Abstract
Cervical cancer is the fourth leading cause of cancer-related deaths among women worldwide, causing over 660,000 new cases and 350,000 deaths in 2022, with a disproportionately high burden in low-resource countries where access to treatment is limited. Human papillomavirus (HPV) is a common sexually transmitted infection that accounts for approximately 95% of cervical cancer cases. Persistent HPV infection can progress to cervical dysplasia, categorized into varying severities (CIN1, CIN2, and CIN3), which significantly increases cancer risk. The mechanism of HPV-induced malignancy involves the disruption of cellular apoptosis by integrating viral genetic material into cervical cells, particularly within the transformation zone. The viral proteins E6 and E7 play pivotal roles in cervical carcinogenesis by inhibiting tumor suppressor proteins, promoting uncontrolled cell proliferation, and evading immune responses, ultimately driving progression toward malignancy. Timely detection and intervention are essential for managing HPV-related cervical cancers. Preventative measures such as HPV vaccination have demonstrated substantial efficacy. Six vaccines targeting high-risk (HR) HPV strains are recommended before sexual activity or exposure. Despite these advancements, barriers, such as misinformation, logistical challenges, and limited healthcare infrastructure, persist, particularly in underserved regions. Advances in diagnostic and therapeutic technologies have offered new avenues for addressing these challenges. Next-generation sequencing and CRISPR gene editing are emerging as promising tools for HPV-related cancer treatment that enable precise and targeted interventions. Furthermore, artificial intelligence (AI) and imaging innovations have significantly enhanced diagnostic accuracy and personalized care. Pap smears and HPV DNA testing are indispensable tools for early detection. To tackle HPV-related cervical cancer globally, a multifaceted approach is required. Public health education, vaccination programs, research, and international collaboration are crucial. Public health campaigns should combat misinformation, strengthen vaccination programs, and focus on novel therapies, screening technologies, and next-generation sequencing.
Keywords
Cervical cancer, human papillomavirus, HPV vaccinesIntroduction
Cervical cancer remains one of the most significant global health challenges, with an estimated 660,000 new cases reported in 2022, making it the fourth most common cancer among women worldwide. The highest burden of cervical cancer is concentrated in low- and middle-income countries (LMICs), where access to screening, immunization, and treatment services is often limited [1]. This geographical disparity is further compounded by risk factors, such as high HIV prevalence, gender bias, and poverty, which exacerbate the situation. The incidence and death rates of cervical cancer are disproportionately high in regions such as Sub-Saharan Africa (SSA), Central America, and Southeast Asia [1, 2]. Moreover, cervical cancer disproportionately affects younger women, accounting for 20% of maternal cancer-related deaths [3].
Human papillomavirus (HPV), a common sexually transmitted infection, is the primary cause of cervical cancer affecting the skin, vagina, and throat, with 95% of the cases resulting from persistent infection. HPV infection is typically asymptomatic, and the immune system of most individuals clears the virus. However, in some cases, untreated persistent infections in the cervix can lead to the development of cervical dysplasia, which may progress to cancer within 15–20 years. In women with weakened immune systems, such as those living with untreated HIV, this progression can occur more rapidly within 5–10 years. Factors such as the carcinogenic potential of specific HPV strains, co-infection with other sexually transmitted infections, immune system health, and lifestyle factors such as smoking, early pregnancy, and hormonal contraception further contribute to this process [1]. The introduction of HPV vaccines, including tetravalent, bivalent, and 9-valent vaccines, has proven effective in reducing the incidence of HPV-related diseases and infections [4]. These vaccines target both low-risk and high-risk HPV strains, which are responsible for approximately 70% of genital warts and 90% of cutaneous malignancies (Table 1). Despite the success of vaccination, there remains a significant risk of HPV-related cancers and other health complications [5]. This study aimed to explore the mechanisms of HPV progression to cervical cancer, assess associated public health implications, and propose preventive measures to address this global health burden.
Types of HPV vaccines
| HPV vaccine type | Targeted HPV types | Diseases prevented | Recommended age at vaccination |
|---|---|---|---|
| Bivalent | HPV-16, HPV-18 | Cervical cancer, precancerous lesions | 9 years and older (up to 26 for females, 45 for males) |
| Quadrivalent | HPV-6, HPV-11, HPV-16, HPV-18 | Cervical cancer, precancerous lesions, genital warts | 9 years and older (up to 26 for females, 45 for males) |
| 9-valent | HPV-6, HPV-11, HPV-16, HPV-18, HPV-31, HPV-33, HPV-45, HPV-52, HPV-58 | Cervical cancer, precancerous lesions, genital warts | 9 years and older (up to 26 for females, 45 for males) |
Methodology
A comprehensive narrative review of HPV-induced cervical cancer was conducted from sources including Scopus, PubMed, and Google Scholar, with no restrictions on publication dates, to ensure a thorough exploration of both historical and recent advancements in HPV research. A structured search strategy employed keywords and Medical Subject Headings (MeSH) terms such as “human papillomavirus”, “HPV”, “cervical cancer”, “HPV vaccines”, AND “screening techniques”, utilizing Boolean operators (AND and OR). The inclusion criteria encompassed peer-reviewed studies focusing on the epidemiology, prevention, screening, and treatment of HPV-induced cervical cancer published in English, and articles not published in English and non-academic sources were excluded. A snowball search strategy was used to identify additional articles from the reference lists of the included articles. From the gathered articles, a thematic analysis was performed to synthesize recurring patterns, with the findings presented narratively under appropriate sections to highlight gaps in the study. However, it is important to note that, as a narrative review, this approach may introduce potential bias in the selection of studies, as it is dependent on the authors’ subjective judgment in choosing relevant literature. From the gathered articles, a thematic analysis was performed to synthesize recurring patterns, with the findings presented narratively under appropriate sections to highlight gaps in the study. The limitations of this methodology, including the risk of selection bias and lack of systematic review rigor, should be considered when interpreting the findings.
Overview of HPV and cervical cancer
HPV is a common sexually transmitted infection that most sexually active individuals frequently acquire at some point in their lives with no obvious symptoms. There are over a hundred different types of HPV, with varied degrees of risk, making up the virus. Approximately 40 of these types are spread through intercourse, and the remaining cause warts on the hands and feet [8]. These sexually transmitted HPV strains specifically target mucosal membranes, such as the damp regions surrounding the genitalia and anus. Not all HPVs that spread through sexual contact cause serious health problems. HPV is classified as a small, double-stranded DNA virus and is primarily divided into two groups: high-risk HPVs, which are associated with a variety of anogenital cancers, including cervical, anal, vulvar, vaginal, and penile cancers, and low-risk HPVs, which cause cutaneous and anogenital warts. Approximately 70% of cervical malignancies are caused by high-risk HPV strains such as HPV types HPV-16 and HPV-18. Other high-risk types are HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58 [9, 10]. Conversely, 90% of genital warts are caused by low-risk HPV strains such as types HPV-6 and HPV-11, which rarely result in cancer. These warts can develop weeks or months after intercourse with an infected partner, which usually resemble bumps and occasionally cauliflowers [8]. HPV infection is the primary cause of cervical cancer, which occurs six times more frequently in women living with HIV than in those without HIV infection [11]. Without treatment, persistent HPV infection in the cervix is responsible for 95% of cervical cancer cases [11, 12]. Several factors can affect cancer progression, including the carcinogenic potential of the HPV strain, an individual’s immune system health, co-infections with other sexually transmitted diseases (STDs), the number of childbirths, early age at first pregnancy, use of hormonal contraceptives, and other lifestyle or health-related factors [1].
Global impact of HPV-induced cervical cancer
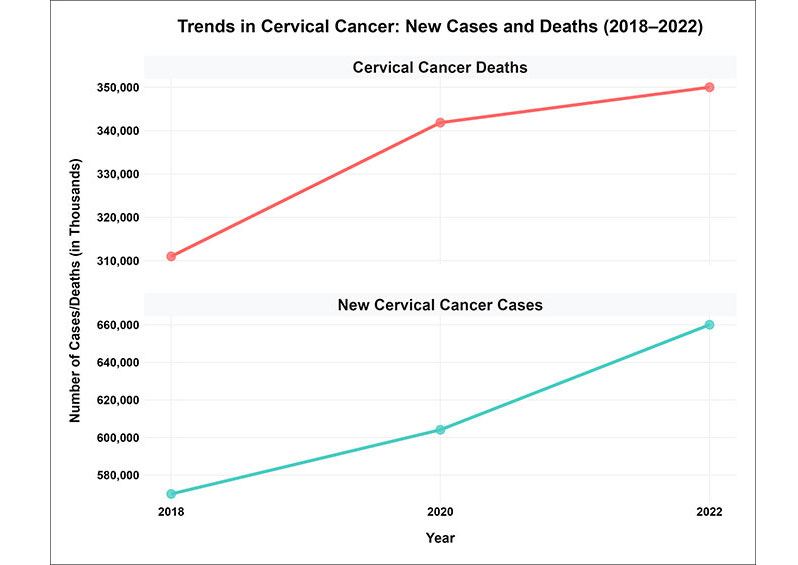
Cervical cancer remains a significant global health challenge, with the World Health Organization (WHO) reporting approximately 660,000 new cases and 350,000 deaths by 2022 (Figure 1) [13]. This marks an increase from earlier estimates, including 604,127 new cases and 341,831 deaths in 2020 [4], and 570,000 new cases and 311,000 deaths in 2018 [14]. This burden disproportionately affects regions such as SSA, China, and India, which bear a significant portion of cases. According to Martel et al. [15], cervical cancer accounts for approximately 90% of malignancies in women infected with HPV. By 2019, the number of female cancer cases associated with HPV infections had escalated to 620,000, highlighting the persistent threat posed by this disease [15]. Despite advancements in prevention strategies, including HPV vaccination, the burden remains high, particularly in LMICs. These regions face barriers, such as limited access to vaccination, cervical screening, and treatment facilities, which exacerbate health inequities. As of 2022, cervical cancer is the fourth most common cancer among women, necessitating the urgent need for globally coordinated efforts to enhance access to preventative measures and treatments, particularly in resource-limited settings [16].
Prevalence and mechanism of HPV infection leading to cervical cancer
The prevalence of HPV infection and its associated cervical cancer is alarming, particularly in middle-aged women [14, 17]. The geographic distribution of cervical cancer is highly uneven, with over 85% of deaths occurring in low-resource regions [18]. SSA has the highest incidence rate, accounting for > 90% of the global burden [19]. This regional disparity underscores the interplay among socioeconomic factors, healthcare access, and disease burden. HPV is the leading cause of cervical cancer and remains a significant global public health concern globally [20]. The prevalence of HPV among women with normal cervical cytology showed a slight increase from 10.4% in 2007 to 11.7% in 2010. In 2019, the global adjusted prevalence rate was 9.9%. In 2022, the burden of HPV-related disease will continue to grow, with an estimated 660,000 new cases and 350,000 deaths reported. Oceania and Africa had the highest prevalence rates at 21.8% and 21.1%, respectively, followed by Europe (14.2%), the Americas (11.5%), and Asia (9.4%) [21].
Cervical dysplasia refers to abnormal changes in cervical cells caused by persistent HPV infection. The severity of dysplasia is graded as follows: cervical intraepithelial neoplasia grade 1 (CIN1; mild dysplasia) involves minor abnormalities in a small portion of the tissue, which often resolve on their own. CIN2 (moderate dysplasia) and CIN3 (severe dysplasia) showed progressively more significant abnormal changes in cervical tissue. There is a greater chance that aberrant cells in CIN2 and CIN3 will develop cancer, requiring careful observation or excision. These cellular alterations were identified using a smear test and examined under a microscope [22]. HPV disrupts apoptosis, which is crucial for cell turnover and immune function, and impairs cell activity. HPV contributes to this process by integrating its genetic material into the DNA of cervical cells, thereby disrupting their normal function. The cervix, which connects the uterus to the vagina, consists of two main parts: the ectocervix and endocervix. The ectocervix, visible during gynecological examinations, is covered by thin, flat squamous cells, whereas the endocervix, located internally, is lined with glandular cells. These two cell types meet in the transformation zone, which is a critical site where cervical cancer often originates. These cells undergo malignant transformation [23, 24]. HPV proteins E6 and E7 are essential for the promotion of cervical cancer growth. The E6 protein binds to and disrupts an essential tumor suppressor protein in cervical cells. Next, this complex interacts with a different protein that prevents tumor growth. Consequently, the body’s attempt to eradicate HPV E6 unintentionally destroys this vital tumor-suppressive protein, increasing the risk of cancer development. The HPV E7 protein targets another tumor-suppressing protein, inhibiting its activity and increasing the likelihood of cancer development [25].
Types and stages of cervical cancer development
Cervical cancer is categorized into two main types based on the originating cell type: squamous cell carcinoma (SCC) (80–90% of cases) and adenocarcinoma (AC) (10–20% of cases). SCC arises from ectocervical squamous cells, whereas AC originates from the glandular cells of the endocervix [26]. Both types often develop at the squamocolumnar junction, which is a critical region of the cervix. Cervical cancer progresses through four stages, with treatment options varying by stage (Table 2). Stage 1 involves a localized disease that is treatable primarily through surgery. By stage 4, the cancer spreads to distant organs, requiring multimodal therapies including surgery, chemoradiotherapy, targeted therapies, and immunotherapy [27].
Stages of cervical cancer development
| Stage | Description | Treatment |
|---|---|---|
| Stage 1 | Localized disease | Surgery |
| Stage 2 | Cancer spreads to nearby tissues | Surgery, radiation therapy |
| Stage 3 | The cancer spreads further into the pelvis or to lymph nodes | Chemoradiotherapy |
| Stage 4 | Cancer spreads to distant organs | Multimodal therapies (surgery, chemoradiotherapy, targeted therapies, immunotherapy) |
Mechanisms of HPV infection and replication
Entry and initial infection
HPV infection begins with the virus targeting the basal cells, typically following microabrasions, and progresses through cellular differentiation in the parabasal layer. However, the specific receptors for HPV entries remain elusive [28]. Candidates such as heparan sulfate proteoglycans (HPSGs), syndecan (Sdc) types Sdc2 and Sdc4, and the annexin A2/S100A10 heterotetramer (A2t) have been proposed as critical mediators of viral attachment and entry [26–29]. Moreover, epidermal growth factor receptor (EGFR), α6-integrin, CD63, and tetraspanin CD151 [29] are cell membrane receptors linked to HPV binding. Additionally, HPV enters cells via endocytosis without the aid of dynamin, clathrin, caveolin, or lipid rafts [30]. Once internalized, HPV exploits the endocytic pathways to avoid degradation and facilitates trafficking to the nucleus.
HPV genome trafficking and cellular interaction
When HPV binds to HPSGs, the RG-1 epitope at the N-terminus of the L2 protein is exposed, causing the L1 protein to alter its shape and display L2 on the capsid surface. When the RG-1 epitope is cleaved upstream by furin protease, L2 attaches to the HPV episome and is trafficked downstream [31]. p120-catenin and γ-secretase are two examples of chaperones that L2 associates with throughout this trafficking phase [32, 33]. As such, L2 engages in various protein interactions during endosomal entrance to assist its introduction into vesicular membranes, ultimately enhancing the vesicular trafficking of the L2-HPV episome. To maintain the integrity of the L2-HPV episome, it has been shown that L2 interacts with sorting nexin 17 (SNX17) to limit vesicle acidification and prevent rapid lysosomal destruction of vesicle contents [34]. Furthermore, L2 binds to Vps26, Vps29, and Vps35, which are essential for retrograde trafficking of the L2-HPV episome to the trans-Golgi network (TGN) [4]. The L2-HPV episomes subsequently move from the endosomal compartments to the TGN, where they remain until mitosis begins [28].
Viral genome amplification and immune evasion
L2 aids L2-HPV episome transport along microtubules during mitosis through interactions with motor proteins Karyopherin alpha2 (KPNA2), dynein light chain (DYNLT3), and Ran-binding protein 10 (RanBP10). L2 then attaches mitotic chromosomes to enable L2-dependent chromosomal anchoring of the L2-HPV episome in open mitosis [35]. ND10/PML bodies, which are highly transcriptionally active areas, are where HPV episomes are located after being transported into the nucleus of daughter cells in a mitosis-dependent manner owing to this anchoring process [36]. The E6, E7, E1, and E2 genes are expressed upon entry into the nucleus and are essential for the initial phase of viral genome amplification. The progression of HPV-related cancers is closely associated with viral persistence and oncogenic activity. The expression of E6 and E7 oncogenes disrupts critical tumor suppressor pathways, leading to cellular transformation and uncontrolled proliferation. E6 degrades p53 through its interaction with E6-associated protein (E6AP), inhibiting apoptosis, and DNA repair, whereas E7 promotes the degradation of retinoblastoma protein (pRb), freeing E2F to drive cell cycle progression. This deregulation fosters genomic instability, which is a key step in the progression to malignancy [37, 38]. HPV transcription is triggered by p97 and p105 promoters of HPV-16 and HPV-18, respectively. By attaching to its response elements in the long control region (LCR), the DNA-binding domain of the E2 protein subsequently represses the transcription of early viral genes [39].
Concurrently, the E1 protein functions as an ATP-dependent DNA helicase and is thought to be the only encoder [40]. E1 and E2 work together to create hexameric complexes that attach to the replication origin, unwind DNA before the replication fork, and facilitate the recruitment of the replication machinery. To avoid immune surveillance and create chronic infections, the virus replicates its episomes at low copy numbers (50–100 episomes per nucleus) [41]. Further evidence suggests that E1 suppresses the expression of genes linked to immunological responses, such as IFNβ1, IFNλ1, and interferon-stimulated genes (ISGs) [42]. As the infection progresses, the integration of the HPV genome into the host cell’s genome often occurs, especially in high-risk HPV types. This integration results in deregulated E6 and E7 expressions, further amplifying oncogenic potential and contributing to the development of invasive cancer. The production of reactive oxygen species (ROS) and DNA strand breaks during integration exacerbate genomic instability and promote tumorigenesis. These events are hallmark features of HPV-driven cancers, including cervical, oropharyngeal, and anogenital cancers [42, 43]. The host cell division program affects HPV genome amplification and the production of E6 and E7 is required for mitosis in infected cells. E6 and E7 oncoproteins drive the HPV replicative cycle, and their activity is essential for oncogenesis and viral replication. More specifically, the E7 oncoprotein is vital for sustaining cell cycle progression and encouraging the G1 to S phase transition. This is accomplished by its interaction with the E3 ubiquitin ligase Cullin 2, which promotes the degradation of pRb and the tumor suppressor protein pRb [44].
These events allow transcription factor E2F to be freed from its inhibitor pRb, which promotes the expression of target genes, including cyclin E, cyclin A, and p16INK4A [44]. The creation of viral progeny, generally produced from bicistronic mRNA, is ensured by the activities of the viral oncogenes E6 and E7 in HPV-infected cells. Thus, E6 may balance the E7’s promotion of excessive cellular growth. E6 interacts with the tumor suppressor protein p53 and, when combined with E6AP, creates a complex that helps the proteasome to degrade p53. The function of p53 in apoptosis induction and DNA damage repair is compromised by this interaction [44]. Furthermore, E6 and E7 support the HPV replicative cycle by modulating many signaling pathways, such as PI3K/Akt, Notch, and Wnt/β-catenin [45]. Viral propagation is further aided by the E5 protein, which stimulates cell division in higher layers of the epithelium. E5 inhibits endosomal acidification and promotes recycling of the EGFR receptor through its interaction with the 16-kDa subunit of vacuolar ATPase (vATPase). Viral replication is accelerated by the activation of EGFR and its downstream effectors. Additionally, E5 helps in immune evasion by blocking the MHCI presentation of antigens. By interfering with the trafficking of MHCI heavy chains to the cell membrane indirectly, it accumulates in the endoplasmic reticulum and Golgi apparatus [46].
HPV episomes can multiply by hundreds of copies per cell during this stage of infection. The E4 protein is essential for breaking down the cytokeratin network, which makes cells more brittle and encourages the release of offspring virions [47]. It has been demonstrated that E4 causes keratin to become hyperphosphorylated and ubiquitinated at residues lysine 8 (K8) and serine 73 (S73). This suggests that these alterations aid the breakdown of keratin by proteasomes, which disrupts the network [48]. Viral capsid proteins are assembled in terminally differentiated cells and are ultimately driven by the late promoters, p670 and p811, in HPV-16 and HPV-18, respectively. An icosahedral shell of 360 L1 molecules organized into 72 pentameric capsomers, with L2 positioned in the center, encases the HPV genome [49]. The generation of DNA strand breaks, ROS, and genomic instability during infection can aid the integration of the viral genome into the host cellular genome. The deregulated expression of the E6 and E7 oncogenes is frequently the result of this integration, and this is a crucial step in the development of cervical cancer. Cellular transformation is caused by E6 and E7 interactions with tumor suppressor proteins and elements of cellular signaling pathways [50, 51].
Roles of viral oncoproteins
E1 and E2 proteins
The early phases of HPV infection are marked by the expression of E1 protein, which is highly conserved across HPV strains (Table 3). E2 proteins play a multifaceted role in viral replication and transcription while also influencing other critical processes. Depending on the promoter environment, E2 can either activate or repress viral gene expression, highlighting its regulatory complexity [52]. The two functional domains of E2 proteins are the C-terminal conserved domain, responsible for DNA binding and dimerization, and the N-terminal conserved domain, crucial for transactivation (TA) and DNA replication [53]. Together, the E1 and E2 proteins are indispensable for the initiation and regulation of HPV replication.
Roles of viral oncoproteins
| Oncoprotein | Function | Source |
|---|---|---|
| E1 | - Highly conserved across HPV strains.- Indispensable for initiating and regulating HPV replication. | [52] |
| E2 | - Multifaceted role in viral replication and transcription.- Influences other critical processes.- Can either activate or repress viral gene expression. | [53] |
| E4 | - Translated as an E1^E4 fusion protein.- Leucine cluster motif is crucial for keratin binding.- C-terminus facilitates self-association, forming structures reminiscent of amyloid fibers.- Plays a pivotal role in regulating the cytokeratin network to aid viral release and dissemination. | [54] |
| E5 | - Significant in HPV-mediated carcinogenesis.- Supports cellular hyperproliferation and cancer progression in both high-risk (HR-HPV) and low-risk (low-riskHPV) forms.- Influences E6 and E7 activities. | [55, 56] |
| E6 | - Relatively large (18 kDa).- Contains two zinc finger domains.- Primarily found in the nucleus.- Orchestrates the transformation of normal cells into malignant ones, significantly contributing to cervical carcinogenesis. | [57, 58] |
| E7 | - Approximately 100 amino acids long.- Comprises three conserved regions (CR1, CR2, and CR3).- A key player in cervical carcinogenesis through interactions with various host factors. | [59, 60] |
E4 protein
The HPV E4 open reading frame (ORF) is located within the E2 gene, specifically in the region coding for the flexible hinge domain of E2. Through alternative splicing, the E4 protein is translated as an E1^E4 fusion protein, incorporating the first amino acids from the E1 protein, including the start codon [54]. The leucine cluster motif in E4 is crucial for keratin binding, whereas its C-terminus facilitates self-association, forming structures reminiscent of amyloid fibers. These fibers play a pivotal role in regulating the cytokeratin network to aid viral release and dissemination [48].
E5 protein
Although β-HPV lacks the encoded E5 protein, the E5 oncoprotein remains significant in HPV-mediated carcinogenesis. Found in the endoplasmic reticulum, E5 is a small, 83-amino-acid, hydrophobic, membrane-bound protein encoded at the 3´ end of the early region of the viral genome and expressed from spliced mRNA [55]. According to Moody and Laimins [50], E5 comprises three domains: N-terminal, central, and C-terminal domains. Its N-terminal domain is highly hydrophobic and supports cellular hyperproliferation and cancer progression in both high-risk HPV and low-risk HPV forms. Additionally, E5 influences E6 and E7 activities, amplifying their oncogenic potential [56].
E6 protein
Compared with other HPV proteins, the E6 protein is comparatively large (18 kDa) and approximately 150 amino acids in length. It contains two zinc finger domains, each characterized by four Cys-X-X-Cys motifs. E6 has C- and N-terminal domains and is primarily found in the nucleus. Interactions between the PDZ-binding motif in the C-terminal domain and various cellular proteins affect numerous cellular activities [57]. HPV genome integration into the host genome frequently disrupts the E2 gene, a negative regulator of E6 transcription, resulting in E6 upregulation. E6, in conjunction with E7, orchestrates the transformation of normal cells into malignant cells, thereby significantly contributing to cervical carcinogenesis [58].
E7 protein
The E7 protein, which is approximately 100 amino acids long, comprises three conserved regions: CR1, CR2, and CR3. CR1 resembles the T antigens of SV40 and adenovirus, whereas CR2 shares homology with the related viral proteins. CR3, which is common to all HPV types, encodes a zinc finger domain containing two Cys-X-X-Cys motifs separated by 29 amino acids [59]. E7 plays a key role in cervical carcinogenesis by interacting with various host factors [60].
Prevention and control measures
Gardasil was the first HPV vaccine approved by the United States Food and Drug Administration (FDA) in 2006, which was developed by the pharmaceutical company Merck & Co., Inc. [61, 62]. Six licensed vaccines are currently available, marking a significant milestone in preventing cervical cancer and other HPV-related diseases [63]. As of 2024, WHO reported 144 nations out of the 194 WHO Member States to have incorporated HPV vaccination into their national vaccination programs, of which 27 countries are from Africa, while 34 countries have launched cervical cancer surveillance schemes in Africa as reported by WHO in 2023 [64, 65].
Administering these vaccines before the initiation of sexual activity is optimal, although they can also be effective after HPV exposure. These vaccines are prepared using recombinant DNA technology and cell culture techniques, incorporating pure L1 structural proteins that self-assemble into virus-like particles (VLPs) specific to HPV types. They do not contain active biological agents or viral DNA, thus ensuring that they cannot transmit HPV. The formulations include adjuvants but are free from preservatives and antibiotics [6, 66]. The nonavalent HPV vaccine includes VLPs targeting high-risk types HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58. Both nonavalent and quadrivalent vaccines protect against anogenital warts caused by HPV types HPV-6 and HPV-11. All HPV vaccines incorporate VLPs into high-risk types HPV-16 and HPV-18. Vaccination is recommended for girls aged nine years and above, with approval extending to age 26 for females and 45 for males [67]. HPV vaccination effectively prevents premalignant cervical lesions and malignancies by targeting various high-risk HPV strains. Prefilled syringes and single- or double-dose vials can be used for vaccine administration. The inclusion of adjuvants in these vaccines is crucial because they enhance the immune response and ensure long-lasting immunity. The adjuvants help boost the body’s immune reaction to VLPs, thereby improving the overall effectiveness of the vaccine [68]. To ensure safety, quality, and effectiveness, WHO has established stringent requirements, including nucleic acid-based assays and type-specific criteria for anti-HPV type HPV-16/18 serological testing. These guidelines assist manufacturers and regulatory agencies in producing and evaluating high-quality vaccines [58]. Currently, HPV vaccination programs are included in national immunization policies in 125 countries (64%) for girls and in 47 countries (24%) for boys. Notable progress has been made in expanding access to vaccinations worldwide [68]. This widespread implementation highlights the global commitment to reducing HPV-related diseases and is detailed in WHO’s comprehensive vaccination data [69].
Vaccine efficiency and coverage rates
Licensed HPV vaccinations are most effective in those not already infected based on clinical trials before their licensure [70]. The effectiveness of these vaccines decreases with increased sexual activity and potential for HPV exposure. While individuals as young as nine years old were recruited in research assessing antibody responses, young women between the ages of 15 and 26 made up the bulk of trial participants. Because of the lack of information regarding the safety and effectiveness of HPV vaccination in children under the age of nine, younger children might not receive these shots [71]. This initiative aligns with the WHO’s Global Strategy to Accelerate the Elimination of Cervical Cancer, aiming to increase HPV vaccination rates and reduce cervical cancer incidence worldwide. Since the establishment of a Global Strategy to Accelerate the Elimination of Cervical Cancer, 30 additional countries, including high-risk regions such as Bangladesh, Indonesia, and Nigeria, have introduced the HPV vaccine.
Licensed HPV vaccinations are most effective in individuals not previously infected, as demonstrated by clinical trials conducted before their licensure [64]. Vaccine effectiveness diminishes with increased sexual activity and potential HPV exposure. While antibody response studies included participants as young as nine years old, the majority of clinical trials focused on young women aged 15 to 26. Due to limited data on the safety and efficacy of HPV vaccination in children under nine years, vaccinations are typically not administered to this age group [71]. In alignment with the WHO’s Global Strategy to Accelerate the Elimination of Cervical Cancer, over 30 additional countries, including high-burden regions such as Bangladesh, Indonesia, and Nigeria, have introduced HPV vaccination programs to reduce the incidence of cervical cancer and address the disparities in vaccine access globally [72].
Currently, 140 countries include HPV vaccination in their national immunization programs [70, 71]. In 2022, 21% of girls worldwide received at least one dose of the HPV vaccine, marking a recovery of immunization rates to pre-pandemic levels. Reaching the target of immunizing every girl in the world against HPV by 2030 is possible if the current rate of advancement keeps up [73]. To attain a 90% coverage objective and manage pandemic-related disruptions, the WHO partnered with the Global Alliance for Vaccines and Immunization (GAVI) and other partners to launch systematic activities. The goals of these initiatives are to speed up catch-up immunizations, revive HPV vaccination programs, and reach girls who missed their vaccinations due to pandemic disruptions [71, 74]. For instance, Nigeria added the HPV vaccine, which comes in a single dosage, to its regular immunization schedule in October 2023. The government of Nigeria intends to vaccinate 7.7 million girls, making it the most extensive HPV vaccination campaign in the area in a single dose [75].
Challenges and innovations in HPV-related cervical cancer prevention and treatment
Standard treatment facilitators include bringing treatment closer to patients and providing transportation or vouchers. The cost of transportation and the distance to the medical facility, along with other practical obstacles, prevented women from obtaining therapy, with most of them ignorant of essential details, including making an appointment for follow-up [76, 77]. Following their interviews, many women sought additional information about their treatment, including the hospital to visit, the cost of therapy, and what to expect. Within the healthcare system, some women have claimed to have encountered logistical challenges [78, 79]. This study draws insights from multiple countries, highlighting both shared and context-specific challenges in accessing HPV-related cervical cancer treatment [78, 80]. While certain barriers—such as transportation difficulties and misinformation—are common across different settings, the extent of their impact varies depending on healthcare infrastructure, socioeconomic factors, and public health policies [81, 82]. Often, participants were unclear about the role of HPV in cervical cancer. During the in-depth interviews (IDIs), assumptions emerged, rather than openly admitting ignorance. Misinterpretation of test results and treatment plans often prevents women from receiving the appropriate care [77, 80]. For example, myths include the idea that women should not undergo treatment for HPV throughout their reproductive years and uncertainty about the nature of the treatment, such as whether daily medicine similar to HIV is required [77, 81]. Frequent questions included, “Is HPV curable?” and “Does being HPV positive equate to a cervical cancer diagnosis?” These misconceptions highlight the important obstacles to successful cancer therapy and prevention. By analyzing data from different healthcare settings, this study underscores the need for tailored intervention strategies that consider country-specific healthcare policies and cultural perspectives [83, 84]. Addressing these diverse barriers is essential for improving HPV-related cervical cancer prevention and treatment globally [85]. Throughout the IDIs, fear surfaced as a common topic. This included anxiety related to screening, results (“I understood my result, but still felt some fear”), cancer, disease, and treatment [82]. In particular, participants’ anxiety about receiving a cancer diagnosis prevents them from thinking about available treatment alternatives [77, 82]. It is important to note that while these issues were frequently raised, the study’s findings do not necessarily suggest that all women experience these barriers in the same manner. These interpretations reflect a broad understanding of the challenges, but the data do not directly establish the extent to which each factor contributes uniquely to treatment delays or misconceptions. For example, while anxiety about diagnosis is significant, the study did not provide direct evidence linking this fear to treatment avoidance across all participants [83, 84].
Technological advances in the diagnosis and treatment of HPV-induced cervical cancer have dramatically improved the diagnostic and therapeutic outcomes [85]. Next-generation sequencing (NGS) is an advanced tool in genomics research that simultaneously sequences millions of DNA fragments and offers detailed insights into genome structure, genetic variations, and gene activity. Recent advancements in NGS have emphasized faster and more accurate sequencing, reduced costs, and enhanced data analysis, offering significant breakthroughs in disease understanding and personalized healthcare [86]. CRISPR/Cas is a fast, cost-effective, and efficient gene editing strategy. CRISPR/Cas can knock out the E6 and E7 oncogenes in HPV, reactivate tumor suppressor pathways (p53 and Rb), and inhibit cancer cell growth [87]. Despite preclinical success, challenges, such as vector delivery and off-target effects, remain. These issues can be addressed by liposome packaging, improved bioinformatics tools, and advanced sequencing methods. The future of CRISPR/Cas in cancer treatment is promising, with ongoing trials for various diseases, including the first CRISPR-based HPV treatment focusing on editing the E6 and E7 oncogenes [88–90]. However, although the potential of CRISPR/Cas and NGS is promising, these findings should be interpreted with caution. Current evidence primarily stems from preclinical studies, and further clinical trials are necessary to directly demonstrate the efficacy and safety of these technologies in treating HPV-induced cervical cancer. As research progresses, the real-world application of these innovations will become clearer, although they offer considerable hope for future cancer treatments [85, 90].
Furthermore, novel imaging techniques such as optical coherence tomography (OCT) and reflectance confocal microscopy (RCM) have improved the visualization and assessment of cervical lesions, allowing for early identification and accurate diagnosis [80]. The accurate determination of the size and depth of melanoma infiltration is vital. However, the current techniques have limitations. This study introduces a combined 18 MHz ultrasound and photoacoustic tomography device that provides high-resolution 3D skin lesion images. The device strongly correlates with histological measurements, highlighting its potential as a non-invasive diagnostic tool [91]. These technological breakthroughs are critical for improving the treatment and outcomes of HPV-induced cervical cancer. Healthcare delivery and medical practice can undergo significant changes owing to the powerful and innovative field of artificial intelligence (AI) in computer science [92]. The healthcare industry stands to benefit greatly from AI, which can improve patient outcomes, personalize treatment programs, and increase the accuracy of diagnoses. AI algorithms outperform traditional methods in analyzing large datasets, identifying patterns, and predicting disease progression. AI aids cancer detection, prognosis, and treatment planning by examining complex medical imaging and genomic data [93]. Furthermore, AI-powered technologies can improve clinical workflows, minimize administrative costs, and facilitate decision-making, resulting in more efficient and effective healthcare delivery. As AI technology progresses, its incorporation into various healthcare applications promises to transform patient care and medical research. While AI’s potential is significant, the currently available data focus largely on theoretical applications and preliminary studies. The actual impact of AI on cervical cancer diagnosis and treatment requires further empirical validation in a clinical setting. Therefore, while AI offers substantial promise, the broad claims about its transformative effects should be approached with careful consideration of existing limitations and ongoing research [92, 94, 95].
Screening, detection, and public health strategies for HPV-related cervical cancer
HPV infections, which are oncogenic, account for most cervical malignancies. These infections can cause premalignant lesions to develop, ultimately leading to invasive carcinomas [17]. Nearly all cervical malignancies are caused by high-risk HPV infections. Pap smears are used in cervical cancer screening to identify cytological alterations in early HPV infection [96]. Cytological alterations include abnormal cells and precancerous and malignant tumors. When a Pap smear indicated the possibility of cancer, HPV DNA testing was performed either as a follow-up or by co-testing using the same cytology sample. Screening sensitivity and specificity have been improved using this method [96]. Cervical SCC (CSCC) (approximately 70%), cervical AC (CAC) (approximately 25%), and mixed histological tumors (30%) are histologically linked cervical malignancies associated with HPV. In contrast, less than 1% of newly diagnosed cases are non-HPV-associated cervical malignancies, including cervical neuroendocrine carcinoma, small-cell carcinoma, and large-cell carcinoma [96]. In contrast to AC, which develops from glandular epithelial cells of the endocervix, SCC is derived from squamous epithelial cells of the ectocervix [97]. According to recent research, the incidence of SCC is decreasing, whereas that of AC is increasing in several nations [87, 88]. The cohort effect and less effective cytological screening for AC explain the increase in AC incidence among younger women [97]. Although there is growing evidence that patterns of dissemination, prognostic variables, treatment outcomes, and AC epidemiology differ from those of SCC, it can be hypothesized that these differences are due to evolving screening practices. Despite these variations, cervical cancer is classified and treated using similar protocols [98].
By classifying invasion into three separate patterns, the Silva-classification approach has been used to diagnose CAC associated with HPV [98, 99]. Although HPV analysis and p16 immunohistochemistry (IHC) are not necessary for diagnosis or categorization, p16 expression is regarded as a surrogate marker for HPV association [99]. CSCC associated with HPV is frequently associated with precancerous lesions, and no precancerous lesions have been reported in infrequent instances of non-HPV-related CSCC [99]. HPV-related CSCC was verified by HPV DNA testing according to WHO recommendations. However, p16 IHC is also recommended because there is limited morphological differentiation between HPV-related and non-HPV-related cases [99]. Cervical cancer is believed to progress on a continuum, starting with moderate CIN1, moving on to microinvasive lesions and more severe neoplasia (CIN2 or CIN3), and ending with invasive disease [100]. The data indicate that while CIN1 is common, the direct progression from CIN2 or CIN3 to invasive cancer remains a subject of ongoing research, with some data suggesting that this progression may be influenced by other factors, such as immune response or co-infections [101, 102].
Cervical cancer often shows no early symptoms, leading to a delayed diagnosis. Early diagnosis through screening is essential to identify precancerous cellular alterations before the complete development of cancer. According to previous studies, HPV-infected cervical cells can develop into precancerous lesions over five to ten years. High-risk HPV strains, which are precursors of cervical cancer, can be detected using HPV testing [103]. Cervical cancer is usually easier to cure if discovered early. If symptoms appear later than expected, treatment may become more challenging for managing cervical cancer [104]. According to the American Cancer Society, cervical cancer screening and testing should begin at approximately 25 years. People between the ages of 25 years and 65 years should undergo a primary HPV test every five years. Screening may differ from the criteria mentioned above for various reasons, including an individual’s unique risk factors and medical history [104]. Although test choices are becoming increasingly individualized, individuals must be examined for cervical cancer regularly regardless of the test they choose. WHO recognizes HPV-related illnesses, especially cervical cancer, as major global health concerns. WHO recommends that routine HPV vaccination should be included in national immunization programs. This recommendation is subject to several conditions: public health priorities should include HPV-related disease prevention, vaccine implementation should be feasible, sustainable funding should be available, and local vaccination strategies should be cost-effective. The follow-up Pap test recommendations vary from every 3–6 months to annually or every three years. The varying intervals for follow-up testing are based on factors such as the initial test results, although there is debate regarding the optimal frequency, which may vary by region and population. Doctors can increase women’s adherence to follow-up testing by talking to them in a way that considers their sociocultural context, asking about worries about their partners’ reactions, stressing the value of follow-up, and ensuring that they understand the procedure and timetable for follow-up recommendations [105]. Knowledge is a critical predisposing factor for changing behavior and has a significant impact on health-seeking behaviors. Acquiring knowledge could potentially improve attitudes, disbelief, and misconceptions significantly as well as improve screening [106]. Healthcare practitioners play a key role in raising awareness and providing information that improves screening adherence [107].
Data from the 2016 Centers for Disease Control National Immunization Survey-Teen (NIS-Teen) show that compared to 59.3% of teenagers living in non-metropolitan statistical areas (non-MSAs), 70.1% of teenagers living in MSAs’ main cities had received one or more doses of the HPV vaccine [108]. Limited access to healthcare in rural areas contributes to lower vaccination rates [109]. In addition to misinformation, one of the most common barriers was discovered in a study of teenagers in rural regions who had not received vaccinations. Prompt advice from clinicians is essential for initiating vaccinations because it encourages many patients to adopt healthy practices [110, 111]. Although these findings are consistent with the observed trends, it is still unclear whether targeted education campaigns in rural areas will effectively overcome these barriers, as other factors such as vaccine accessibility may also play a significant role [112]. Notably, even though this is a strong predictor of HPV vaccine series completion, 66% of physicians said that they did not have enough time to lecture parents and teenagers about the HPV vaccine [113]. A community-based strategy may address some of these challenges, as it is not reliant on patients attending clinical sessions. Community-based interventions such as educational media can motivate patients to be vaccinated [114, 115].
These interventions, which can be designed and implemented by educators, public health workers, nurses, or counselors who reside in or are not affiliated with these communities, focus on removing barriers related to inadequate education or misinformation and the provider’s lack of time for education [116]. Community-based education programs have the potential to be more successful in debunking myths and highlighting the importance of vaccinations because they are not restricted by time or location. Thus, community-based educational initiatives may work better in rural regions than in cities [117]. Most research on HPV vaccines has focused on individual, parent, and provider interventions; however, community-based interventions have not been well studied in this sector. Additionally, studies contrasting community-based interventions in rural and urban environments are lacking [92]. The stigma, scarcity of healthcare providers, and restricted access to education that characterize rural areas may make community-based therapies more advantageous than those in cities, although they are still understudied. While some studies suggest that community-based education may be more effective in rural areas, it remains to be seen whether these interventions can be successfully scaled to larger urban populations. To have a meaningful economic impact on public health, we must determine the relative efficacy of community-based education programs in rural and urban environments [116, 118].
A limited number of studies utilizing community-based education interventions have been conducted in the last ten years to boost HPV vaccine uptake or intention to take up in towns and cities. Community-based interventions include radio, newspapers, video advertising, websites, social media campaigns, educational sessions, school-based reminders, and radionovelas [119, 120]. Based on variables such as vaccination uptake, willingness to vaccinate, information gained, and/or impression of reduced barriers to vaccination, nine of the ten treatments reported in the literature showed noteworthy efficacy effects. Although promising, it is necessary to recognize that the long-term sustainability of these interventions remains uncertain, and further studies are needed to evaluate their impact across different regions and populations. Of these, eight were conducted in rural regions. The efficacy of community-based treatments in rural and urban settings has not yet been compared.
Recommendations for future directions
HPV-induced cervical cancer is a global public health concern, especially in LMICs, which have limited access to prevention and treatment. Despite advances in HPV vaccines and screening, significant gaps remain in the prevention, diagnosis, and treatment of cervical cancer, particularly for individuals with advanced disease. A comprehensive approach is necessary, aligning with the WHO’s Cervical Cancer Elimination Initiative (CCEI) pillar 3, which emphasizes the treatment of invasive cervical cancer using chemotherapy, surgery, and radiotherapy (RT) [121, 122]. Recent studies have highlighted the need for public health education, technological advancements, and global collaboration [123]. The following guidelines address these issues and provide a complete plan to mitigate the effects of HPV-induced cervical cancer. Focusing on development, technological advancements, AI potential, and firm policies can significantly reduce the disease burden. Additionally, efforts to strengthen treatment capacity for those exposed to HPV without vaccination and for individuals with advanced disease should be prioritized.
Policy recommendations and global initiatives
Policy suggestions and global activities are critical for the successful treatment of HPV-induced cervical cancer (Figure 2). Governments should adopt and enforce comprehensive HPV vaccination programs to ensure widespread coverage and accessibility to all eligible populations. Equally important is the establishment of national and regional centers of excellence for cervical cancer treatment, enabling access to chemotherapy, surgery, and RT for advanced disease management [124]. International collaboration is essential, with organizations such as WHO driving efforts to standardize screening techniques, improve healthcare infrastructure, and fund research into novel diagnostics and treatments. Policies should also include public education initiatives to raise awareness of HPV prevention and the significance of frequent screening [125]. Funding should support low-cost HPV vaccines and diagnostics, particularly for low-income areas. Incorporating treatment strategies into global initiatives will bridge the gap in cervical cancer care and ensure equitable access to life-saving interventions, even for patients with invasive diseases. These integrated efforts have the potential to drastically reduce the incidence and mortality rates of cervical cancer all over the world [126].
Research and development
Research and development (R&D) is critical in the fight against HPV-induced cervical cancer. Efforts should focus on the development of new treatments, vaccines, and diagnostics, with an emphasis on comprehensive strategies that address the full spectrum of disease progression, including advanced cases [108]. Technologies such as CRISPR, NGS, and improved immunotherapy are promising and should be integrated into therapeutic frameworks. Furthermore, the exploration of therapeutic combinations involving chemotherapy, surgery, and RT is vital for managing invasive cervical cancer, particularly for patients who were not vaccinated or presented with advanced disease [127, 128]. Investigating the molecular mechanisms, including the pathways of HPV-mediated carcinogenesis, will pave the way for targeted therapies and novel preventative approaches. Collaborative R&D activities, backed by solid funding and international alliances, should prioritize translating scientific findings into clinical applications, such as personalized medicine and improved treatment regimens, to significantly enhance patient outcomes [88, 89, 129].
Emerging treatments and vaccines
Emerging therapies and vaccines are critical for the treatment of HPV-related cervical cancers. Immunotherapies and targeted gene-editing technologies such as CRISPR/Cas9 hold promise for selectively targeting and eradicating HPV-infected cells [130]. Furthermore, advances in vaccine research have broadened protection against a broader spectrum of HPV strains, increasing the efficacy of preventative measures. New vaccinations are being developed to provide longer-lasting protection, make administration more accessible, and expand demographic coverage. Continued research and clinical trial investments are required to bring these new medicines and vaccines to wider clinical usage, reducing the worldwide cervical cancer burden [88–90].
Innovative screening techniques
Innovative screening methods for HPV-induced cervical cancer have revolutionized early identification and treatment. Liquid-based cytology and high-risk HPV DNA testing improve the accuracy and reliability of screening programs, allowing for the earlier detection of precancerous lesions. Furthermore, developments in molecular diagnostics, such as NGS and digital colposcopy, provide precise genetic insights and improve the visibility of cervical abnormalities [131]. Self-sampling methods are gaining popularity, as they allow women in resource-constrained environments to participate in screening programs. While screening innovations address early detection, it is critical to integrate strategies for diagnosing and staging advanced cervical cancer, enabling timely and effective treatment interventions. These developments are crucial for lowering the incidence and mortality of cervical cancer using effective and accessible screening methods [90, 132].
Global collaboration
Global collaboration is critical in the fight against HPV-induced cervical cancer, affecting research, policy, and health care delivery. A global partnership allows for the sharing of knowledge, resources, and best practices for prevention, screening, and treatment [133]. Collaborative programs, such as the GAVI and the WHO’s Global Strategy to Accelerate Cervical Cancer Elimination, aim to increase vaccine coverage, particularly in low-income countries [134]. Multinational research consortiums also help produce breakthrough diagnostic tools and cures suited to different global situations. These coordinated efforts strengthen healthcare systems and promote equity in cervical cancer prevention and care with the ultimate objective of eliminating cervical cancer globally [135].
International partnership and funding
International partnerships and funding are pivotal for addressing the global burden of HPV-induced cervical cancer. Collaborative efforts among governments, non-governmental organizations, and international health agencies facilitate the sharing of expertise, resources, and technology essential for prevention, screening, and treatment initiatives. Global cervical cancer management efforts are governed by recommendations established by organizations such as the International Agency for Research on Cancer (IARC) and WHO, which are crucial for coordinating research objectives [136, 137]. Initiatives such as the Global Fund to Fight AIDS, tuberculosis, and malaria, and bilateral aid programs provide financial support for the construction of screening infrastructure, procurement of vaccines, and creation of healthcare capacity in low-resource settings. These collaborations highlight the importance of working together to accomplish global health objectives and lower the incidence and mortality rates of cervical cancer [138, 139].
Unified strategies to combat HPV-induced cervical cancer
Unified measures to combat HPV-induced cervical cancer include vaccination, improved detection technologies, and increased access to treatment. Vaccination programs that target HPV strains, such as HPV types HPV-16 and HPV-18, which are most associated with cervical cancer, are critical for primary prevention. Integrating these programs into national immunization schedules worldwide, particularly in LMICs, has the potential to drastically lower future cancer burden. At the same time, advances in screening tools, such as HPV DNA testing, and cytology-based approaches, such as Pap smears, ensure the early detection and treatment of precancerous lesions. Access to cost-effective therapeutic alternatives, including surgical treatment, chemotherapy, and RT, particularly brachytherapy, must be prioritized as essential components in reducing mortality rates, especially for advanced and invasive cases of cervical cancer [85, 140].
Conclusions
The global prevalence of HPV infections is alarming, particularly in low-resource areas, such as SSA, where over 85% of cervical cancer deaths occur. HPV-related cervical dysplasia progresses to cancer through viral proteins E6 and E7, disrupting key tumor suppressor functions. This disparity highlights the need for effective public health interventions such as vaccination and early screening programs in underserved areas. Policymakers should prioritize HPV prevention, screening, and treatment strategies, particularly in regions with limited access to healthcare, to reduce cervical cancer mortality and improve health outcomes. HPV vaccination is effective in preventing cervical cancer and anogenital warts, but challenges remain in low-resource settings owing to logistical barriers, misinformation, and anxiety about cancer diagnoses. Innovations, such as CRISPR/Cas gene editing, NGS, and AI-powered diagnostic tools, hold promise for improving cervical cancer treatment and prevention. Community-based educational initiatives can enhance vaccine uptake and reduce misinformation. The global fight against HPV-induced cervical cancer requires comprehensive approaches, including improved vaccination, screening, and treatment accessibility, particularly in low-resource settings. Emphasizing public health education, AI advancements, and international collaboration, along with investing in emerging therapies and diagnostics, can significantly reduce cervical cancer incidence and mortality, particularly in low-income countries.
Abbreviations
| AC: | adenocarcinoma |
| AI: | artificial intelligence |
| CAC: | cervical adenocarcinoma |
| CIN1: | cervical intraepithelial neoplasia grade 1 |
| CSCC: | cervical squamous cell carcinoma |
| E6AP: | E6-associated protein |
| EGFR: | epidermal growth factor receptor |
| GAVI: | Global Alliance for Vaccines and Immunization |
| HPSGs: | heparan sulfate proteoglycans |
| HPV: | human papillomavirus |
| IDIs: | in-depth interviews |
| IHC: | immunohistochemistry |
| LMICs: | low- and middle-income countries |
| NGS: | next-generation sequencing |
| pRb: | retinoblastoma protein |
| R&D: | research and development |
| ROS: | reactive oxygen species |
| RT: | radiotherapy |
| SCC: | squamous cell carcinoma |
| Sdc: | syndecan |
| SSA: | Sub-Saharan Africa |
| TGN: | trans-Golgi network |
| VLPs: | virus-like particles |
| WHO: | World Health Organization |
Declarations
Author contributions
RAA: Conceptualization, Data curation, Methodology, Supervision, Writing—review & editing. BOA: Data curation, Methodology, Writing—review & editing. ARA: Data curation, Writing—review & editing. MMA: Methodology, Validation, Writing—review & editing. OJO: Project administration, Data validation, Methodology, Writing—review & editing. TK: Data curation, Writing—review & editing. BMU: Data curation, Supervision, Writing—review & editing.
Conflicts of interest
Not applicable.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
The authors have not received any funding for this study.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.