Affiliation:
1Oral Medicine and Periodontology Department, Faculty of Dentistry, Cairo University, Cairo 11251, Egypt
Email: mai.zakaria@dentistry.cu.edu.eg
ORCID: https://orcid.org/0000-0002-2846-6094
Affiliation:
2Surgery and Oral Medicine Department, Oral and Dental Research Institute, National Research Centre, Giza 12311, Egypt
ORCID: https://orcid.org/0000-0001-7246-9666
Affiliation:
1Oral Medicine and Periodontology Department, Faculty of Dentistry, Cairo University, Cairo 11251, Egypt
ORCID: https://orcid.org/0000-0002-6345-2579
Explor Med. 2025;6:1001283 DOI: https://doi.org/10.37349/emed.2025.1001283
Received: December 24, 2024 Accepted: February 03, 2025 Published: February 18, 2025
Academic Editor: Gaetano Isola, University of Catania, Italy
Aim: The present study was designed to assess the effectiveness of topical application of sulfasalazine in combination with topically applied corticosteroids versus using topical steroids alone as the standard control in management of symptomatic oral lichen planus (OLP).
Methods: The trial involved 46 participants having symptomatic OLP. Subjects included were divided into two equal groups at random. Group C (control group), in this group patients were treated with topically applied corticosteroids only as the standard treatment of OLP. In Group T (test group) topical sulfasalazine was used in combination with topical corticosteroids in management of the OLP cases. The patients used the topical applications four times per day in an alternate sequence (in Group T). The treatment schedule was continuous for 4 weeks with one visit weekly as a follow-up. Oral hygiene measures were strictly applied with total elimination of plaque with calculus deposits removal as they implement inflammation intra-orally and exaggerate together distribution and signs of OLP lesions. Each group underwent pain assessment and sign score recordings both before and after the used management strategies.
Results: The results of all patients reported no unwanted reactions or complications using both treatment strategies. Both study groups reported a significant decrease in the pain scale and sign score recordings over time as shown within the intragroup findings. Group T experienced a significantly higher reduction in pain scale (starting at two weeks) and sign score results (starting at three weeks) as compared to Group C.
Conclusions: Based on the data presented in this study, combination of topical sulfasalazine with topical corticosteroids is an efficient treatment in management of OLP in terms of decreasing pain scale and sign score values (Clinical Trials.gov with registration number NCT06060301).
Oral lichen planus (OLP) is a chronic disorder with an inflammatory nature, its pathogenesis includes a T-cell-mediated response against epithelium due to unidentified antigen(s). It affects 0.1–4% of the population all over the world [1]. Patients’ follow-up is mandatory to identify malignant changes into squamous cell carcinoma, taking place nearly in 0.2–4% of patients according to World Health Organization (WHO) reports [2].
OLP is twice as common in women as in men, and it is often manifested in the fifth and sixth decades of life. Clinical types of OLP include plaque-like, papular, reticular, bullous, erosive, and atrophic ulcerative lesions. OLP is typically found symmetrically on both sides of the cheek mucosa. It is not as much common on the tongue, lips, and gingival tissues. The white keratotic form has no symptoms thus not requiring treatment. However red lesions associated with pain and burning sensation need management [3].
OLP has been treated with a diversity of therapeutic approaches. It is difficult to employ a distinct, comprehensive therapeutic treatment method since disease activity is changing between remissions and exacerbations. The main goals of current therapy approaches are to lessen mucosal ulcerations and pain sensations. Because of the illness’s refractory nature, current medications are still unable to fully treat it. The management of OLP includes a combination of topical and systemic therapeutic techniques. Up until now, corticosteroids have been the standard treatment for OLP symptoms; however, chronic use of these medications has resulted in a number of undesirable side effects, comprising atrophy of the mucosal tissues, overgrowth of the candida, suppression of adrenal glands, elevated blood pressure, gastrointestinal distress, and elevated blood glucose levels. Corticosteroids are mostly administered systemically or intralesionally but still, their effect is frequently inadequate [4].
Topical steroid resistance in OLP appears to require an alternative effective therapeutic approach with the fewest adverse effects, as it is becoming unsatisfactory for various patients [5].
Sulfasalazine works well for immune-related inflammatory diseases such as Behcet’s disease, rheumatoid arthritis, and Crohn’s disorder, and it is also widely utilized in the management of inflammatory bowel disease. Notwithstanding its efficacy, the anti-inflammatory mechanism remains unclear. Sulfasalazine taken orally breaks down in the gut into 5-aminosalicylic acid (5-ASA) and sulfapyridine. It is well-recognized that sulfapyridine has antibacterial properties [6] whereas 5-ASA possesses anti-inflammatory properties [7]. Dermatological conditions for instance psoriasis, alopecia areata, and even lichen planus (LP) can be effectively treated with sulfasalazine [8].
For the treatment of LP, sulfasalazine has been recommended as a therapeutic alternative with few side effects [9]. Nevertheless sulfasalazine oral delivery has no effect on mucosal LP, and thus topical use of this medication has been tried showing its efficacy in management of OLP refractory cases [10].
To the authors’ knowledge, the influence of topical sulfasalazine with corticosteroid in management of OLP has not been studied. Therefore, in the current contemplation, we evaluated the efficiency of topical sulfasalazine in combination with topical corticosteroids in individuals with symptomatic OLP versus using topical corticosteroids alone. The study was based on the null hypothesis that there are no differences in effectiveness of different treatments used between the two study groups.
This is a randomized clinical study to assess the efficiency of topical sulfasalazine in combination with topical corticosteroids in management of OLP within adult Egyptian subjects presented to the Oral Medicine Department at the Faculty of Dentistry, Cairo University.
The trial procedures were clarified before beginning the management of the participants. For their agreement, each of them signed an informed consent form (submitted to the Ethical Committee for ethical approval). This study was carried out on oral atrophic-erosive lesions in patients having OLP starting from March 2023 till September 2024. OLP patients were diagnosed using the diagnostic criteria approved by the WHO [11].
The present study was carried out in compliance with the World Medical Association guidelines of ethics for research involving human participants (Declaration of Helsinki, 1978, as amended in 2024). The Research Ethical Committee of the Faculty of Dentistry, Cairo University accepted the study protocol, which was given the 201222 code. Additionally, it was listed on Clinical Trials.gov with registration number NCT06060301.
In order to apply a two-sided statistical test of the null hypothesis—that there is no difference between the various tested groups—a power analysis was considered with sufficient power. The anticipated sample size (n) was 36 instances, or 18 cases per group, using an alpha (α) level of 0.05, a beta (β) level of 0.2 (i.e., power = 80%), and an effect size (d) of 1.00 that was determined using the findings of a prior study [10]. To account for potential dropouts at various follow-up periods, the sample size was expanded by 25% to reach 46 cases or 23 cases in each group. G*Power 3.1.9.7 was utilized to determine the sample size [12].
The study eligibility criteria were patients aged 30–65 years old having atrophic-erosive type OLP. Smokers, pregnant or lactating women, hepatitis C virus (HCV) antibodies positive patients, and diabetic and hypertensive patients were not included in the study. Participants who have used topical or systemic steroids throughout the past two months, patients on lichenoid reaction-producing medications, and patients having skin LP or amalgam restorations next to their oral lesions were omitted from the study.
Patients were gathered sequentially from the outpatient clinic of the Oral Medicine Department, Faculty of Dentistry, Cairo University. Every patient had a comprehensive oral examination and the medical history was obtained.
In this study, 46 patients in total were included and equally distributed at random (using sealed envelopes) into two groups. For three to five minutes, the twenty-three patients in the test group (Group T) used topical sulfasalazine, which was made by dissolving one commercially available tablet of sulfasalazine (Colosalazine EC 500 mg, made by the Alexandria Company for Pharmaceuticals & Chemical Industries, Alexandria, registration No. E.D.A. Reg. No. 59979/2022) in five mL of distilled water used as a mouth wash and then spitted combined with commercially available topical corticosteroids (triamcinolone acetonide 0.1%, Kenacort-A Orabase, Turkey, manufacturing license No. 19.01,2011-229/23). This combination was applied 4 times daily for the test group in an alternate sequence. The 23 patients classified as the control group (Group C) received only 4 times topical application of corticosteroids gel per day. In both study groups, the subjects used the recommended topical management four times daily for duration of four weeks. The treatment for each group was given by one of the study’s researchers (MZ) who was not involved in the assessment of the outcomes.
Following each application, patients were stated to avoid eating or drinking for at least half an hour. The subjects were advised to discontinue the medication if any negative unwanted effects appeared and to visit the clinic the next day after communicating with the researchers. Other than the prescribed medications, the patients were not permitted to utilize any other medications for the lesions. Because the patients in the two groups had different forms of commercially available medications it was not possible to blind the patients for their treatments.
The treatment schedule was continuous for 4 weeks with one visit weekly as a follow-up. All subjects in the study groups did sufficient oral hygiene routine procedures with total elimination of plaque using commercially existing tooth paste free from sodium lauryl sulfate and they had wash-out duration for 2 weeks with calculus deposits removal as they implemented inflammation intra-orally and exaggerated together distribution and signs of OLP lesions. Participants were instructed to prevent traumatic handling of soft tissues by consuming toothbrushes with soft-typed bristles. Foods and beverages that were hot, spicy, acidic, or hard were avoided.
Every patient in each group was evaluated using the sign scoring scale of Thongprasom et al., 1992 [13] where: 5 (white striae with an erosive area > 1 cm2). 4 (white striae with an erosive area < 1 cm2). 3 (white striae with an atrophic area > 1 cm2). 2 (white striae with an atrophic area < 1 cm2). 1 (mild white striae only) and 0 scored for (no lesions, normal mucosa). Pain was assessed using the numeric analogic scale (NAS) [14] where patients requested to amount the severity of their pain utilizing numbers from 0 to 100 considering that 0 indicated no pain and 100 indicated the severest pain ever felt. Both recordings were reported by one of the study’s authors (IER) who was blinded about the treatment given for each patient to avoid performance bias before starting the treatment at baseline and every one week for a period of 4 weeks which was the study duration.
Fisher’s exact test was used to analyze categorical data, which were displayed as frequency and percentage values. The mean, standard deviation (SD), median, and interquartile range (IQR) values were used to represent numerical data. By examining the data distribution and applying Shapiro-Wilk’s test, they were examined for normalcy. The independent t-test was used to analyze age data, which had a normal distribution. Friedman’s test and the Mann-Whitney U test were used to analyze other non-parametric data, and the Nemenyi post hoc test was used to analyze intragroup comparisons. False discovery rate (FDR) was used to modify p-values for multiple comparisons. The Spearman’s rank-order correlation coefficient was used to analyze the correlations. For all tests, the significance level was set at p < 0.05. R statistical analysis software, version 4.3.2 for Windows (R Core Team 2023), was used to conduct the statistical analysis.
The research was conducted on 46 randomly and equally allocated cases to each study group. There were nine males and fourteen females with a mean age of 48.04 (± 8.56) years in the test group, and ten males and thirteen females with a mean age of 48.30 (± 8.93) years in the control group. Age and gender distribution did not significantly differ between the two groups (p > 0.05) as shown in Table 1. No unwanted reactions or complications with topical use of sulfasalazine have been reported in both groups.
Demographic data and intergroup comparisons
| Parameter | Control | Test | p-value | |
|---|---|---|---|---|
| Gender [n (%)] | Male | 10 (43.48%) | 9 (39.13%) | 0.960ns |
| Female | 13 (56.52%) | 14 (60.87%) | ||
| Age (years) | Mean ± SD | 48.30 ± 8.93 | 48.04 ± 8.56 | 0.920ns |
| Median (IQR) | 48.00 (15.00) | 48.00 (14.00) | ||
SD: standard deviation; IQR: interquartile range; ns: non-significant. Significance at (p < 0.05)
As indicated in Tables 2 and 3, there was a significant decrease in sign score and pain scale by time (p < 0.001) among both groups. Values evaluated at baseline were considerably more than those assessed at other times (p < 0.001) in the control group, according to post hoc pairwise comparisons for sign scores. Moreover, the findings revealed that values found one week later were considerably greater than those obtained at subsequent times (p < 0.001). The values evaluated at later intervals beginning with one week, as shown in Table 2, did not, however, differ significantly.
Sign score comparisons between and within groups
| Interval | Measurement | Control | Test | Test statistic | p-value |
|---|---|---|---|---|---|
| Baseline | Mean ± SD | 4.52 ± 0.67A | 4.52 ± 0.67A | 264.50 | 0.970ns |
| Median (IQR) | 5.00 (1.00)A | 5.00 (1.00)A | |||
| 1 week | Mean ± SD | 3.91 ± 0.85B | 3.87 ± 0.81B | 271.50 | 0.879ns |
| Median (IQR) | 4.00 (2.00)B | 4.00 (1.50)B | |||
| 2 weeks | Mean ± SD | 3.43 ± 0.79C | 3.22 ± 0.80C | 307.00 | 0.311ns |
| Median (IQR) | 4.00 (1.00)C | 3.00 (1.00)C | |||
| 3 weeks | Mean ± SD | 3.39 ± 0.84C | 2.26 ± 0.75D | 436.50 | < 0.001* |
| Median (IQR) | 4.00 (1.00)C | 2.00 (1.00)D | |||
| 4 weeks | Mean ± SD | 3.09 ± 1.04C | 1.17 ± 0.58E | 487.00 | < 0.001* |
| Median (IQR) | 3.00 (2.00)C | 1.00 (0.50)E | |||
| Test statistic | 71.30 | 88.39 | |||
| p-value | < 0.001* | < 0.001* | |||
SD: standard deviation; IQR: interquartile range; ns: non-significant; * significant (p < 0.05). Significant differences exist between values in the same vertical column that have dissimilar superscripts. Mean values having the same superscript letter are not significantly different
Pain scale comparisons between and within groups
| Interval | Measurement | Control | Test | Test statistic | p-value |
|---|---|---|---|---|---|
| Baseline | Mean ± SD | 8.70 ± 0.70A | 8.70 ± 0.70A | 264.50 | 0.980ns |
| Median (IQR) | 9.00 (1.00)A | 9.00 (1.00)A | |||
| 1 week | Mean ± SD | 6.74 ± 0.96B | 6.57 ± 0.79B | 286.50 | 0.612ns |
| Median (IQR) | 7.00 (1.00)B | 6.00 (1.00)B | |||
| 2 weeks | Mean ± SD | 5.04 ± 0.98C | 4.52 ± 0.73C | 348.50 | 0.052ns |
| Median (IQR) | 5.00 (2.00)C | 4.00 (1.00)C | |||
| 3 weeks | Mean ± SD | 3.39 ± 1.03D | 2.26 ± 0.62D | 424.50 | < 0.001* |
| Median (IQR) | 3.00 (1.00)D | 2.00 (1.00)D | |||
| 4 weeks | Mean ± SD | 1.78 ± 0.90E | 0.57 ± 0.59E | 449.50 | < 0.001* |
| Median (IQR) | 2.00 (1.50)E | 1.00 (1.00)E | |||
| Test statistic | 91.05 | 91.81 | |||
| p-value | < 0.001* | < 0.001* | |||
SD: standard deviation; IQR: interquartile range; ns: non-significant; * significant (p < 0.05). Significant differences exist between values in the same vertical column that have dissimilar superscripts. Mean values having the same superscript letter are not significantly different
All post hoc pairwise evaluations were statistically significant (p < 0.001) for the sign scores measured in the test group and for the pain scale values measured in both groups, as indicated in Tables 2 and 3.
There was no significant difference in the sign scores and pain scale between the two groups when comparing the study groups from baseline to two weeks (p > 0.05). But at the third week, the scores of the test group were significantly lesser than those of the control group (p < 0.001), as indicated in Tables 2 and 3.
Concerning the percentage of change, starting from 2 weeks, the reduction from baseline in the test group was higher, yet it was only statistically significant for the sign score values starting from 3 weeks (p < 0.001). On the other hand, as indicated in Table 4, the test group experienced a significantly higher reduction in pain scale recordings beginning at 2 weeks (p < 0.001). This is presented in Figures 1 and 2.
Intergroup comparisons of the percentage of reduction in measured scores
| Parameter | Interval | Measurement | Control | Test | Test statistic | p-value |
|---|---|---|---|---|---|---|
| Sign score | Baseline–1 week | Mean ± SD | 13.70 ± 11.40 | 14.57 ± 11.07 | 272.50 | 0.861 |
| Median (IQR) | 20.00 (25.00) | 20.00 (25.00) | ||||
| Baseline–2 weeks | Mean ± SD | 24.49 ± 11.51 | 29.42 ± 11.11 | 331.50 | 0.113 | |
| Median (IQR) | 20.00 (5.00) | 25.00 (20.00) | ||||
| Baseline–3 weeks | Mean ± SD | 25.94 ± 10.32 | 51.01 ± 12.03 | 491.50 | < 0.001* | |
| Median (IQR) | 20.00 (5.00) | 50.00 (20.00) | ||||
| Baseline–4 weeks | Mean ± SD | 33.41 ± 16.92 | 75.00 ± 11.28 | 514.00 | < 0.001* | |
| Median (IQR) | 25.00 (30.00) | 75.00 (12.50) | ||||
| Pain scale | Baseline–1 week | Mean ± SD | 22.66 ± 7.37 | 24.60 ± 5.19 | 293.00 | 0.527 |
| Median (IQR) | 22.22 (3.89) | 25.00 (2.78) | ||||
| Baseline–2 weeks | Mean ± SD | 42.24 ± 8.89 | 48.18 ± 5.73 | 368.50 | 0.020* | |
| Median (IQR) | 44.44 (16.67) | 50.00 (5.56) | ||||
| Baseline–3 weeks | Mean ± SD | 61.27 ± 10.55 | 74.06 ± 6.68 | 446.00 | < 0.001* | |
| Median (IQR) | 62.50 (13.89) | 75.00 (11.11) | ||||
| Baseline–4 weeks | Mean ± SD | 79.95 ± 9.57 | 93.70 ± 6.43 | 476.50 | < 0.001* | |
| Median (IQR) | 77.78 (15.00) | 88.89 (11.11) |
SD: standard deviation; IQR: interquartile range; * significant (p < 0.05)
A statistically significant positive correlation (rs = 0.770, p < 0.001) was observed between the two measured sign scores and pain scales as shown in Table 5.
Correlation between sign and pain scores
| Variables | Correlation coefficient (95% CI) | p-value |
|---|---|---|
| Sign score and pain scale | 0.770 (0.711:0.818) | < 0.001* |
CI: confidence interval; * significant (p < 0.05)
The clinical improvement of OLP cases following the combined treatment using topical sulfasalazine with topical corticosteroids was recorded as shown in Figure 3.
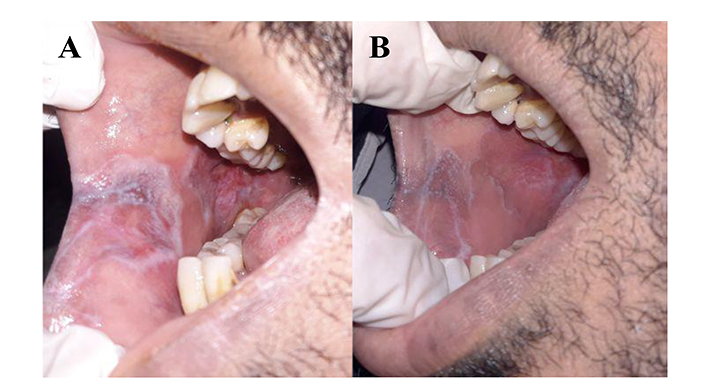
Improved case of oral lichen planus (OLP) affecting buccal mucosa. (A) Baseline; (B) after 4 weeks of combination treatment with topical sulfasalazine and topical corticosteroid
Although the precise pathologic mechanism underlying OLP, a chronic inflammatory disease, is unknown, evidence suggests that OLP is a disorder with autoimmune origin driven by T cells [15]. In particular, topically applied steroids are regarded as the first-line for OLP management. There have been trials with other topical medications like tacrolimus, pimecrolimus, cyclosporine, and aloe vera [16]. These, however, did not have as much of an impact as corticosteroids. In light of the fact that topical steroid resistance can become inconvenient for certain patients, it appears that finding an effective treatment strategy with the fewest negative effects is still imperative [3].
In addition to being used to treat Crohn’s disease and ulcerative colitis, sulfasalazine is a second-line treatment for rheumatoid arthritis. Similarly, sulfasalazine is proposed as a therapeutic alternative for the treatment of LP because it does not have any serious adverse effects.
Sulfasalazine administered orally, however, had no effect on mucosal LP with any observed improvement in all LP patients; this was explained according to the assumption that cytokine expression is not precisely equivalent in these two clinical manifestations of the same disease [17, 18].
Accordingly, the existing trial was done to investigate the influence of topical application of sulfasalazine combined with topical corticosteroids in the management of OLP patients as a test group compared to those managed with only topical steroids as a control group.
Sulfasalazine employs its anti-inflammatory effect by controlling dysregulated arachidonic acid metabolism hindering lipoxygenase enzyme; this will result in decreased production of pro-inflammatory leukotrienes. It also impedes the production of various inflammatory mediators and the expression of some adhesion molecules contributing to the process of OLP pathogenesis, which explains the reduction of sign score and pain scale values in the current study over time. The patients in this contemplate reported decreased pain and burning sensation during the treatment period. This all was in line as explained by Jeong et al. [10].
The current research revealed a significant reduction in sign score and pain scale over time concerning both intragroup relations. Additionally, pain scale values were significantly changed in both control and test groups. Meanwhile, a significant improvement in sign score values was more obvious in the test group. As regards the percentage of change reduction of sign score and pain scale in the test group it was significant in comparison to the control group. These findings are in line with the data of the previous investigation done by Jeong et al. [10] regarding the pain scale. However, regarding the reduction of sign score the current study is against that one which showed a non-significant decrease in the sign score with topical sulfasalazine treatment. This can be attributed to the previous study’s limited sample size [10].
Besides, the findings of the current research are against the outcome of the previous trials showing that sulfasalazine treatment had no effect on mucosal lesions of LP [17, 18]. This may be attributed to the difference in route of administration between these investigations using systemic oral route compared to the topical application in the current study. When taken orally, sulfasalazine is broken down by the gut microbiota’s azoreductase enzyme into 5-ASA and sulfapyridine. A number of bacteria have azo-reductase activity, including Bifidobacterium lactis, Streptococcus salivarius, and Lactobacillus acidophilus [19]. Most oral microbiota in the oral cavity is made up of Streptococcus species, including S. salivarius [20]. Thus, a medication topically administered to the oral mucosa was proposed as a potential treatment for OLP.
Furthermore, Langerhans and endothelial cells in oral and cutaneous LP express certain adhesion molecules, including vascular cellular adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin adhesion molecule 1 (ELAM-1). In rat heart transplants, sulfasalazine inhibits nuclear factor-κβ transcription, which lowers the expression of adhesion molecules (VCAM, ICAM, and ELAM). Additionally, this medication suppresses the production of interleukin-12 in macrophages, inhibits the production of interleukin-2 and lymphocyte proliferative responses, and stops the immunological response of T-helper1 cells [10].
To the authors’ knowledge, the present study assesses the efficacy of topical sulfasalazine with corticosteroid in management of OLP in a randomized clinical trial study design which has not been presented before.
The current study’s limitation was a short follow-up period relative to the chronic nature of OLP which is characterized by remission and exacerbation. Accordingly, depending on this study’s outcomes we recommend designing researches investigating only the effect of sulfasalazine in the management of OLP and more follow-up period. Despite the sample size calculation done for the number of patients to be recruited studies involving more number of the patients for conclusion generalization.
In conclusion, based on the data presented in this study, combination of topical sulfasalazine with topical corticosteroids is an efficient treatment in management of OLP in terms of decreasing pain scale and sign score values.
5-ASA: 5-aminosalicylic acid
ELAM-1: E-selectin adhesion molecule 1
ICAM-1: intercellular adhesion molecule 1
LP: lichen planus
OLP: oral lichen planus
VCAM-1: vascular cellular adhesion molecule 1
WHO: World Health Organization
MZ: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. BM: Writing—review & editing. IER: Formal analysis, Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The Research Ethical Committee of the Faculty of Dentistry, Cairo University accepted the study protocol, which was given the 201222 code. The present study was carried out in compliance with the World Medical Association guidelines of ethics for research involving human participants (Declaration of Helsinki, 1978, as amended in 2024). This work was done in years 2023–2024.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets used and/or analyses used during the current study are available from the corresponding author on reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2084
Download: 25
Times Cited: 0
