 27.1 years; patients with MASLD had a higher risk of all-cause mortality (HR: 1.13, 95% CI: 1.01–1.27) and subjects with MetALD showed the highest risk of all-cause death (HR: 1.68, 95% CI: 1.10–2.57) [68]. Another large study compared all-cause mortality in patients with MASLD and MetALD to those without fatty liver followed for a median of 26.8 years [69]. The data showed, after adjusting for demographic factors, that both patients with MASLD (HR: 1.234, 95% CI: 1.118–1.362) and those with MetALD (HR: 1.690, 95% CI: 1.213–2.355) had a higher risk of all-cause mortality; however, after adjusting for other relevant factors, the high risk of all-cause mortality was found only in patients with MetALD (HR: 1.368; 95% CI: 1.019–1.838). These findings highlight how harmful alcohol is and importance of avoiding its consumption in patients with MASLD.
Mortality in patients with MASLD has been shown to be mainly due, in descending order, to CVD, followed by extrahepatic cancer, liver disease and diabetes [70]. A large meta-analysis showed that the stage of liver fibrosis is a predictor of all-cause mortality, thus representing the main predictor of MASLD outcomes [71]. Two recent studies highlighted a differential impact of individual CMRF on mortality and that hypertension, hyperglycemia, hypertriglyceridemia, and low HDLc levels were associated with an elevated risk of all-cause mortality [72, 73]. While some studies, conducted in subjects with NAFLD, did not show a significant increase in all-cause mortality [69, 74], probably also due to the heterogeneity of the population, the majority of data and meta-analysis seem to indicate that patients with MASLD and in particular those with MetALD have an increased risk of mortality. Further studies will be needed to confirm this particular aspect of the natural history of MASLD and whether the various combinations of CMRFs impact differently on overall mortality.
Liver disease
There are no specific studies on the natural history of liver disease in patients with MASLD. However, it has been shown that 99% of patients with NAFLD meet the diagnostic criteria for MASLD, so it has been suggested that data on the natural history of NAFLD should be valid also for patients with MASLD [75]. Two meta-analyses of studies conducted on paired liver biopsies showed that MASLD can evolve to MASH in 12% to 40% (Figure 2) [3, 4]. Approximately 35% of patients with MASH develop liver fibrosis, of which 10–25% evolve in advanced fibrosis or cirrhosis within an average period of 4.5 years [76, 77]. The fibrosis progression appears to be more consistent in patients with MASH in the early stages (F0–F1) than in those with F3 towards cirrhosis (14% vs. 2%, respectively) [65]. It has been reported that MASLD, regardless of the presence of MASH, can evolve to fibrosis in approximately 40% of cases (Figure 2). The great variability between studies on disease progression could be attributed to different impact that single or multiple overlapping of CMRFs may have had on the disease.
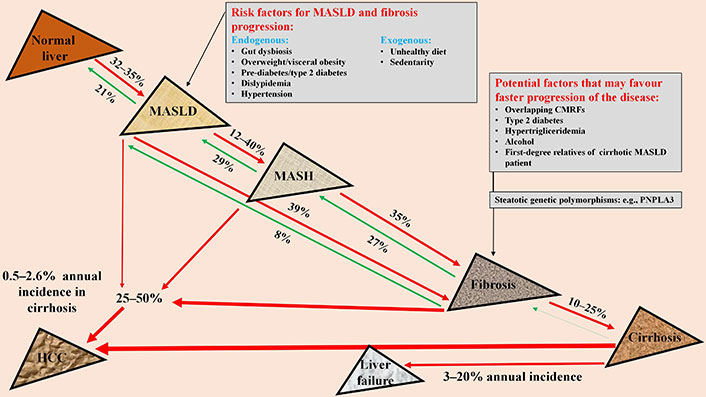
Natural history of liver disease in MASLD. MASLD: metabolic dysfunction-associated steatotic liver disease; CMRFs: cardio-metabolic risk factors; PNPLA3: patatin-like phospholipase domain-containing protein 3; MASH: metabolic dysfunction-associated steatohepatitis; HCC: hepatocellular carcinoma. Red arrow: disease progression; green arrow: disease regression; arrow thickness: different impact on disease progression
All studies have demonstrated the bidirectionality in MASLD progression, i.e., its evolution but also the possibility of spontaneous regression of the disease burden. A significant number of MASLD patients with or without fibrosis or MASH may experience regression of disease stage over time, although the rate of regression appears to be lower than the rate of progression (Figure 2). In addition, patients with advanced fibrosis were more likely to have fibrosis regression than patients with mild fibrosis [3]. A meta-analysis using liver biopsy studies evaluated the rate of progression of one stage of liver fibrosis in MASL and MASH patients and was estimated to be 14 and 7 years, respectively [4]. A recent meta-analysis of 54 liver biopsy studies including 26,738 patients evaluated the time to progression and regression of liver fibrosis in patients with MASLD [3]. The progression of one stage of liver fibrosis was reported to be 9.9, 10.3, 13.3 and 22.2 years for baseline fibrosis of F0, F1, F2 and F3, respectively. On the other hand, the regression of one stage of liver fibrosis was reported to be 21.3, 12.5, 20.4 and 40.0 years for baseline fibrosis of F4, F3, F2 and F1, respectively. In most cases, the progression to advanced fibrosis in MASLD is slow, with a rough estimate of 25–50 years, although in about 20% of cases the progression to advanced fibrosis or cirrhosis can be particularly rapid, even 4.5–6 years. It should be noted that 40–50% of patients show stationarity of the stage of the disease over time [4].
The cause of this bi-directionality is unknown, but it can be hypothesized that fluctuation in CMRFs levels over time act as a fuel for disease, although ad-hoc studies are needed. Recent data seem to indicate that the number of CMRFs (Table 2), type 2 diabetes, hypertriglyceridemia and the use of moderate amounts of alcohol, as well as being a first-degree relative of a cirrhotic patient with MASLD play a significant role in causing a high burden of steatosis and rapid progression to fibrosis and cirrhosis (Figure 2) [61, 78, 79]. Carrier status of the patatin-like phospholipase domain-containing protein 3 (PNPLA3) polymorphism in individuals with MASLD has been identified as an additional risk factor for faster fibrosis progression. The PNPLA3 gene encodes an enzyme that breaks down triacylglycerol. The I148M variant reduces its activity, causing steatosis, inflammation and liver fibrosis [80].
Patients with cirrhosis have a progression to liver failure between 3% and 20% per year [81, 82]. A recent meta-analysis showed that patients with MASLD and type 2 diabetes have a higher propensity for hepatic decompensation [aHR: 2.15 (1.39–3.34), P = 0.0006] [83]. The stage of liver fibrosis plays an important role in prognosis and in particular there is a directly proportional increase between fibrosis stages and liver-related mortality [mortality rate ratio: stage 1 = 1.4 (95% CI: 0.17–11.95); stage 2 = 9.57 (95% CI: 1.67–54.93); stage 3 =16.69 (95% CI: 2.92–95.36); and stage 4 = 42.30 (95% CI: 3.51–510.34)] [84]. Cirrhosis with liver failure is associated with an increased risk for all-cause mortality (HR: 6.8, 95% CI: 2.2–21.3) [82].
Hepatocellular carcinoma
HCC is the leading primary liver cancer in patients with MASLD and can develop at any stage of the disease, i.e., in both the non-cirrhotic and cirrhotic phases (Figure 2) [85]. MASLD cirrhosis represents the greatest risk for HCC. Cirrhotic patients have a tenfold higher risk of HCC than non-cirrhotic patients [86], although, 25% to 50% of HCC cases are observed in the non-cirrhotic phase of MASLD (Figure 2) [87]. Estimates of the annual incidence of HCC in subjects with MASLD cirrhosis range from 0.5% to 2.6%, whereas in subjects without cirrhosis the incidence is lower [88, 89]. Obesity and type 2 diabetes have been identified as risk factors for HCC in patients with MASLD. A meta-analysis showed that type 2 diabetes is an independent predictor of incident HCC [aHR: 5.34 (1.67–17.09), P = 0.0048] [83]. Subjects with MASLD who carry the PNPLA3 polymorphism have a three-fold increased risk of developing HCC [90, 91]. It has also been reported that genetic variant of MBOAT7 is associated with an increased risk of HCC. This polymorphism has been demonstrated to predispose to SLD, mitochondrial morphological alteration, progression to cirrhosis and the development of HCC [24, 92].
MASLD and extrahepatic diseases
The close connection between MASLD and metabolic changes in glucose and lipids is often bi-directional, i.e., MASLD in itself can cause metabolic disorders, means that MASLD must be considered not only a liver disease, but a condition that can influence and promote systemic pathologies. A close association exists between type 2 diabetes, CVD and MASLD, the latter having been reported as an independent risk factor for both diabetes and CVD [57, 93]. In addition, substantial evidence exists that MASLD, with its associated metabolic alterations and pathophysiological changes, represents a risk of developing chronic kidney disease (CKD), endocrine diseases and extrahepatic cancer [93–95].
Cardio-vascular disease
Studies have shown a close association between MASLD and atherosclerosis, heart failure and atrial fibrillation, and have found that MASLD is an independent risk factor for CVD morbidity and mortality as well as being an important predictor of CV events [93, 96, 97].
A recent meta-analysis evaluated the risk of myocardial infarction in people with MASLD [98] and showed that the risk of myocardial infarction was higher in patients with MASLD compared with those without MASLD (HR: 1.26, 95% CI: 1.08–1.47, P = 0.003). The authors concluded that MASLD increases the risk of myocardial infarction independent of traditional risk factors. Moreover, several studies have shown that CVD is the most common cause of death in subjects with MASLD [99, 100]. One recent study [101] conducted in a MASLD population followed for a median period of 13.6 years showed that MASLD is an independent CVD risk factor for coronary artery disease, stroke, heart failure and CV death. Studies have showed that in patients with MASLD, irrespective of the presence of traditional CV risk factors, a close association was found with: (a) subclinical carotid atherosclerosis, documented by intima-media thickness measurement, (b) increased arterial stiffness, a marker of early atherosclerosis and, (c) an elevated coronary calcification score [102, 103]. Furthermore, MASLD was found to be an independent risk factor for the presence of vulnerable non-calcified coronary plaque, which is associated with sudden and unexpected cardiac events [104]. It also showed that CV risk increases with progression of liver disease (advanced fibrosis: aHR: 1.67, 95% CI: 1.47–1.89, and cirrhosis: aHR: 2.15, 95% CI: 1.77–2.61) [101]. A recent study, using a causal mediation analysis, demonstrated a bidirectional temporal relationship between MASLD and hypertension, although MASLD contributes more to increased blood pressure than vice versa, and a positive role of hypertension in mediating the MASLD-CVD association [105]. A study conducted in a large population followed for an average of 9.0 years showed that individuals with MASLD are at high risk of CVD and that the association with alcohol (MetALD) is an additive risk factor for stroke and coronary artery disease [63]. In particular, compared to subjects without steatosis, those with MASLD and MetALD showed a significant higher risk of CVD, subdistribution hazard ratio (SHR) 1.19 (95% CI: 1.15–1.24); and SHR 1.28 (95% CI: 1.20–1.36, P < 0.001), respectively, coronary artery disease, SHR 1.22 (95% CI: 1.15–1.28) and SHR 1.23 (95% CI: 1.12–1.34, P < 0.001), respectively, and stroke, SHR 1.19 (95% CI: 1.13–1.24) and SHR 1.30 (95% CI: 1.20–1.41, P < 0.001) respectively. Of relevance, the authors also evaluated the impact of each single CMRF on the incident risk of CVD among patients joining in the study, comparing subjects with MASLD versus those without steatosis. Data showed that each single CMRF was associated with a significant, although different, increase in CV risk, which was for body mass index (BMI), SHR 1.09 (95% CI: 1.04–1.14), for diabetes, SHR 1.08 (95% CI: 1.05–1.11), for blood pressure, SHR 1.32 (95% CI: 1.27–1.37), for triglycerides SHR 1.20 (95% CI: 1.16–1.24) and for HDLc, SHR 1.15 (95% CI: 1.11–1.18) [63, 103]. In addition, the impact of the number of CMRFs overlapping on the incident risk of CVD in subjects with MASLD was also evaluated and as shown in Table 2, incident CV events increased significantly with the number of CMRFs overlapping [63, 103]. The data indicate that each CMRF and the number of overlapping CMRFs determine not only a different degree of steatosis and liver fibrosis, but also a different risk of CV events; in other words, in a single subject, the risk of development and progression of liver disease and CVD is proportional to the type and number of overlapping CMRFs. A recent study in a large population of patients with MASLD confirmed that the risk of CVD is directly proportional to the number of CMRFs involved and that their improvement is associated with a reduction in CVD risk [106].
The data presented above demonstrate that the risk of CVD is present in all stages of MASLD. However, there is a temporal relationship between the early stage of MASLD and subclinical atherosclerosis, as well as the stage of advanced liver fibrosis and CV events and related outcomes. This appears to indicate that MASLD plays a direct role in increasing the risk of atherosclerosis and CVD. However, the debate on the causal relationship, or simple association related to common etiopathogenetic factors, between MASLD and CVD remains unresolved. Interpretation of the data still requires caution and further evidence is needed to establish causality, which is crucial in clinical practice when making important decisions such as recommending early screening, follow-up or aggressive therapeutic intervention.
The pathophysiological mechanisms by which MASLD becomes a risk factor for CVD are complex and not yet fully clarified. Figure 1 shows the main mechanisms involved and, in addition to insulin resistance and glucose and lipid metabolic disorders, a leading role is played by hepatic hyperproduction and secretion of cytokines with inflammatory activity (IL-6, IL-1b, TNF, etc.) determining a chronic low-level systemic inflammation and an increase in oxidative stress, along with a condition of hypercoagulability [107, 108]. Furthermore, mediators secreted by metabolic organs, such as hepatokines, adipokines (adiponectin, leptin and resistin), extracellular vesicles surrounded by a lipid bilayer, and gut-derived factors (such as trimethylamine N-oxide, LPS, bile acids, short-chain fatty acids and N,N-trimethyl-5-aminovaleric acid) are involved in the pathogenesis of CVD in patients with MASLD [109]. However, it must be emphasized that it is not easy to determine the overall effect of these organ-secreted metabolic factors in the development of CVD, considering the complex interactions with multiple organs throughout the body and therefore further ad hoc studies need to be done. In any cases, studies in patients with MASLD have evaluated the potential role of excessive secretion of hepatokines (e.g., fetuin-A and angiopoietin-like proteins—ANGPTLs) that characteristically occurs in the pathogenesis of atherosclerosis and CVD, since they are able to act directly on vascular cells, as well as modulate insulin resistance, systemic inflammation and dyslipidemia [110]. It has been shown that elevated levels of fetuin-A were associated with an increased risk of atherosclerosis. The mechanisms by which fetuin-A promotes atherosclerosis are multiple and include inflammatory reaction of endothelial cells, macrophage foam formation and smooth muscle cell proliferation and collagen deposition [111]. ANGPTs are associated with an increased risk of atherosclerosis because they are able to induce endothelial dysfunction and promote the development of dyslipidemia through the suppression of lipoprotein lipase (LPL) activity [112]. This milieu causes endothelial dysfunction inducing plaque formations. In addition, patients with MASLD show an increase in VLDL secretion into circulation, promoting an atherogenic lipid profile, which is worsened by the intestinal dysbiosis present in MASLD that further impacts lipid atherogenic metabolism [113], inducing and worsening the atherosclerotic process [30, 114]. The identification of the mechanisms involved in the cardiometabolic pathophysiology associated with MASLD may be of great clinical importance for the development of diagnostic markers and for identifying new possible therapeutic strategies aimed at reducing the risk of life-threatening CV events and improving survival.
Chronic kidney disease
Recently, a specific and extensive review of the literature has been conducted on the close correlation between MASLD and the risk of developing CKD [94]. Some studies and a meta-analysis have shown that subjects with MASLD are at high risk of developing CKD, and, in particular over a median period of 10 years, subjects with MASLD have a 1.5–2.0 times higher risk of developing CKD than subjects without steatosis [115, 116]. Furthermore, recently, in a large-scale study, it was demonstrated that, during a median follow-up of 12.8 years, patients with MAFLD had a 2-fold higher risk of developing end-stage renal disease compared to those without steatosis [117]. It has been shown that the stage of liver fibrosis was associated with an increased risk of developing incident CKD [118], and it was also been observed that, during a 5-year follow-up period, patients whose degree of fibrosis worsened had a greater risk of developing CKD than those whose degree of fibrosis improved [119]. These data were corroborated by a multicenter study [82] conducted on liver biopsies and a recent meta-analysis [116] which demonstrated that the risk of CKD increased with the progression of liver disease and the degree of fibrosis.
The precise pathophysiological mechanisms linking MASLD with CKD are complex and not yet fully understood and, they are extensively discussed in the specific review mentioned above [94]. In short, since the same CMRFs are involved in etiopathogenesis of both MASLD and CKD there is a causal correlation between the two conditions and, it is conceivable that in individuals with MASLD the development of CKD occurs through a complex interaction of metabolic and hemodynamic alterations, lipid nephrotoxicity and a possible genetic predisposition. These close etiopathogenetic links between the two conditions mean that the greater the degree of MASLD, the greater the risk of developing CKD. Thus, MASLD is able to increase the risk of developing CKD through the same mechanisms mentioned for CVD, in particular the low degree of systemic inflammation (Figure 1), as well as insulin resistance, glucose metabolism alterations and atherogenic dyslipidemia, each one amplified by the progression of MASLD and the degree of liver fibrosis. The liver disease in association with metabolic alterations causes accumulation of lipids in the kidney altering renal hydrostatic pressure and, causing renal inflammation and fibrosis that represent the basis of CKD. On the other hand, the development and progression of CKD contribute to worsening liver disease. CKD, with its functional insufficiency, produces the release of high concentrations of uremic toxin into the circulation that causes inflammation of the adipose tissue and increases intestinal permeability, contributing to worsening the liver condition [94]. Therefore, MASLD and CKD interact in their mutual development and evolution.
Endocrine diseases
Apart from obesity and insulin resistance, which are two cornerstones in the development of MASLD, there are extensive evidences of a complex endocrine dysregulation in patients with MASLD, although there is no conclusive evidence of a causal relationship. The main endocrine disorders reported in MASLD are low levels of growth hormone (GH), sex and thyroid hormones (THs), and these alterations are likely to promote both the development and progression of MASLD [95]. Low serum and hepatic levels of GH and insulin-like growth factor (IGF)-1 have been reported in patients with MASLD and particularly in subjects with MASH [120]. In addition, reduced levels of GH and IGF-1 have been reported to be closely related to intrahepatic fat storage and progression of MASLD [121, 122]. A case report and a small prospective study seem to support the role of GH in MASLD as they have shown that GH replacement therapy improved hepatic steatosis, function, and fibrosis [123, 124]. It has been suggested that the effect of GH on steatosis may be mediated by its role in inducing weight loss, although some data suggest a possible direct effect of GH on reducing hepatic fat content [125].
THs activate the basal metabolism and stimulate both lipogenesis and lipolysis. Hepatic metabolism is directly influenced by THs through specific hepatic receptors, thyroid hormone receptor alpha (THRα) and THRβ, especially the latter which is richly expressed in the liver. THs promote hepatic lipogenesis through the THRα and activate fatty acid oxidation through the THRβ. In addition, the liver also contains receptors for thyroid-stimulating hormone, which can directly cause steatosis [126, 127]. An association between MASLD and hypothyroidism has been reported [128, 129], and relative intrahepatic hypothyroidism has been demonstrated [130]. Most studies and meta-analysis have demonstrated a close association between development of MASLD and hypothyroidism and between the latter and advanced degree of fibrosis [129, 131–137]. An association between hypothyroidism and cirrhosis has also been reported [138]. Data suggest that in MASLD patients THRβ activity is often reduced and therefore, in part, MASLD/MASH may be a condition characterized by low hepatic THs levels and consequent hepatic hypothyroidism with increased hepatic triglyceride deposition and higher level of insulin resistance. Of particular note and importance was the evidence that there is an inverse correlation between THRβ mRNA levels and the severity of MASLD [139], and that the administration of resmetirom, a selective agonist with high affinity for THRβ is able to significantly improve steatosis, inflammation and liver fibrosis [140].
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is reported to be associated with MASLD and a three-fold increased risk of having MASH and more advanced fibrosis compared to patients without OSA [141, 142]. However, there are currently no data to support that patients with OSA are at increased risk of liver disease progression or development of HCC.
Extrahepatic cancer
A recent meta-analysis evaluating 10 longitudinal cohort studies involving a large number of subjects assessed the risk of incident extrahepatic cancer in patients with MASLD, showing that these patients have an increased risk of developing extrahepatic cancers during an average follow-up of 5.8 years [143]. Specifically, for the following cancers, the random-effects HRs (95% CI) were: esophageal cancer (5 studies evaluated), 1.93 (1.19–3.12); stomach cancer (6 studies), 1.81 (1.19–2.75); pancreatic cancer (3 studies), 1.84 (1.23–2.74); colorectal cancer (8 studies), 1.64 (1.24–2.19); thyroid cancer (2 studies), 2.63 (1.27–5. 45); lung cancer (5 studies), 1.30 (1.14–1.48); urinary cancer (4 studies), 1.33 (1.04–1.70); breast cancer (4 studies), 1.39 (1.13–1.71); female genital cancer (4 studies), 1.62 (1.13–2.32); prostate cancer (5 studies), 1.16 (0.82–1.64); and hematological cancer (2 studies), 1.47 (0.69–3.12) [143]. These data were fully confirmed by another recent meta-analysis that included 64 studies and a large population of patients with MASLD [144]. Furthermore, this latter meta-analysis showed that in patients with MASLD, extrahepatic cancers are eight times more frequent than HCC and do not correlate with the stage of disease or liver fibrosis. A very recent large retrospective study further confirmed that during a 10-year follow-up, subjects with MASLD have a higher incidence of extrahepatic cancer [145].
An important pathogenetic event in the development of cancer is chronic inflammation, particularly the activation of NF-κB [146]. Other mechanisms that may contribute to the higher risk of extrahepatic cancer include alterations in adipocytokines, such as adiponectin and leptin, increased oxygen free radicals, IGF-1, and insulin resistance (Figure 1).
Data from the literature show that patients with MASLD have an increased long-term risk of developing extrahepatic cancers, particularly gastrointestinal, breast and gynecological cancers. However, it must be emphasized that almost all data derive from observational cohort studies and do not allow to establish causality between the two events. Therefore, further studies are needed to understand the complex relationship between MASLD and the development of extrahepatic cancer.
MASLD: management and therapeutic approaches
Management
MASLD is characterized by the contribution of multiple CMRFs, a complex pathophysiology, as well as multiorgan involvement and complex outcomes, which are reflected in the management and treatment of the disease, which cannot be only hepatocentric but must be holistic and multidisciplinary in order to improve or eliminate the risk of morbidity and mortality associated with the disease (Figure 3).
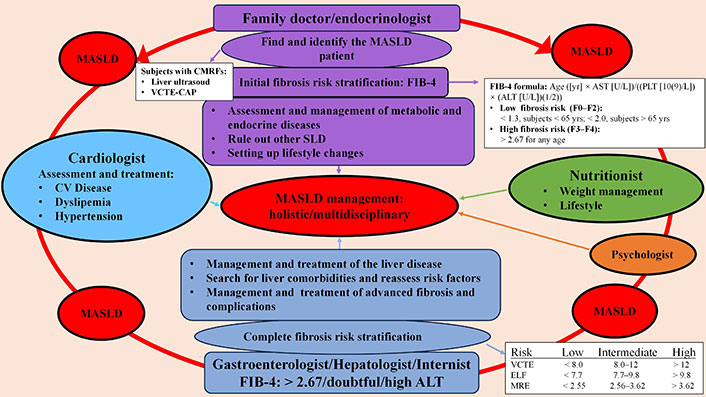
Management holistic/multidisciplinary of patients with MASLD. MASLD: metabolic dysfunction-associated steatotic liver disease; CMRFs: cardiometabolic risk factors; VCTE-CAP: vibration-controlled transient elastography-controlled attenuation parameter; CV: cardio-vascular; FIB-4: fibrosis index based on 4 factors; SLD: steatotic liver diseases; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ELF: enhanced liver fibrosis; MRE: magnetic resonance elastography
The first fundamental step is to identify the “elephant in the room”, i.e., subjects with SLD, which may be incidental or specifically investigate in those with CMRFs (Table 1). In the first phase of the identification process, given the epidemiological characteristics and the metabolic nature of the pathology, a fundamental role is played by the family doctor and the endocrinologist, who must acquire adequate basic skill of the pathology in order to be able to start an early identification process of the disease in subjects at risk and promote its adequate management (Figure 3). People with CMRFs should be screened for SLD using non-invasive tests such as liver ultrasound or vibration-controlled transient elastography (VCTE)-controlled attenuation parameter (CAP). Individuals found to have an SLD need to be assessed to exclude secondary causes, through quantification of alcohol consumption and appropriate laboratory assessment [147, 148], and once the presence of at least one CMRF (Table 1) has been established, a diagnosis of MASLD can be made.
The patient with MASLD should undergo clinical and instrumental evaluation of the CV system, the endocrinological status, in particular of prediabetes and diabetes status and thyroid function, and of all the comorbidities usually associated with MASLD whose diagnostic procedures are reported elsewhere [149]. Furthermore, all patients should be evaluated for possible alcohol abuse or use of potentially steatotic drugs. As reported elsewhere, a careful clinical and laboratory evaluation should be made [148], as well as of the liver fibrosis, assessed through the simple non-invasive test fibrosis index based on 4 factors (FIB-4) score, which can identify subjects at low or high risk of fibrosis (Figure 3) [150]. Subjects at low risk of liver fibrosis should be managed by the family doctor or endocrinologist with appropriate advance treatment of CMRFs and modification of lifestyle and diet, also through the help of a professional nutritionist and if necessary, a psychologist. These patients should be reassessed periodically, every 2–3 years, for the status of liver fibrosis [148]. On the other hand, patients with MASLD at high risk for liver fibrosis (Figure 3) should be referred to an experienced hepatologist for management [148]. The hepatologist, beyond a complete evaluation of CMRFs, alcohol use and quantification, lifestyle and other causes of SLD, performs a precise risk stratification, using non-invasive tests, such as VCTE or enhanced liver fibrosis (ELF) or magnetic resonance elastography (MRE). If the fibrosis risk is low (Figure 3), a hepatic therapeutic approach is not necessary, but a change in lifestyle and diet and an advanced specific treatment of CMRF are strongly indicated [148]. Of great importance is defining the follow-up intervals of these patients that can vary depending on the CMRFs involved and the number of overlapping risk factors. In fact, in case of type 2 diabetes or if are present two or more CMRF, the follow-up must be close (every 1–2 years), due to the higher risk of disease progression, while in other cases the follow-up can be longer (every 2–3 years). If the risk of fibrosis is intermediate or high, the degree of liver damage should be carefully assessed, particularly if diabetes or multiple superimposed CMRFs are present, and specific liver treatment should be considered, in addition to that previously reported for low-risk subjects. If noninvasive tests results are indeterminate, an elevated alanine aminotransferase (ALT) value persists, or concurrent or concomitant diagnoses are possible, a liver biopsy may be indicated [148]. If liver biopsy shows fibrosis stage 0–1, the patient should be reevaluated after 1–2 years according to the management proposed above for low-risk subjects, while if the biopsy shows a fibrosis stage 2–3, the follow-up should be closer (every year) and liver treatment should be started as well as, in case of cirrhosis a specific treatment must be started [148].
Therapeutic approaches
Besides the recently approved resmetirom by the US Food and Drug Administration (FDA) [151], there is currently no other pharmacological therapy for MASLD and even drugs that have demonstrated some effect in the treatment of MASLD are considered off-label. The scarce availability of pharmacological treatments is mainly due to the heterogeneity of the population fitting diagnostic criteria and the complexity of the pathophysiology of MASLD. Although it offers many drug targets, identifying the right ones is a challenge and it is also likely that more than one drug will be needed for therapeutic management. Therefore, considering the etiopathogenetic role of CMRFs in determining MASLD and its progression, it is of fundamental importance to treat them early, achieving the best possible control. Aggressive therapeutic management of CMRFs is of primary importance, since even the excellent control of a single CMRF can reverse the stage of MASLD-associated liver disease, as well as to improving CV and metabolic outcomes. Management of CMRFs, CVDs, CKDs, endocrine diseases and cancers in patients with MASLD requires a multidisciplinary approach as part of the holistic disease management.
Non-pharmacological treatments
Currently, the main first-line therapeutic approach for MASLD is based on non-pharmacological treatments that include personalized lifestyle changes, particularly weight loss through a hypocaloric diet and increased physical activity [152]. Moreover, in select cases, bariatric surgery may play an important role as it has the greatest impact on weight loss and the fastest reversal of hepatic fibrosis. However, inveterate behavioral changes for patients with MASLD are difficult and challenging not only to achieve, but especially to maintain weight loss. Patients are often reluctant and sometimes unsure of how to lose weight. Traditional lifestyle and dietary interventions are generally effective in achieving modest and often clinically insignificant results and therefore often fail, which is a major hurdle for clinicians and patients to overcome. The most frequently reported causes of failure are lack of time, lack of understanding of the indications and difficulty in accessing resources for lifestyle interventions, boredom with change, poor self-management skills, and the inability or impossibility of health workers to provide real-time supervision and psychological support [153, 154]. Recent reports have highlighted the potential of using digital therapeutics (DTx) to remove many of the barriers to weight loss mentioned above [155]. DTx uses mobile apps, wearable devices, and online platforms to deliver personalized interventions [156]. Although the use of DTx in the treatment of MASLD is still experimental, early data seem promising. In fact, they have shown significantly higher adherence rates compared to traditional interventions, ranging from 42% to 100%. In addition, data seem to indicate that lifestyle intervention programs with DTx induce 3-fold greater long-term weight loss compared to standard of care [157]. The results of these preliminary studies are encouraging and support a possible use of DTx in lifestyle intervention programs in patients with MASLD, although further confirmatory studies on large unselected populations are needed.
Weight loss
Weight loss diet represents, at the moment, the only treatment able to positively interact with all pathogenic mechanisms. In particular, weight loss is able to reduce adipose tissue stress, insulin resistance, lipotoxicity, inflammation and mechanotransduction [158]. Furthermore, it improves diabetes, dyslipidemia and hypertension and a healthy diet also impacts positively on intestinal dysbiosis. Based on the above, it is clear that a healthy diet, aimed at weight reduction, is a cornerstone of the ideal management of MASLD at any stage (Table 3) and should be pursued and recommended even in association with pharmacological treatment. In people who are reluctant to follow a diet, it is necessary to be convincing and dedicate enough time to explain the benefits and the importance of a therapy with no adverse effects, involving a nutritionist in order to personalize the diet, as well as a psychologist. Furthermore, even a little weight loss can be of great benefit. Indeed, a weight loss of 3–5% has been shown to improve steatosis, of 7–10% to improve liver inflammation, and greater than 10% to improve fibrosis as well [148, 159–161]. The Mediterranean diet should be preferred with reduced intake of excess saturated fats, refined carbohydrates, fructose and sugary drinks [162]. Coffee consumption is permitted since a meta-analysis has shown that its regular use is associated with lower risk of MASLD and liver fibrosis [163]. On the contrary, the use of alcohol must be strongly discouraged, especially in high-risk patients, since it promotes SLD and progression of liver fibrosis, as well as worsening both hepatic and extrahepatic events [148].
Therapeutic approaches for different stages of MASLD
| Effective treatment† | Improve MASL | Improve/resolution MASH | Improve liver fibrosis/cirrhosis |
|---|---|---|---|
| -Diet and increased physical activity-Bariatric surgery-PPAR receptor agonists: pioglitazone, lanifribranor, saroglitazar-Incretin receptor agonists: liraglutide, semaglutide, tirzepatide-SGLT-2 inhibitors: dapagliflozin, empagliflozin-Thyroid hormone receptor-β agonists: resmetirom | -Diet and increased physical activity-Bariatric surgery-PPAR receptor agonists-Incretin receptor agonists-SGLT-2 inhibitors-Thyroid hormone receptor-β agonists | -Diet and increased physical activity-Bariatric surgery-PPAR receptor agonists-Incretin receptor agonists-Thyroid hormone receptor-β agonists | -Diet and increased physical activity-Bariatric surgery-PPAR receptor agonists-Thyroid hormone receptor-β agonists |
† With the exception of resmetirom, drug treatments are currently considered off-label for MASLD. Some drugs are approved for treatment of MASLD-associated comorbidities such as type 2 diabetes and obesity. MASLD: metabolic dysfunction-associated steatotic liver disease; MASH: metabolic dysfunction-associated steatohepatitis; PPAR: peroxisome proliferator-activated receptors; SGLT-2: sodium-glucose cotransporter 2
Physical activity
Exercise has been shown to improve MASLD, regardless of weight loss [164, 165]. The recommended physical activity for patients with MASLD is at least 150 minutes/week of moderate intensity or, alternatively, 75 minutes/week of vigorous intensity. Aerobic exercise with the addition of resistance training is suggested [166]. As shown in Table 3, exercise is effective in improving all stages of MASLD, although data regarding the effect between physical activity and liver fibrosis are still limited. Exercise and diet for weight loss have a synergistic effect on the treatment of MASLD, so combining them is a good idea, although both diet and exercise must be adapted to each patient’s clinical condition and abilities. As shown in Figure 4, the mechanisms underlying the positive effects of physical activity in MASLD are complex and related to cross-talk between multiple organs and tissues and reflect changes in energy balance, cytokines, hepatokines, gut hormones, and myokine secretion that reduce inflammatory activity, circulating lipids, hepatic fat deposits and peripheral insulin resistance [167]. Physical activity reduces de novo hepatic lipogenesis, improves glycemic control, and enhances lipoprotein clearance, thereby reducing hepatic fat [168]. Evidence shows that physical activity can reverse gut dysbiosis in patients with MASLD. This is achieved through an increase in α-diversity and a shift in β-diversity of the gut microbiome, as well as a reduction in inflammation and intestinal permeability [169]. It has been proposed that the exercise specialist should be included in the multidisciplinary team treating MASLD [166].
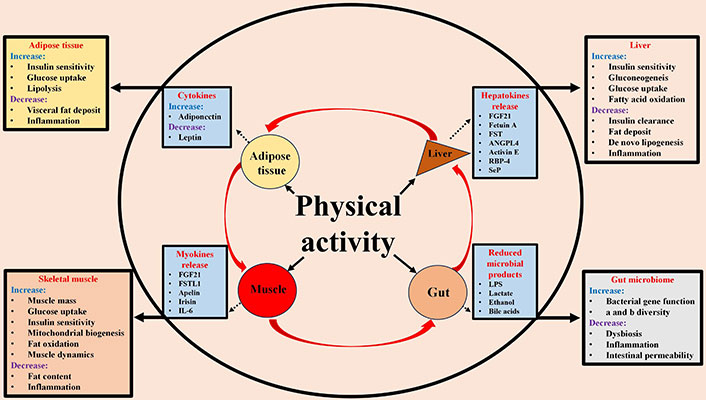
Positive effects of physical activity on the pathogenetic mechanisms of MASLD through liver-adipose tissue-muscle-gut cross talk. FGF21: fibroblast growth factor 21; FSTL1: follistatin-like 1; FST: follistatin; ANGPL4: angiopoietin-like protein 4; RBP-4: retinol binding protein 4; SeP: selenoprotein P; LPS: lipopolysaccharide
Bariatric surgery
Bariatric surgery has been shown to be safe in patients with MASLD and it is able to improve steatosis, inflammation and liver fibrosis (Table 3) [170]. Obviously, the use of bariatric surgery finds specific indications, namely in obese patients with BMI ≥ 35 kg/m2 with MASH, fibrosis and comorbidities, or obese patients with BMI > 40 kg/m2. After bariatric surgery, resolution of steatosis was reported from 66% to 88%, while regression of inflammation and ballooning from 50% to 84% and resolution of liver fibrosis from 40% to 68% [170]. The effects are long-lasting, at least for 5 years, and appear to be related to weight loss [171–173]. Furthermore, it reduces the risk of major hepatic, including HCC, and CV outcomes [174, 175].
Bariatric surgery is contraindicated for patients with decompensated cirrhosis, including those with a past history or portal hypertension (> 10 mmHg), due to the elevated post-surgical mortality rate [176, 177].
The complex mechanisms by which bariatric surgery reverses MASLD include regulation of food intake, gut hormone secretion, bile acid signaling, and reduction of visceral adiposity and adipose tissue inflammation [170].
Pharmacological treatment of CMRFs
Obesity
In the treatment of obesity with associated MASLD, drugs for weight loss should be considered, although as a second line to lifestyle changes. Drugs that may reduce body weight and have shown some effect in MASLD studies include glucagon-like peptide-1 (GLP-1) analogues (e.g., liraglutide, dulaglutide, semaglutide), tirzepatide [a dual agonist of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP)] and orlistat. These drugs are able to induce a reduction in body weight of 6 to 11% in 12 months in association with lifestyle changes [178]. GLP-1 induce weight loss, glycemic and satiety control, splanchnic vagal effects, and modifying insulin secretion [179]. GLP-1 adverse effects include nausea, diarrhea, and constipation. Orlistat is a reversible inhibitor of pancreatic lipase blocking the intestinal absorption of dietary fats and thus promoting weight loss. Orlistat, in limited studies, has shown modest and uncertain impact in MASLD improving biochemical markers and an inconstant effect on histological parameters, such as steatosis, inflammation, and ballooning, but did not affect fibrosis. The effects have been linked to weight loss. Although orlistat may improve biomarkers in patients with MASLD, it cannot be considered a drug of choice for the management of MASLD at this time [180]. Tirzepatide has been shown to be effective in resolving liver inflammation in patients with MASH and moderate or severe fibrosis [181]. More data are needed to confirm the efficacy and safety of tirzepatide in patients with MASH.
Type 2 diabetes
Type 2 diabetes is one of the main factors determining the progression of fibrosis, liver failure and HCC development in MASLD [182]. In addition to lifestyle changes, aggressive and innovative drug treatment is mandatory. The ideal therapy for patients with type 2 diabetes and MASLD should aim at adequate glycemic control, improvement of liver damage and fibrosis as well as other metabolic factors and overall CV risk. Therefore, the treatment of diabetes associated with MASLD should have multi-target effects and be adapted to the evolutionary stage of the hepatic disease. The glycemic targets in patients with NAFLD are the same as those dictated by the international guidelines for the treatment of type 2 diabetes, where, in general, a glycated hemoglobin value < 7% can be considered acceptable.
The antidiabetic drugs that have been shown to have a multi-targeted effect are metformin, pioglitazone, GLP-1 receptor agonists, dipeptidyl-peptidase-4 (DPP-4) inhibitors and sodium-glucose cotransporter 2 (SGLT-2) inhibitors [183]. All of these drugs have a marked effect on glucose metabolism, significantly reducing blood sugar and insulin resistance, and a different effect on weight loss. While pioglitazone has been associated with an increase in body weight and DPP-4 inhibitors show no effect, the others are able to determine a more or less significant reduction in body weight. With the exception of DPP-4 inhibitors, all of these drugs are able to reduce atherosclerotic CVD [183, 184]. In addition, SGLT-2 inhibitors have been shown to improve mortality in patients with heart failure [183, 184]. The effect of these drugs on MASLD is variable and is described in more detail in the next chapter. However, for DPP-4 inhibitors there is no significant evidence of improvement liver condition, while for metformin there is evidence that it can improve steatosis. Furthermore, it has been demonstrated that metformin activates AMP-activated protein kinase (AMPK) both at the liver and muscle level and therefore seems able to increase muscle glucose uptake, modulate lipid circulation and activate fatty acid oxidation reducing VLDL synthesis and fat accumulation in the liver, increasing insulin sensitivity [185]. For all these effects, metformin could have a positive role in the treatment of MASLD but further studies are needed. Therefore, in the absence of contraindications, metformin, in addition to lifestyle modifications, should be used as a therapy for type 2 diabetic patients with early MASLD. This is supported by its efficacy in lowering blood glucose without inducing hypoglycemia, by the fact that it does not increase weight or even cause weight loss, by its excellent tolerability, by its low cost, by its reduction in CV risk and by its probable reduction in HCC risk [186].
In MASH patients with type 2 diabetes associated with conditions such as high waist circumference, high triglycerides and low HDL cholesterol, suggesting a high level of insulin resistance, pioglitazone could be the drug of choice. However, the use of pioglitazone may be limited by its adverse effects, such as weight gain, fluid retention, and heart failure, although these side effects can be mitigated by using low doses of the drug (15–30 mg) or prevented by combining it with an SGLT-2 inhibitor [187]. In patients with type 2 diabetes associated with obesity and MASH, GLP-1s should be used, whose safety and efficacy have been demonstrated [179, 188].
Hypercholesterolemia
Hypercholesterolemia should be treated with statins as first-line agents. If the therapeutic goal is not achieved, statins may be used in combination with other lipid-lowering agents, such as ezetemibe, proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors, inclisiran, bempedoic acid, fibrates, omega-3 fatty acids, or icosapent ethyl. Statins are safe in MASLD, although they should be used with caution in patients with cirrhosis, particularly in decompensated cirrhosis [148].
Hypertriglyceridemia
Hypertriglyceridemia should be treated with fibrates, omega-3 fatty acids, and eicosapentaenoic acid, alone or in combination. The combination of statins with fibrates should be avoided due to the high risk of statin-induced myopathy [148].
Hypertension
Hypertension can be treated with angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, although their use should be avoided in decompensated cirrhosis. Other antihypertensive drugs may be used according to the clinical condition, especially calcium antagonists, beta blockers and diuretics [148].
Pharmacological treatment of hepatic disease
The possible pharmacological treatment goals of MASLD are to reduce steatosis, reduce or resolve MASH, improve or stabilize fibrosis with the intent of preventing progression to cirrhosis. Table 3 reports the main therapeutic approaches that have shown to be effective in different phases of MASLD. There are currently no approved drugs for the treatment of MASLD other than resmetirom, although some drugs ordinarily used for the treatment of type 2 diabetes and obesity have shown some efficacy in improving certain parameters at different stages of MASLD (Table 3).
Thyroid hormone receptor-β agonists
Resmetirom is the first drug approved by the US FDA for the treatment of patients with MASH and moderate to advanced liver fibrosis [150] and thus it can be freely used in the countries where it has been approved. The drug promotes lipid metabolism in the liver by inducing hepatic hyperthyroidism, and also prevents liver damage by reducing lipotoxicity. The randomized study, resmetirom versus diet and exercise, conducted in patients with MASLD and F2–F3 fibrosis, on matched liver biopsies, over a period of 12 months, showed that resmetirom at a dose of 80 mg or 100 mg taken orally once daily, significantly reduced MASH and fibrosis approximately in 28% and 25% of cases, respectively, compared to 10% and 14%, respectively, in controls [189]. The evidence that resmetirom can improve both MASH and fibrosis, at least by one degree stage, makes it an important weapon in patients with MASLD considered at risk, although in a limited number of patients, in whom it reduces the risk of progression to cirrhosis. Moreover, as resmetirom shows few and mild side effects, nausea and diarrhoea, it has become an attractive option in patients with MASLD at risk, but should always be combined with diet, exercise and other CMRFs-targeted therapies to implement and optimize treatment. In this regard, the possible pharmacological interactions need to be taken into consideration. A potential risk of interaction is the association between resmetirom and statins which could increase the toxicity of the latter and should be avoided. Resmetirom should also be avoided in subjects with decompensated cirrhosis. Last but not least, it must be remembered that resmetirom has a high expected cost of between $40,000 and $50,000 per year and, therefore, as its efficacy is only in a limited number of patients, a post-hoc and cost-effectiveness analysis in different stages of the disease and under conditions of actual hepatic hypothyroidism is desirable before treating the ‘elephant in the room’ as a whole.
Peroxisome proliferator-activated receptor agonists
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors activated by fatty acids and act as lipid sensors with multiple metabolic effects. Pioglitazone improves steatosis, and can resolve MASH, while the efficacy on fibrosis is less clear. It also improves insulin resistance, diabetes, reduces CV risk and stroke [190–192]. Side effects of treatment are significant, including weight gain, risk of heart failure, and bone loss, making the drug difficult to hand in MASLD.
Lanifribranor, a pan-PPAR agonist, in a phase 2 study, was shown to improve both MASH and fibrosis and therefore represents a potential drug for the treatment of MASLD [193].
Saroglitazar is a dual PPAR-α/γ agonist and has been shown to improve steatosis and MASH, but results regarding improvement in fibrosis are uncertain [194].
GLP-1 receptor agonist
The GLP-1 receptor agonists (GLP-1 RAs; e.g., liraglutide, dulaglutide and semaglutide) are effective in reducing steatosis and MASH, while they appear to have modest effect on fibrosis [195, 196]. In a recent study conducted in a large cohort of patients with MASLD and type 2 diabetes [197], GLP-1 receptor agonists were shown to reduce the risk of progression to cirrhosis, its complications and overall mortality when used at an early stage of MASLD. The preventive effect occurs after 18–24 months of treatment and increases over time. On the other hand, GLP-1 receptor agonists did not show a significant effect when used in MASLD patients with cirrhosis on the prevention of its complications, the development of HCC and mortality [197]. The data seem to support the early use of GLP-1 in patients with type 2 diabetes and MASLD without cirrhosis, although further long-term studies are needed.
Tirzepatide, a dual GLP-1 and GIP agonist, was evaluated in a randomized phase 2 study of patients with MASH and F2–F3 fibrosis treated for 52 weeks and showed a resolution of MASH without worsening fibrosis [181]. Treatment with tirzepatide, compared to semaglutide, showed a greater effect in reducing HbA1c and an even longer-lasting weight loss [198]. Further studies with this promising drug are needed.
Sodium-glucose cotransporter 2 inhibitors
Dapagliflozin and empagliflozin have been studied in the treatment of MASLD and data showed that they improved steatosis, but had no significant effect on MASH and fibrosis [199].
Fibroblast growth factor 21
Fibroblast growth factor 21 (FGF21) regulates most phases of metabolism and protects cells from stress. Efruxifermin is an analogue of FGF21 that has been evaluated in a randomized phase 2b trial in patients with MASH and F2–F3 fibrosis. Data from this study showed that after 24 weeks of treatment with efruxifermin, a significant improvement in both MASH and fibrosis was observed compared to the control population. Therefore, the drug represents a promising candidate for the treatment of MASLD patients considered at risk and is being evaluated in a phase 3 study [200].
Conclusions
MASLD, a highly endemic liver and systemic disease worldwide, is a nosological entity defined by a complex etiopathogenesis involving multiple CMRFs which are responsible for the high variability of its natural history and affect its management. Emerging data highlight the different impacts of each CMRFs on the development of MASLD and throw the number of overlapping CMRFs determines the rate of progression of the disease. The bidirectionality of disease progression may be linked to changes that can occur naturally or induced for other purpose in CMRFs over the time. Therefore, although the term MASLD encompasses a single disease, it actually occurs as a different disease depending on each patient with its own progression and outcomes. Therefore, further studies are needed to understand the impact of individual CMFRs and their overlap on disease progression, in order to subcategorize patients and implement management strategies in the follow-up of those at higher risk. The degree of liver disease and particularly the stage of fibrosis predicts liver outcomes, such as liver failure and mortality, and also predicts a high risk of all-cause mortality. HCC is a major complication that, although most likely associated with advanced fibrosis or cirrhosis, can occur at all stages of the disease, often in association with certain metabolic conditions. Therefore, monitoring the development of HCC in patients with MASLD remains difficult and further studies are needed to improve prevention strategies.
MASLD, through overproduction of atherosclerotic dyslipidemia and chronic hepatic and low-grade systemic inflammation, modulates an increased risk of CV, renal and endocrine diseases and is also associated with a high risk of developing extrahepatic cancer. Therefore, its management requires a holistic approach with evaluation of extrahepatic pathologies and aggressive advanced treatment of CMRFs through a multidisciplinary collaboration. Currently, weight-loss diet and physical activity are still considered the first-choice etiopathogenetic treatments effective in all phases of MASLD. The hepatologist, among skills, needs to acquire the ability to encourage their patients to start and follow a healthy diet and exercise. If pharmacological treatments are prescribed, they should always be combined with diet and exercise to optimize therapeutic management. Apart from resmetirom, recently approved by the US FDA, there are no other drugs licensed for the treatment of MASLD. Many drugs were assessed in clinical trials and are currently used to treat MASLD comorbid, such as type 2 diabetes and obesity, showing partial and variable efficacy in the treatment of MASLD as well. Resmetirom, in patients with advanced fibrosis, is able to reduce, in a certain percentage of cases, both MASH and liver fibrosis. There are several ongoing clinical trials on drugs targeting different pathogenetic mechanisms aiming to implement, in the near future therapeutic strategies for MASLD.
Abbreviations
| aHR: | adjusted hazard ratio |
| ARBs: | angiotensin II receptor blockers |
| BMI: | body mass index |
| CKD: | chronic kidney disease |
| CMRFs: | cardio-metabolic risk factors |
| CV: | cardio-vascular |
| CVD: | cardio-vascular disease |
| DPP-4: | dipeptidyl-peptidase-4 |
| DTx: | digital therapy-delivered interventions |
| ER: | endothelial reticulum |
| FDA: | Food and Drug Administration |
| FGF21: | fibroblast growth factor 21 |
| FIB-4: | fibrosis index based on 4 factors |
| GH: | growth hormone |
| GIP: | glucose-dependent insulinotropic polypeptide |
| GLP-1: | glucagon-like peptide-1 |
| HCC: | hepatocellular carcinoma |
| HDLc: | high-density lipoprotein cholesterol |
| IGF: | insulin-like growth factor |
| LPS: | lipopolysaccharides |
| MASH: | metabolic dysfunction-associated steatohepatitis |
| MASLD: | metabolic dysfunction-associated steatotic liver disease |
| MetALD: | metabolic alcoholic liver disease |
| NAFLD: | non-alcoholic fatty liver disease |
| OSA: | obstructive sleep apnea |
| PNPLA3: | patatin-like phospholipase domain-containing protein 3 |
| PPARs: | peroxisome proliferator-activated receptors |
| SGLT-2: | sodium-glucose cotransporter 2 |
| SHR: | subdistribution hazard ratio |
| SLD: | steatotic liver disease |
| THR: | thyroid hormone receptor |
| THs: | thyroid hormones |
| VCTE: | vibration-controlled transient elastography |
| VLDL: | very low-density lipoprotein |
Declarations
Author contributions
LEA: Conceptualization, Project administration, Methodology, Data curation, Writing—original draft. AM: Methodology, Writing—review & editing, Validation. LR: Methodology, Data curation, Writing—review & editing, Validation. RN: Methodology, Data curation, Writing—review & editing, Validation. AI: Methodology, Writing—review & editing, Validation. FCS: Methodology, Supervision, Writing—review & editing, Validation. All authors read and approved the submitted version.
Conflicts of interest
Ferdinando Carlo Sasso who is the Editorial Board Member of Exploration of Medicine had no involvement in the decision-making or the review process of this manuscript. The other authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.