Affiliation:
1Department of Oral Medicine and Periodontology, Faculty of Dentistry, Cairo University, Cairo 11251, Egypt
ORCID: https://orcid.org/0000-0003-1773-2227
Affiliation:
1Department of Oral Medicine and Periodontology, Faculty of Dentistry, Cairo University, Cairo 11251, Egypt
ORCID: https://orcid.org/0000-0002-7087-6003
Affiliation:
2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo 11562, Egypt
3School of Life and Medical Sciences, University of Hertfordshire Hosted by Global Academic Foundation, New Administrative Capital 11835, Egypt
ORCID: https://orcid.org/0000-0002-9454-7458
Affiliation:
5Department of Oral Medicine and Periodontology, Faculty of Dentistry, The British University in Egypt, El Sherouk City 11837, Egypt
ORCID: https://orcid.org/0000-0003-2541-7243
Affiliation:
1Department of Oral Medicine and Periodontology, Faculty of Dentistry, Cairo University, Cairo 11251, Egypt
Email: Basma.abdelalim@dentistry.cu.edu.eg
ORCID: https://orcid.org/0000-0001-6516-5461
Explor Med. 2025;6:1001290 DOI: https://doi.org/10.37349/emed.2025.1001290
Received: November 27, 2024 Accepted: February 03, 2025 Published: March 03, 2025
Academic Editor: Gaetano Isola, University of Catania, Italy
Aim: This randomized clinical trial aimed to investigate the chemo-preventive role of thymoquinone in Nigella sativa extract in managing oral leukoplakia clinically, histologically, and at the molecular level.
Methods: A total of 48 patients with oral leukoplakia were randomly allocated to three groups; Group A received local muco-adhesive tablets with Nigella sativa extract in 10 mg/kg dose, for three months, Group B same intervention but with Nigella sativa extract in 5 mg/kg dose, and lastly Group C was the placebo group. The outcomes assessed were clinical improvement, histologic improvement (degree of dysplasia), and molecular biomarkers Ki-67 and caspase-3 by immunohistochemistry.
Results: There was a statistically significant decrease in lesion size in Groups A and B compared to the placebo group. At the molecular level, there was a statistically significant decrease in the expression of Ki-67 in both the Nigella sativa groups compared to the placebo group. While there was a statistically significant increase in caspase-3 in Group A only compared to the other groups.
Conclusions: Thymoquinone in Nigella sativa extract is a promising chemo-preventive agent that can be used in the management of oral leukoplakia (the trial is registered on clinicaltrials.gov identifier: NCT03208790).
The incidence and mortality rates of oral cancer (OC) are growing globally, as reported by the International Agency for Research on Cancer and the World Health Organization (WHO), with a higher prevalence in developing countries [1]. According to the latest WHO data published in 2020, OC deaths in Egypt reached 0.15% of total deaths [2]. Oral potentially malignant disorders (OPMDs) are mucosal lesions that have a statistically high risk of malignant transformation, however, the risk varies depending on many patient- or lesion-related aspects [3, 4].
The estimate of world prevalence of OPMDs is 4.47% (95% CI = 2.43–7.08), as reported in a systematic review including 22 epidemiological studies. The prevalence may vary among different populations. For instance, oral leukoplakia was found to be more prevalent in Asians and males [5]. Leukoplakia is the most common OPMD, although the prevalence varies in different geographic regions according to the definition used to classify leukoplakia [6]. The treatment objective is based mainly on the precancerous nature of the lesion and as the lesions are mostly asymptomatic, the primary aim of management is to prevent malignant transformation [7].
Surgical excision of progressive OPMDs has been considered the standard of care, but we need alternative preventive approaches in patients with OPMDs in multiple sites or with those at sites where surgical excision is not convenient. Besides, multiple recurrences in post-surgical management have urged the search for topical chemo-preventive treatment for OC in the previous decades [8].
Chemoprevention is the approach of using medical treatment to prevent the process of carcinogenesis to stop the progression of OPMDs into frank malignancy [9]. This approach has succeeded in halting the progression of breast, prostate, and colon cancer [10].
Nigella sativa (NS) has been used as a traditional drug for eras. The reports showed that NS and its active ingredient thymoquinone (TQ) have the potential to be used as a treatment for dental and oral conditions [11]. Moreover, TQ might be useful as a protective agent for normal tissues exposed to cytotoxic agents [12, 13]. It was reported that the basic oil and TQ were shown to be effective against cancer in different body parts including prostate, colorectal, breast, sarcoma, and leukemia, and their safety was proven [14–17]. TQ targets many cell signaling pathways at the molecular level. Many researchers recommended that TQ has effectively inhibited cancer initiation and could eradicate a wide range of malignant cells [16]. It was also reported that TQ can increase the effectiveness of various chemotherapeutic drugs and make cancer cells more sensitive to radiation [18]. In an animal model, TQ was found to enhance the anticancer benefits of chemotherapy drugs like cisplatin (CIS), as well as helping to prevent harmful effects of the drug on healthy body organs [19].
Thus, the main objective of this study is to evaluate the chemo-preventive potential of TQ, the active component of NS extract, in the management of oral leukoplakia. The study aimed to assess its effects clinically through lesion size reduction, histologically by evaluating changes in the degree of dysplasia, and at the molecular level by analyzing the expression of proliferation (Ki-67) and apoptosis (caspase-3) biomarkers. Additionally, it sought to determine the efficacy of different dosages of TQ in achieving these outcomes compared to a placebo, providing insight into its role as a non-surgical treatment option for potentially malignant oral disorders.
The trial design was 3 arms randomized controlled clinical trial, parallel groups with a 1:1:1 allocation ratio.
According to an earlier article [20], the clinical response rate of the placebo control group was 4%. If the true clinical response rate of the experimental group was 50%, we required studying 13 in each group to discard the null hypothesis that the exposure rates for cases and controls are the same with probability (power) 0.8. The probability error type I accompanying this test is 0.05 of this null hypothesis. We employed an uncorrected chi-square statistical test to assess this null hypothesis. The number was increased to 16 in each group to compensate for potential losses throughout the follow-up. Version 3.1.2 PS: Power and Sample Size Calculation software (Vanderbilt University, Nashville, Tennessee, USA) was implemented to manage the calculation of the size of the sample.
The patients included in the present study were recruited from the outpatient clinic (Diagnostic Center) of the Faculty of Dentistry, Cairo University.
The study started in September 2019 and ended in December 2022. Patients showed homogenous non-dysplastic leukoplakia or leukoplakia with mild dysplasia, confirmed clinically and histologically. Lesions should be ≥ 2 cm in size to allow for incisional biopsy. We excluded patients who received earlier cures for the premalignant conditions whether surgical or non-surgical, patients who showed lesions with moderate or severe dysplasia, carcinoma in situ, any type of existing malignancy, or any other type of oral leukoplakia rather than the homogenous (verrucous, speckled or hairy leukoplakia) due to ethical considerations in this phase of the clinical study. Also, pregnant or lactating females, patients known to have hypersensitivity to the drug of intervention, and those who couldn’t be committed to the follow-up appointments were excluded.
The research was approved by the Ethics Committee of Scientific Research, Faculty of Dentistry, Cairo University (number: 17 8 3). The study was conducted in accordance with the Declaration of Helsinki (version 2013). Patients who fulfilled the inclusion criteria and were willing to participate were enrolled in the study after signing an Arabic approval consent form in which the treatment plan, all the data needed to be known to patients, and complications that could be met were discussed.
All data, information, patient information, family history, and social and medical history were kept in the files owed to each patient with constrained access to the main supervisor and principal investigator for quality control, assessment, and analysis.
Randomization: Enrolled patients were randomly distributed (Simple Randomization) between the 3 groups using an online randomization program: https://www.random.org (done FMZ). The software of the computer provided random sequences, and accordingly, opaque folded papers containing the assigned numbers were placed in tamper-proofed sealed opaque envelopes (done by FMZ) using packing tape. The patients were recruited by GN, allowed to choose from the envelopes containing their numbers, and allocated to each group accordingly. Blinding was done by a third party at the Faculty of Pharmacy, Cairo University. The participants, clinician (GN), pathologist (AF), outcome assessor (BE), and statistician were blinded to the type of treatment administered. This was achieved by using similar adhesive tablets (in shape, size, and color) for the participants and the investigator in all groups. FMZ had the blinding code, and all other contributors had no access.
Group A was assigned to mucoadhesive tablets of NS extract at a dose of 10 mg/kg q.d.s. for 3 months. Group B was assigned to mucoadhesive tablets of NS extract at a dose of 5 mg/kg q.d.s. for 3 months. Group C was assigned to mucoadhesive tablets of placebo q.d.s. for 3 months.
After completing the study, patients then have been followed up at intervals based on the individual’s risk assessment and considering patient compliance.
The Nigella Sativa L. (Ranunculaceae), seed oil extract was prepared by NAWAH Scientific, Cairo, Egypt. Briefly, it was extracted by n-hexane. The seeds were crushed and the ratio of the seeds: solvent was 9:1. The solvent was removed by lyophilization by Lyophilizer Novalyphe-NL 500, Savant, Holbrook, NY, USA at −45°C under a vacuum of 7 × 10−2 mBAR [21]. Then, High-Performance Liquid Chromatography (HPLC) was carried out to quantify TQ in NS extract, results showed that the concentration of TQ was 0.702 mg/g of seed oil (Supplementary material).
The objective was to prepare NS buccal tablets having good mucoadhesion force, sustain the mucoadhesion with the mucosa for 6 hours (the release period), and have a maximum release extent after 6 hours with a suitable release rate (Supplementary material).
Patients were subjected to detailed clinical extra and intra-oral examination. Lesion photographs were taken of patients in all groups before and after treatment. The lesion area measurement was adapted from the method used by Aragón-Sánchez (2017) [22]. Image J 1.53a software (National Institutes of Health, Rockville, MD; https://imagej.net/) was used to visualize the clinical photographs. The clinical improvement as a percentage of lesion size change after treatment compared to the original size was calculated as follows:
In terms of clinical lesion remission according to size change, the classification of clinical response was described as a complete response when no evidence of a lesion (approx. 100%) was detected by gross inspection, a partial response when detecting a decrease of more than 50% of the lesion size (approx. 75%), a stable response when detecting a decrease of less than 50% of the baseline size and no response, when detecting the progression of the lesion as an unequivocal size increase of any lesion during the treatment period or the appearance of a new lesion.
Tissue biopsy is considered the gold standard for diagnosing malignant and potentially malignant lesions as previously stated [23–25]. The samples were prepared by using 10% formalin for fixation, then were implanted in blocks of paraffin and cut into semi-thin sections (4 μm thickness) in glass slides. Staining of the slides was done using hematoxylin and eosin (H&E) for histopathological examination. To assess the degree of dysplasia before (for diagnosis) and after treatment (for re-evaluation). For histological evaluation, four stages were described as no dysplasia, mild dysplasia, moderate dysplasia, and severe dysplasia. We evaluated the histologic response in terms of stability of the category, regression, or progression of dysplastic changes.
Immunohistochemical staining for antibodies to caspase-3 and Ki-67 was done with respect to instructions of the manufacturer (Thermo Scientific, Thermo Fisher Scientific, Anatomical pathology, Fremont, CA 94538, USA). A universal kit (DAKO, Denmark) was used. The percentage of the reactive areas was calculated with respect to a standard that calculates the edge of the area 11,434.9 μm2 with amplification (× 400).
In the statistical analysis of the study record, IBM SPSS advanced statistics (Statistical Package for Social Sciences), version 21 (SPSS Inc., Chicago, IL) was engaged. Mean and standard deviation as well as median and range were implemented for the numerical values. While for the categorical values numbers and percentages were employed. Both the Shapiro-Wilk test and the Kolmogrov-Smirnov test were employed to analyze the research data. Comparisons between the 3 groups were done using the one-way ANOVA for numeric variables which were distributed in a normal manner, whereas for numeric variables that were non-normally distributed Kruskal-Wallis test was employed. Relation between categorical values variables was interpreted using the chi-square test. The statistically significant result was implemented when the p-value was less than 0.05. The whole tests were believed to be two-tailed.
The current study was carried out on 48 subjects suffering from oral leukoplakia with no or mild dysplasia. The patients were divided into three groups. Each group consisted of 16 patients. One patient was lost from Group A and one from Group B due to transportation issues (as they lived in remote areas), while two patients were lost to follow-up in Group C due to no change in clinical response.
The participants’ flow chart (Figure 1) displays the included groups; the numbers of participants who were randomly assigned to each group received intended treatments and were analyzed for the primary and secondary outcomes.
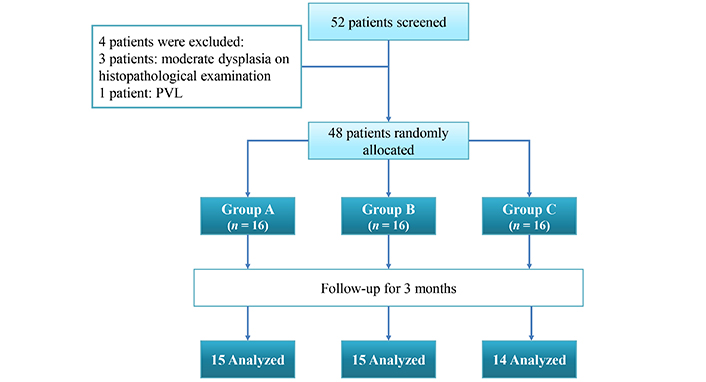
Flowchart showing patient recruitment and analysis. PVL: proliferative verrucous leukoplakia
Descriptive analysis of age, gender, dysplasia at baseline, and smoking habits of all included patients are demonstrated in Table 1. The results of Pearson’s chi-squared test and the one-way ANOVA for demographic data comparison in all the study groups showed no statistically significant difference. Lesions localization varies, lesions were found on the buccal mucosa in 33 patients (68.8%), the floor of the mouth in 2 patients (4.1%), the tongue in 4 patients (8.3%), the palate in 4 patients (8.3%) and labial mucosa in 5 patients (10.4%). The lesions were multiple and bilateral in 20 patients (41.7%) and solitary in 28 (58.3%).
Showing baseline demographic and characteristics for each group
| Variables | Mean (SD) | Male, N (%) | Female, N (%) | No dysplasia, N (%) | Mild dysplasia, N (%) | Smokers, N (%) | Non-smokers, N (%) | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Age | Group A | 50.37 (8.67) | 0.399 | ||||||
| Group B | 54.93 (8.83) | ||||||||
| Group C | 51.62 (11.45) | ||||||||
| Gender | Group A | 14 (93.3) | 1 (6.7) | 0.567 | |||||
| Group B | 14 (93.3) | 1 (6.7) | |||||||
| Group C | 12 (85.7) | 2 (14.3) | |||||||
| Dysplasia stage at baseline | Group A | 8 (53.3) | 7 (46.7) | 0.529 | |||||
| Group B | 9 (60.0) | 6 (40.0) | |||||||
| Group C | 10 (71.4) | 4 (28.6) | |||||||
| Smoking | Group A | 11 (73.3) | 4 (26.7) | 0.418 | |||||
| Group B | 8 (53.3) | 7 (46.7) | |||||||
| Group C | 10 (71.4) | 4 (28.6) | |||||||
SD: standard deviation. Significance level at p-value < 0.05
Table 2 shows that there was no statistically significant difference between the study groups regarding the total lesion size at baseline. However, post-treatment results showed significant differences.
Analysis of total lesion size at baseline and percentage of change in size in response to treatment in each of the 3 groups and Kruskal-Wallis test results for inter-group comparison
| Variables | Mean rank | Median | 25th–75th interquartile range | p-value | |
|---|---|---|---|---|---|
| Total lesion size at baseline in mm2 | Group A (n = 15) | 26.23 | 664 | 336.5–861 | 0.834 |
| Group B (n = 15) | 23.78 | 629 | 304.7–765.7 | ||
| Group C (n = 14) | 23.50 | 531.5 | 386.5–697.2 | ||
| Percentage of size change in response to treatment | Group A (n = 15) | 28.13a | 16.8 | 14.7–50.3 | < 0.001* |
| Group B (n = 15) | 29.40a | 31.6 | 17–37.06 | ||
| Group C (n = 14) | 9.07b | 2.04 | 0.76–2.04 | ||
* Significance level at p-value < 0.05; different superscript letters in the same column designate a statistically significant difference in pairwise comparison
Concerning the clinical response, there was a statistically significant difference between the three studied groups. Table 2 shows the results of the percentage changes in lesion size after treatment in the three included groups expressed as median, range, and mean rank. In Group A, there was no statistically significant difference in percentage of clinical improvement regarding lesion size compared to Group B. Both groups showed a statistically significant higher percentage of lesion size changes (decrease) after treatment compared to Group C.
It was noted that none of the patients in the three study groups showed complete lesion remission or disease progression. Most of the patients remained stable [Group A, 11 patients (73.3%), Group B, 13 patients (86.7 %) and Group C, 14 patients (100%)] however partial response was noticed in both Groups A and B [Group A, in 4 patients (26.7%) and Group B, in 2 patients (13.3 %)]. The difference between groups tested by the chi-square test was statistically non-significant, p-value = 0.112. Clinical pictures before and after treatment are shown in Figure 2.
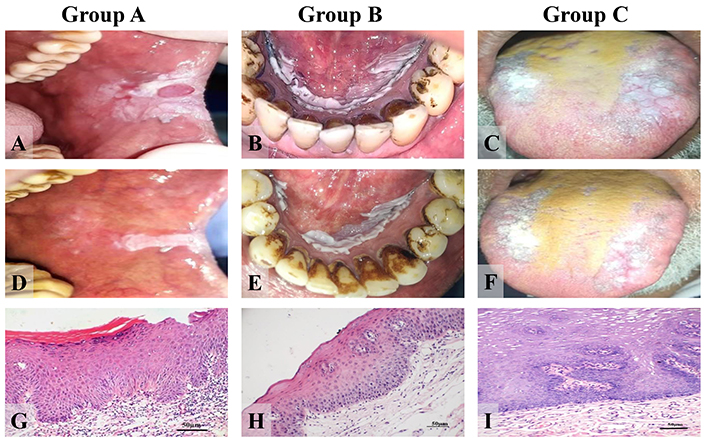
Clinical photos and photomicrographs showing one patient presentation in each group (clinical before and after and histopathological presentation before treatment). Group A: (A) clinical photograph of the buccal mucosa at the left side of a 52-year-old man displaying oral leukoplakia (homogenous) with a reactive lesion in the middle (irritational fibroma); (D) clinical picture of the same patient 3 months after treatment with thymoquinone 10 mg/kg; (G) histopathological picture of the same patient before treatment displaying mild dysplasia (H&E) (× 200); Group B: (B) clinical photograph of the floor of the mouth of a 56-year-old man displaying oral leukoplakia (homogenous); (E) clinical photograph of the same patient 3 months after treatment with thymoquinone 5 mg/kg; (H) histopathological picture of the same patient before treatment displaying mild dysplasia (H&E) (× 200); Group C: (C) clinical photograph of the dorsum of the tongue in a 55-year man displaying oral leukoplakia (homogenous); (F) clinical photograph of the same patient after 3 months of placebo administration; (I) histopathological picture of the same patient with no dysplasia earlier to placebo administration (H&E) (× 200). H&E: hematoxylin and eosin
No change in the dysplasia degree after treatment in all patients except for one patient in Group A. In this patient, the histopathology of the lesion before treatment showed features of mild dysplasia while, after treatment, no dysplasia was detected. The difference between groups in the degree of dysplasia after treatment was non-significant statistically where the p-value > 0.05.
As shown in Table 3, a comparison of means within the groups reveals that in Groups A and B there was a statistically significant decrease in the expression of Ki-67 after treatment. While in Group C there was an insignificant difference in Ki-67 expression after treatment. Regarding the expression of caspase-3, only in Group A, there was a statistically significant increase in its expression after treatment compared to the expression before treatment. In Groups B and C there was insignificant difference in expression of caspase-3 after treatment.
Showing the results of paired t-test for expression of Ki-67and caspase-3 before and after treatment in each study group
| Group | Paired differences | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Standard error mean | 95% Confidence interval of the difference | |||||
| Lower | Upper | |||||||
| Group A (n = 15) | Pair 1 | Expression of Ki-67 before–Expression of Ki-67 after | 4.97 | 2.63 | 0.67 | 3.52 | 6.43 | < 0.001* |
| Pair 2 | Expression of caspase before–Expression of caspase-3 after | –9.28 | 6.70 | 1.73 | –13.00 | –5.57 | < 0.001* | |
| Group B (n = 15) | Pair 1 | Expression of Ki-67 before–Expression of Ki-67 after | 4.56 | 1.46 | 0.37 | 3.75 | 5.37 | < 0.001* |
| Pair 2 | Expression of caspase before–Expression of caspase-3 after | –2.65 | 8.48 | 2.19 | –7.35 | 2.04 | 0.246 | |
| Group C (n = 14) | Pair 1 | Expression of Ki-67 before–Expression of Ki-67 after | 0.81 | 2.69 | 0.72 | –0.74 | 2.37 | 0.279 |
| Pair 2 | Expression of caspase before–Expression of caspase-3 after | –1.86 | 5.42 | 1.44 | –4.99 | 1.26 | 0.221 | |
SD: standard deviation; * significance level at p-value < 0.05
As shown in Table 4, comparing the means of the groups after treatment, there was a statistically significant decrease in the expression of Ki-67 in both NS groups in comparison to the placebo group. While there was a statistically significant increase in Group A only in comparison to both Group B and the placebo group in caspase-3 levels. Immunohistochemical analysis before and after treatment for both Ki-67 and caspase-3 are shown in Figures 3 and 4.
Showing the results of one-way ANOVA for expression of Ki-67and caspase-3 after treatment in study groups
| Group | Mean | SD | 95% Confidence interval for mean | F | p-value | ||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Expression of Ki-67 after intervention | Group A (n = 15) | 3.53a | 1.22 | 2.85 | 4.20 | 26.90 | < 0.001* |
| Group B (n = 15) | 2.12b | 0.70 | 1.73 | 2.50 | |||
| Group C (n = 14) | 5.55c | 1.70 | 4.57 | 6.53 | |||
| Expression of caspase-3 after intervention | Group A (n = 15) | 27.86a | 7.10 | 23.93 | 31.80 | 7.18 | 0.002* |
| Group B (n = 15) | 17.73b | 8.77 | 12.87 | 22.58 | |||
| Group C (n = 14) | 21.30b | 6.04 | 17.81 | 24.79 | |||
SD: standard deviation; * significance level at p-value < 0.05; different superscript letters in the same column designate a statistically significant difference in pairwise comparison
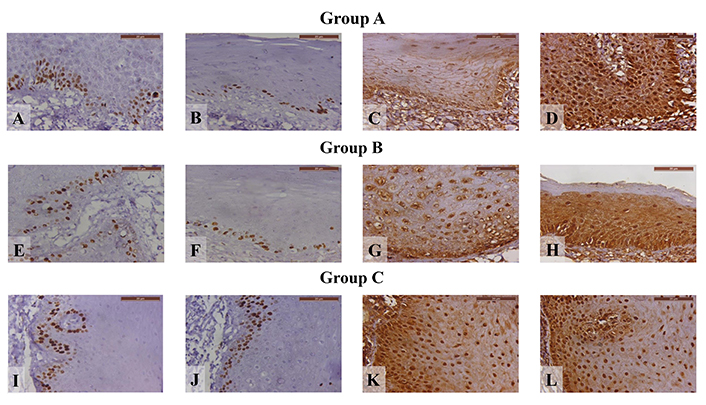
Photomicrographs displaying Ki-67 immunohistochemical staining for various treatment stages in different groups. (A) Group A before treatment; (B) Group A after treatment; (C) Group A before treatment, demonstrating caspase-3 immunohistochemical staining; (D) Group A after treatment, demonstrating caspase-3 immunohistochemical staining; (E) Group B before treatment; (F) Group B after treatment; (G) Group B before treatment, demonstrating caspase-3 immunohistochemical staining; (H) Group B after treatment, demonstrating caspase-3 immunohistochemical staining; (I) Group C before treatment; (J) Group C after treatment; (K) Group C before treatment, demonstrating caspase-3 immunohistochemical staining; (L) Group C after treatment, demonstrating caspase-3 immunohistochemical staining (× 400)
Regarding the side effects, we observed that 4 patients (26.7%) in Group A and 1 patient (6.7%) in Group B reported feeling nausea after application of the drug and it lasted for a short time. No patients discontinued the treatment due to this side effect, but 3 patients reported a reduction of times of application per day.
We evaluated the degree of nausea that was reported by our patients and found that it was mild (causing loss of appetite without alteration in eating habits). Thus, we advised them to avoid applying the treatment immediately after eating and better to be 30 minutes after meals. In addition, we advised them to report if it got worse so as to interfere with their dietary habits, where we would have had to consider stopping treatment. However, nobody reported such a level.
Chemoprevention is a low-risk approach to halt the process of cancer development or prevent cancer recurrence. Thus, local chemo-preventive interventions could provide an alternative early, non-invasive management for OPMDs [9].
One of the most promising traditional medicines in the field of carcinogenesis suppression is NS. It is a significant source of various bioactive constituents, the most well-known of which is TQ, as well as constituting an appreciable percentage of the NS extract [26].
The current study aimed at the management of oral leukoplakia as an OPMD with topically applied sustained-release NS extract with its active anticancer component TQ. The advantages of using topical agents for OPMDs are to provide high local and low total systemic doses to minimize toxicity. Also, to guarantee local delivery beyond the clinical margins of the lesion considering field cancerization of the oral mucosae. Carbopol was used as the adhesive compound in the mucoadhesive tablets, as it is physiologically inactive and is not absorbed from the gastrointestinal tract. It helps in limiting the drug to the site of absorption, increasing the concentration at the absorption site, and fastening the bioavailability [27, 28].
Oral leukoplakia was chosen as it is considered the most common premalignant lesion and the most studied OPMD [5, 6]. Another hospital-based cross-sectional study conducted in Egypt stated that leukoplakia prevalence is 3.54% among smokers [29].
Our clinical and molecular results revealed the potential chemo-preventive role of NS in the management of oral leukoplakia. Over the past decade, many researchers revealed great interest in investigating the TQ anticancer potentials. Subsequently, numerous studies have been conducted to investigate and assess the chemo-preventive or anticancer role and the precise molecular mechanism of action of TQ in different tumor cell lines and many different forms of cancer in animal models [28]. However, to our knowledge, there are no previous human clinical trials that investigated the effect of NS in the management of oral leukoplakia, its chemo-preventive role was only studied on cell lines and experimental animals and showed encouraging results on a molecular basis utilizing molecular markers more or less similar to those applied in the present study [30–36].
TQ aims at targeting various signaling pathways in the cell comprising its role in the hindrance of angiogenesis, regulation of enzyme activities, arrest of the cell cycle, apoptosis induction, androgen receptor suppression as well as inhibition of protein kinase, telomerase, and transcription factor. Several experiments based on clinical laboratories suggested that TQ has the potential to inhibit cancer initiation as well as kill a wide range of cancer cells [16].
The benefit of NS extract and its main constituent TQ was investigated both alone and in combination with CIS when applied in the precancerous stage of oral lesions in hamsters buccal pouches after exposure to carcinogen [19]. In this investigation, the severity of dysplastic changes was compared among the studied groups, the group that was exposed to the carcinogen for 6 weeks (untreated) showed mild dysplasia in 100% of cases, whereas the addition of NS extract to other exposed group (treated) resulted in 40% of samples without dysplasia, revealing the potential chemo-preventive effect of NS extract against OC development. However, on the molecular level, the results showed a non-significant difference in immunostaining with caspase-3 between NS extract-treated groups and untreated ones. This was not in accordance with the present results at higher doses. Conversely, in agreement with the results of the present study, there was a statistically significant difference in the expression of Ki-67 between NS extract-treated groups and untreated ones.
A study conducted on the effects and mechanism of TQ in the growth inhibition of bladder cancer both in vitro and in vivo showed that TQ could significantly lower the nuclear expression of nuclear factor kappa B (NF-κB). The expressions of NF-κB and X-linked inhibitor of apoptosis protein (XIAP) were down-regulated in BIU-87 cells after the treatment of TQ. Furthermore, the positive expressions of Ki-67, NF-κB, and XIAP decreased in tumors after the administration of TQ [29]. The effect on Ki-67 expression seems similar to that observed in the present study despite the cells tested not being oral keratinocytes.
An interesting finding in the present study was the positive results obtained in the placebo group, though significantly lower than the improvement in the TQ groups. This could be attributed to the fact that carbopol may protect the underlying cell layer, forming a thick barrier that separates the cells from the environment due to its continuous cross-linking [37]. However, the significant difference between the TQ groups and placebo highlights the effect of the extract.
Other interesting findings were firstly, the superior results obtained by the lower TQ dose in some of the studied clinical parameters. This could be explained by better patient compliance to treatment as the lower dose did not offend the patients by its nauseating taste that some patients complained of with the higher dose, thus it is recommended to add some flavors to mask the taste and odor of the NS in future preparations. Secondly, the stable histopathological findings before and after treatment despite the clinical improvement and the molecular evidence of change in tissue homeostasis (proliferation and apoptosis) after treatment. This finding was in agreement with Neetha et al. [38] who found that a combination of green tea and curcumin had a good clinical response in association with biomarker modulation, however, histologically no reverse of dysplasia was registered, only a decrease in hyperkeratinization. It seems, according to the significant changes on the molecular level noted in the present study, that the duration of our study was sufficient only for such type of change which would yield differences at further levels, leading to an evident histopathologic change.
However, in contrast to our findings regarding the management of leukoplakia, Singh et al. [39] who tested lycopene compared to placebo and Benner et al. [40] who tested vitamin E in the management of leukoplakia (single arm) had both reported significant clinical and histologic improvement. In the study by Singh et al. [39], they included lesions with mild, moderate, and severe dysplastic changes while we only included lesions with no or mild dysplasia. Moreover, Benner et al. [40] continued his treatment for 24 months compared to 3 months in our study.
Another interesting finding in the present study was the positive results obtained in the placebo group, though significantly lower than the improvement in the TQ groups. This could be attributed to the fact that carbopol might protect the underlying cell layer, forming a thick barrier that separates the cells from the environment due to its continuous cross-linking as reported by Singla et al. [37].
One limitation of the studies considered, as well as ours, was the length of follow-up, which in most studies did not exceed fifteen months. This limitation can lead to an underestimation of malignant transformation since less than half (33–42%) of leukoplakias undergo malignant change within two years of diagnosis [41]. Another limitation in the present study is the relatively small sample size although the sample size calculation was detailed and accounts for potential dropout. Consequently, based on our findings we recommend designing studies expanding the sample size to improve the generalizability of the results, maximizing the drug dose, improving the smell and taste of the tablets to improve the patient compliance, as well as increasing the time of treatment, increasing the follow-up period, and using more in-depth molecular indicators for early malignant transformation as miRNA profiling.
In conclusion, the present study revealed the usefulness of the mucoadhesive tablet formulation of NS extract, with its major component, TQ to enhance the apoptotic machinery and at the same time hinder the proliferative activity in oral leukoplakia, as proved by its effects on the biomarkers caspase-3 and Ki-67, respectively. Such effects at the molecular level were translated clinically as regression in the lesion’s size. At the same time, no progression in the degree of dysplasia has been revealed. Altogether, with the known importance of lack of apoptosis and increased proliferative activity in the process of carcinogenesis, the results encourage the clinical application of the material in the management of OPMDs. The guaranteed safety of the material can nominate it as a feasible candidate for further investigation in the field of OC chemoprevention.
CIS: cisplatin
NF-κB: nuclear factor kappa B
NS: Nigella sativa
OC: oral cancer
OPMDs: oral potentially malignant disorders
TQ: thymoquinone
WHO: World Health Organization
XIAP: X-linked inhibitor of apoptosis protein
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001290_sup_1.pdf.
The authors would like to thank Dr. Noha Azab lecturer of the Department of Oral Medicine and Periodontology, at the Faculty of Dentistry, Cairo University for her help in the clinical part and follow-up with the patients.
GN: Investigation, Data curation, Writing—original draft, Resources. FMZ: Conceptualization, Investigation, Data curation, Writing—review & editing, Supervision. AE and AF: Methodology, Writing—original draft. DG: Validation, Writing—original draft, Writing—review & editing. BE: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Methodology. All authors reviewed the results and approved the final version of the manuscript.
The authors declare no conflicts of interest to report regarding the present study.
The study was performed in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Scientific Research, Faculty of Dentistry, Cairo University (approval No. 17 8 3). The aim of the study was explained to all subjects participating in this study and written informed consents were obtained before enrolment.
The informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets used and/or analyses used during the current study are available from the corresponding author on reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
