Abstract
Aim:
This study aimed to evaluate the efficacy of carboxymethyl cellulose (CMC)-based natural mixture of aloe vera gel and coconut oil as a salivary substitute in comparison to the conventional CMC for the management of xerostomia in a sample of patients with Sjogren’s syndrome (SS).
Methods:
This crossover randomized clinical trial included 24 patients diagnosed with SS. Half the patients started with the study treatment (A-Gel) for 2 weeks, then went through a 7-day washout period, then received the control treatment (B-Gel), while the other half had the order reversed. The measured outcomes were subjective oral dryness (Bother 1’ xerostomia index), modified xerostomia quality of life scale (XeQoLS), clinical oral dryness (CODs), and salivary candidal load.
Results:
With a mean age of 46.96 ± 11.86 years, all patients were female and suffered from mouth dryness for 16.58 ± 25.32 months. In light of our findings, both groups’ Bother 1’ xerostomia index values recorded at different intervals showed significant improvements. There was a p < 0.001 difference between the mean value for groups A and B at baseline (7.33 ± 2.39 and 7.29 ± 2.66), one week later (4.17 ± 2.24 and 4.25 ± 2.57), and two weeks later (2.83 ± 2.08 and 2.88 ± 2.07). However, there was no significant difference between the groups. Likewise, for CODs and modified XeQoLS, no significant difference was found between groups; however, both groups showed a statistically significant improvement (p < 0.001). Improvement in the modified XeQoLS was observed in all domains (physical functioning, pain & clinical acceptance). Regarding candidal load, there was no statistically significant difference between groups or even within groups (p > 0.05).
Conclusions:
As salivary substitutes, the CMC natural mixture (aloe vera, coconut oil) and the conventional CMC-based oral gel are equally efficient at minimizing xerostomia symptoms and enhancing SS patients’ quality of life with minimal side effects. Saliva’s natural effect might be substituted with a natural mixture of coconut oil, aloe vera gel, and CMC. The trial is registered on clinicaltrials.gov, identifier: NCT04252209.
Keywords
Sjogren’s syndrome, salivary substitute, aloe vera gel, carboxymethyl cellulose, xerostomiaIntroduction
Saliva is important for maintaining oral health. Alteration in the quantity or quality of saliva can bring about a sensation of oral dryness known as xerostomia [1]. Xerostomia is linked to multiple etiologies. However, it most frequently develops as an adverse effect of medication, secondary to head and neck radiation therapy, and due to Sjogren’s syndrome (SS) [2].
SS is a multisystem autoimmune disease of unknown etiology characterized mainly by hypofunction of salivary and lacrimal glands and maybe with other systemic extra-glandular manifestations [3]. Along the course of the diseases, there is lymphocytic infiltration of the salivary glands leading to marked changes in the salivary composition and salivary flow rate, which negatively affect several main functions of saliva [4].
Xerostomia can result in functional changes like difficulty in speaking, chewing, and swallowing, as well as prosthetic problems like denture instability, intraoral halitosis, and oral mucosal changes like angular cheilitis, burning sensation, fissured, and erythematous tongue. Prolonged dry mouth in SS patients may increase the risk of plaque accumulation, dental caries, and erosions as saliva lacks anti-cariogenic effects. They are also more susceptible to fungal infections, particularly oral candidiasis, considering their diminished salivary volume with reduced levels of antimicrobial agents such as lactoferrin, IgA, and salivary proteins and peptides. The patient’s quality of life is negatively affected by these signs and symptoms [4].
Management of xerostomia by topical therapy involves the use of sialagogues that enhance normal salivary secretion and saliva substitutes that replace missing normal saliva. However, along the progressive course of SS, salivary glands will not respond to sialagogues and need salivary substitutes to control symptoms of oral dryness [5]. In 2019, the EULAR study group stated that the first therapeutic approach for dryness should be symptomatic relief using topical therapies [6].
Saliva substitutes containing carboxymethyl cellulose (CMC) are better at enhancing oral lubrication and decreasing the severity of symptoms associated with xerostomia with negligible side effects [7]. However, it does not completely resemble the properties of human saliva, based on the extensive studies on CMC [8].
Complementary and alternative medicine has been more recognized than conventional medicine regarding its natural, safe properties. Natural products such as coconut oil and aloe vera have antioxidant, anti-plaque, antibacterial, and antifungal properties and are also useful for soothing oral tissues. These products are easily available at reasonable prices with negligible side effects [9–11].
Aloe vera has a positive effect on controlling gingivitis and plaque accumulation [12]. It also inhibits the cyclo-oxygenase pathway, which downregulates the generation of prostaglandin E2 from arachidonic acid and contributes to its anti-inflammatory effects. Aloe vera has been discovered to promote wound healing by increasing the migration of epithelial cells and the synthesis of hyaluronic acid and dermatan sulfate in the granulation tissue of the lesion [13]. It has also been shown to play an important role in the management of oral lesions, such as oral lichen planus, oral submucous fibrosis, radiation-induced mucositis, and burning mouth syndrome (BMS) [14].
Aloe vera was used as a salivary substitute for many years; oral preparations of aloe vera were found to be efficient in reducing symptoms related to xerostomia [15–17]. Atashi and his co-authors investigated the effects of Veramin moisturizing gel [aloe vera jelly, peppermint essential oil, CMC, propylene glycol (PG), and potassium sorbate] on mouth dryness and oral health among intubated patients in ICU [16]. Study findings suggested that Veramin moisturizing gel was more effective than plain CMC in relieving mouth dryness, preventing dental plaque formation, and improving oral health. Unfortunately, aloe vera is a bitter substance, although it has promising properties [18].
Many studies have shown that coconut oil is one of the most popular oils for maintaining oral health. In coconut oil, lauric acid, monolaurin, and other ester derivatives have a significant role in antibacterial activity. The major antimicrobial mechanisms proposed include cell membrane destruction by physicochemical processes. Also, inhibition of microbial signal transduction and transcription [19]. Coconut oil has antiplaque and anti-cariogenic effects; it has 92% saturated medium-chain fatty acids. Its glucolipid component, sucrose monolaurate has an anti-cariogenic property due to reduced glycolysis and sucrose oxidation on S. mutans, which prevents plaque regrowth [20].
Based on previous studies, we assumed that coconut oil would enhance the properties of aloe vera and act as a flavoring agent. It would additionally mask up the bitterness of aloe vera. We formulate a mixture of CMC with aloe vera and coconut oil to enhance the efficacy of CMC to resemble the natural properties of saliva by augmenting its lubricant, antimicrobial, and anti-inflammatory effects.
To the best of our knowledge and based on the last systematic reviews on interventions for dry mouth and hyposalivation in SS by Hamad et al. [21] and Choudhary et al. [22], no studies evaluate the effect of this natural mix for the management of xerostomia in patients with SS. This natural mixture could provide a solution to the problem of scarce products of salivary substitutes in the market of many countries.
Thus, the present study aimed to evaluate the efficacy of a mixture of aloe vera gel, coconut oil, and CMC in comparison to plain CMC on subjective and objective findings of xerostomia in a sample of patients with SS. Additionally, it provides a suitable alternative flavor and benefits for personal preference.
Materials and methods
The current study is a randomized, crossover, superior clinical trial. The protocol was registered on clinicaltrials.gov under the identifier NCT04252209. The study was approved by the Research Ethics Committee in the Faculty of Dentistry at Cairo University (approval number 16 2 20) and was performed in concordance with the Declaration of Helsinki. Informed consent with the details of the clinical trial was obtained from all the participants. A convenience sample of adult SS patients was recruited from the Rheumatology clinic at El Kasr Al-Ainy, Cairo University.
The sample size was calculated based on the sensation of oral dryness [Bother 1’ xerostomia index (BI1)] reported by [23], using a paired t-test and a 5% alpha level of significance, with a power of 80%. The calculated sample size was 24 patients.
Participants
According to the ACR/EULAR 2016 classification criteria [24], the included patients were diagnosed with SS and suffered from xerostomia or symptomatic oral dryness. We excluded patients with xerostomia from other etiologies, such as radiotherapy, diabetes mellitus, viral infections such as HIV, active HCV, or those taking xerogenic medication such as antidepressants, anticholinergics, antihistamines, and anxiolytics and antihypertensives [1].
Random allocation
A random sequence was generated using a computerized random number generator with an allocation ratio (1:1). Subjects were randomly allocated to the AB [A: study treatment (A-Gel); B: control treatment (B-Gel)] or BA sequence. Concealment was done using separate serially numbered envelopes that were sealed, opaque, and kept by Mai Zakaria. After patients were found eligible by Basma Elsaadany, Mervat Eissa, or Alaa Mahmoud, Mai Zakaria would register the patient name and number and reveal the allocated treatment.
The study groups
The study treatment was in the form of a gel containing 10% aloe vera jelly, 10% coconut oil, 3% peppermint essential oil, 3% CMC, 10% PG, and 0.1% potassium sorbate, and water up to 100%. After weighing the calculated amount of each ingredient, CMC was levigated with PG in a mortar till a homogeneous paste was obtained. The calculated volume of purified water was heated to 50°C, and aloe vera jelly and potassium sorbate were dissolved using a magnetic stirrer with a hotplate. This heated solution was added to the CMC-PG paste gradually with continuous levigation till a semi-solid transparent gel was obtained. After cooling to room temperature, coconut and peppermint oils were incorporated into the gel using an undulator mill, then transferred to the collapsible tubes and labeled as “A-Gel”.
The control treatment was a gel containing 3% CMC, 3% peppermint essential oil, 10% PG, 0.1% potassium sorbate, and water up to 100% and was labeled as “B-Gel”.
Patients were advised to use 0.5 teaspoons of the preparation to be applied topically to all surfaces of the oral mucosa 4 times daily after eating and before sleep. Both groups (Group A included all participants received A-gel either at first period or after the washout period; Group B included all participants received B-gel either at first period or after the washout period) were instructed to follow oral hygiene measures such as daily tooth brushing, hydration with sufficient amounts of water, eating fibrous food such as fruits and vegetables, and avoiding any alcohol-containing mouthwash or any other topical oral products. The medication was withdrawn if any allergic reaction occurred.
Blinding
Both gels were prepared by a third-party pharmacist in the Faculty of Pharmacy at Al Azhar University, Cairo, Egypt. Both gels had similar appearance, color, smell, and weight (75 grams), and were placed in identical containers. Basma Elsaadany, Mervat Eissa, and Alaa Mahmoud were responsible for patient recruitment and outcome assessment. Both investigators and participants were blinded to the treatment as well as the statistician. Mai Zakaria was the investigator with the key for unblinding.
The measured outcomes
The primary outcome was subjective oral dryness using the BI1 [25]. It was a patient-centered scale as the patient reported how much he suffered from oral dryness on a scale from 0 to 10. This outcome was assessed 3 times for each treatment: one at baseline, the second after seven days, and the final value at the end of each treatment period.
The secondary outcomes were quality of life, clinical oral dryness, and candidal concentrations. Quality of life was assessed using both parts of the modified Xerostomia Quality of Life Scale (XeQoLS) questionnaire. Part 1 contained eight questions with a continuous response score derived from a 100 mm visual analog scale (VAS), where the positive response was placed on the left and the negative response on the right. This part evaluated physical functions, pain or discomfort, and clinical acceptance. Part 2 contained 12 questions measured by the dichotomous method (yes/no) to evaluate after-use improvement from treatments [8]. Each part was measured 2 times for each treatment, the first one at baseline and the final one at the end of each treatment period.
The clinical oral dryness (CODs) was also measured 2 times for each treatment, first at baseline and finally at the end of each treatment period using the Challacombe Scale. The Challacombe Scale assesses the severity of mouth dryness and treatment outcomes over time. It works as an additive score from 1 to 10, with higher scores representing severe dryness. An additive score of 1–3 indicates mild dryness, 4–6 indicates moderate dryness, and 7–10 indicates severe dryness [25].
Saliva was collected 2 times for each treatment, at baseline and the end of each treatment period, by Alaa Mahmoud, and Candidal load was measured by Sherifa Tarek using the concentrated rinse method for the collected saliva samples. The oral cavity was rinsed with 10 mL of sterile saline, and 7 to 10 mL was collected as the rinse solution. The concentrated rinse solution was prepared by centrifuging it at 2,300 g for 20 min. After the supernatant was removed, the cell pellet was resuspended in 500 μL, which was inoculated onto agar medium in 100 μL aliquots. Candida colonies were counted after incubation at 37°C for 48 h. If there were too many Candidal colonies to be counted, the Candida solutions were diluted tenfold [26].
The following diagram represents patient enrollment, allocation, compliance, follow-up, and analysis during the 5-week study period (Figure 1).
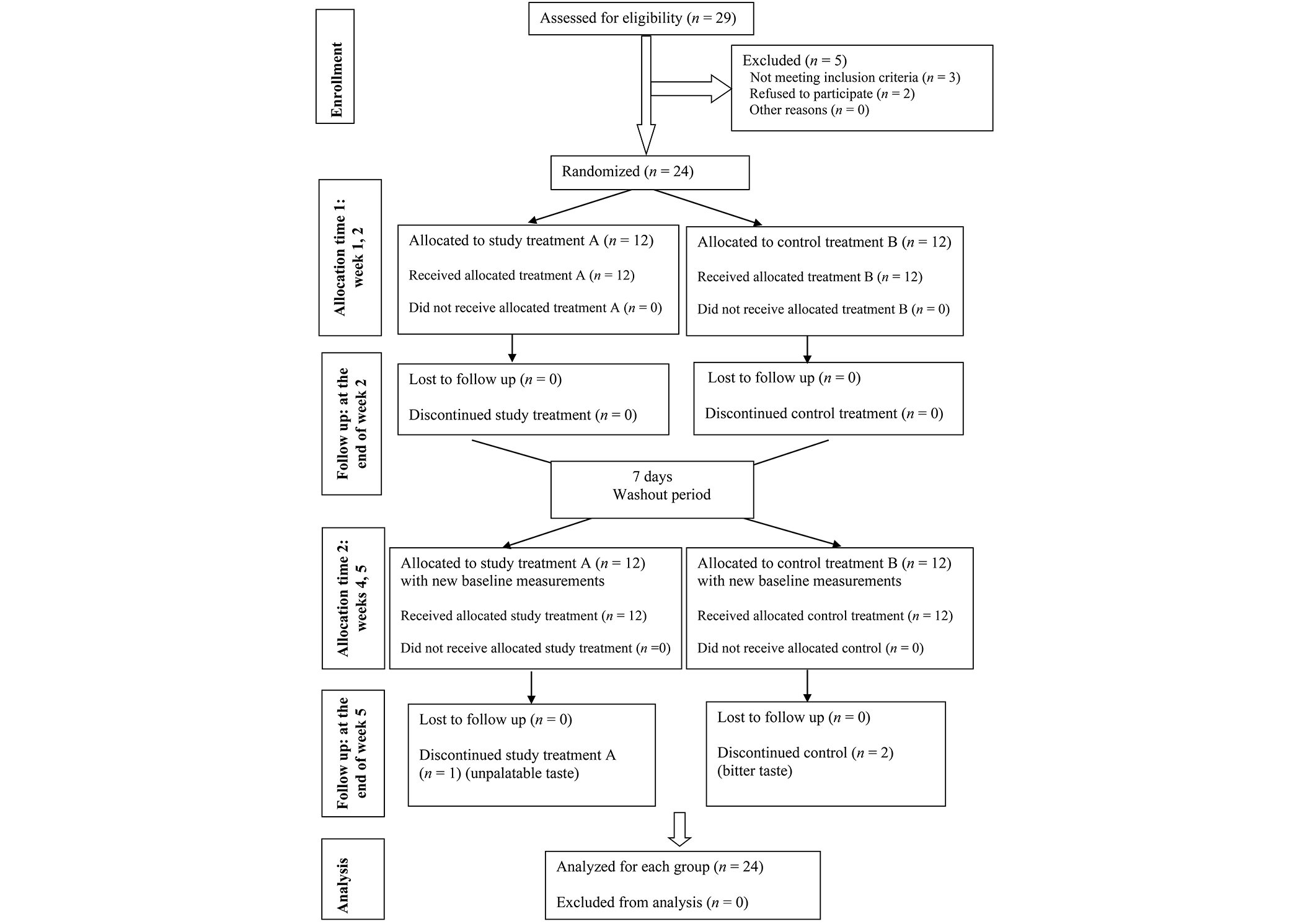
Flow chart of patient enrollment, compliance, and analysis during the 5-week study period
Statistical analysis
Categorical data were presented as frequency and percentage values. Numerical data were presented as mean and standard deviation. They were tested for normality using Shapiro-Wilk’s test and were found to be non-parametric. Inter- and intra-group comparisons were analyzed using mixed linear models. Correlations were analyzed using Spearman’s rank-order correlation coefficient. p-values were adjusted for multiple comparisons using Bonferroni correction. The significance level was set at p < 0.05 for all tests. Statistical analysis was performed with R statistical analysis software version 4.3.1 for Windows.
Results
Demographic data for the included 24 SS cases are shown in Table 1. All patients were females with a mean age of 46.96 ± 11.86 years, suffering from mouth dryness for 16.58 ± 25.32 months, and the majority of them, 11 (45.8%), had mouth dryness secondary to rheumatoid arthritis.
Summary statistics for demographic data
| Parameter | Number (%) | |
|---|---|---|
| Gender | Male | 0 (0.0%) |
| Female | 24 (100%) | |
| Types of Sjogren’s | Primary Sjogren’s | 8 (33.3%) |
| Secondary to rheumatoid arthritis | 11 (45.8%) | |
| Secondary to systemic lupus erythematosus | 5 (20.8%) | |
| Age (years) (Mean ± SD) | 46.96 ± 11.86 | |
| Duration of mouth dryness (months) (Mean ± SD) | 16.58 ± 25.32 | |
SD: standard deviation
The primary outcome in this study was measured using the BI1. Values for both groups are reported in Table 2.
Inter- and intra-group comparisons, mean and standard deviation values of the Bother 1’ xerostomia index (BI1)
| Intervals | BI1 (Mean ± SD) | p-value | |
|---|---|---|---|
| Group A | Group B | ||
| Baseline | 7.33 ± 2.39 | 7.29 ± 2.66 | 0.916ns |
| After 1 week | 4.17 ± 2.24 | 4.25 ± 2.57 | 0.834ns |
| After 2 weeks | 2.83 ± 2.08 | 2.88 ± 2.07 | 0.892ns |
| p-value | < 0.001* | < 0.001* | - |
* significant (p < 0.05). ns: non-significant (p > 0.05); SD: standard deviation. -: no data
As for the secondary outcomes, the quality of life (part 1) showed no statistically significant difference between group A and group B regarding all domains at baseline or after 2 weeks; however, there was a statistically significant difference within each group between baseline and after 2 weeks as shown in Table 3.
Inter- and intra-group comparisons, mean and standard deviation values of modified Xerostomia Quality of Life Scale (XeQoLS) questionnaire part 1 [8]
| Domain | Question | Interval | Quality of life score (Mean ± SD) | p-value | |
|---|---|---|---|---|---|
| Group A | Group B | ||||
| 1. Physical functioning | 1.1 Do you have difficulty chewing because of your dry mouth? | Baseline | 61.30 ± 25.28 | 57.71 ± 27.35 | 0.416ns |
| After 2 weeks | 32.17 ± 25.58 | 31.25 ± 25.25 | 0.885ns | ||
| p-value | < 0.001* | < 0.001* | - | ||
| 1.2 Do you have difficulty swallowing because of your dry mouth? | Baseline | 51.30 ± 41.26 | 53.75 ± 42.00 | 0.307ns | |
| After 2 weeks | 40.43 ± 39.02 | 39.17 ± 39.00 | 0.809ns | ||
| p-value | 0.006* | 0.006* | - | ||
| 1.3 Is speech difficult because of your dry mouth? | Baseline | 56.09 ± 30.41 | 55.00 ± 31.76 | 0.307ns | |
| After 2 weeks | 29.78 ± 27.16 | 30.62 ± 28.14 | 0.487ns | ||
| p-value | < 0.001* | < 0.001* | - | ||
| 1.4 Is taste affected by your dry mouth? | Baseline | 30.00 ± 30.90 | 28.75 ± 30.83 | 0.999ns | |
| After 2 weeks | 23.48 ± 29.79 | 20.00 ± 27.50 | 0.266ns | ||
| p-value | 0.012* | 0.013* | - | ||
| 2. Pain/discomfort | 2.1 How dry is your mouth? | Baseline | 72.61 ± 24.16 | 70.83 ± 27.81 | 0.589ns |
| After 2 weeks | 29.13 ± 20.43 | 29.58 ± 19.89 | 0.784ns | ||
| p-value | < 0.001* | < 0.001* | - | ||
| 2.2 Do you have a burning sensation in your mouth? | Baseline | 29.13 ± 30.88 | 29.58 ± 29.85 | 0.445ns | |
| After 2 weeks | 14.35 ± 20.41 | 13.75 ± 20.18 | 0.328ns | ||
| p-value | 0.002* | 0.002* | - | ||
| 3. Other clinical acceptances | 3.1 Do you have difficulty with sleeping caused by your dry mouth? | Baseline | 57.83 ± 25.40 | 52.50 ± 23.08 | 0.233ns |
| After 2 weeks | 25.87 ± 20.92 | 22.92 ± 22.93 | 0.501ns | ||
| p-value | < 0.001* | < 0.001* | - | ||
| 3.2 How often do you sip liquids for oral comfort when not eating? | Baseline | 59.78 ± 22.59 | 56.88 ± 24.58 | 0.607ns | |
| After 2 weeks | 31.74 ± 20.54 | 26.88 ± 21.46 | 0.208ns | ||
| p-value | < 0.001* | < 0.001* | - | ||
* significant (p < 0.05). ns: non-significant (p > 0.05); SD: standard deviation. -: no data
For the second part of the quality of life questionnaire, there was no statistically significant difference between both groups regarding all questions in the physical functioning, pain/discomfort, personal/psychological functioning, social functioning, and other clinical acceptance domains, as shown in Table 4.
Intergroup comparisons, frequency, and percentage values of modified Xerostomia Quality of Life Scale (XeQoLS) questionnaire part 2 [8]
| Domain | Question | Answer | Quality of life score (Frequency and percentage values) | p-value | |
|---|---|---|---|---|---|
| Group A | Group B | ||||
| 1. Physical functioning | 1.1 Do you have difficulty chewing because of your dry mouth? | Yes | 24 (100%) | 22 (91.7%) | 0.149ns |
| Did the product make chewing easier? | Yes | 22 (91.7%) | 20 (83.3%) | 0.383ns | |
| 1.2 Do you have difficulty swallowing because of your dry mouth? | Yes | 15 (62.5%) | 15 (62.5%) | 0.999ns | |
| Did the product make swallowing easier? | Yes | 8 (33.3%) | 9 (37.5%) | 0.763ns | |
| 1.3 Do you have speech difficulty because of your dry mouth? | Yes | 22 (91.7%) | 21 (87.5%) | 0.637ns | |
| Did the product make talking easier? | Yes | 20 (83.3%) | 20 (83.3%) | 0.999ns | |
| 1.4 Is taste affected by your dry mouth? | Yes | 14 (58.3%) | 13 (54.2%) | 0.771ns | |
| Did the product improve your sensation of taste? | Yes | 8 (33.3%) | 8 (33.3%) | 0.999ns | |
| 2. Pain/discomfort | 2.1 Do you suffer from a dry mouth? | Yes | 24 (100%) | 24 (100%) | NA |
| Did the product make your dry mouth better? | Yes | 24 (100%) | 24 (100%) | NA | |
| 2.2 Do you have a burning sensation in your mouth? | Yes | 14 (58.3%) | 14 (58.3%) | 0.999ns | |
| If you have a burning mouth, did the product improve the burning sensation? | Yes | 13 (54.2%) | 13 (54.2%) | 0.999ns | |
| 2.3 Do you suffer from a dry mouth in the daytime? | Yes | 24 (100%) | 24 (100%) | 0.999ns | |
| Was the product most useful in the daytime? | Yes | 24 (100%) | 24 (100%) | 0.999ns | |
| 3.Personal/psychological functioning | 3.1 Do you visit people less frequently because of your dry mouth? | Yes | 0 (0.0%) | 0 (0.0%) | 0.999ns |
| Did you visit people more than you used to? | Yes | 0 (0.0%) | 0 (0.0%) | 0.999ns | |
| 4. Social functioning | 4.1 Do you avoid speaking to people because of your dry mouth? | Yes | 2 (8.3%) | 0 (0.0%) | 0.149ns |
| Did you speak to people more than you used to? | Yes | 1 (4.2%) | 0 (0.0%) | 0.312ns | |
| 4.2 Do you stay at home more because of your dry mouth? | Yes | 1 (4.2%) | 0 (0.0%) | 0.312ns | |
| Do you get out of the house more than you used to? | Yes | 1 (4.2%) | 0 (0.0%) | 0.312ns | |
| 5. Other clinical acceptances | 5.1 Do you suffer from a dry mouth in the nighttime? | Yes | 24 (100%) | 24 (100%) | NA |
| Did the product stop you from waking in the night? | Yes | 23 (95.8%) | 23 (95.8%) | 0.999ns | |
| 5.2 If you wear dentures, does your dry mouth affect the retention of the dentures? | Yes | 0 (0.0%) | 0 (0.0%) | NA | |
| If you wear dentures, did the product help with the retention of the dentures? | Yes | 0 (0.0%) | 0 (0.0%) | NA | |
ns: non-significant (p > 0.05). NA: not available
As shown in Table 4, the most affected physical function is chewing, followed by speech; all of the participants in group A and more than 90% of group B reported chewing difficulty. While more than 90% of group A and 87.5% of group B reported speech difficulty. Both are highly improved after treatment, either in group A or group B. In the pain/discomfort domain, all of the participants reported dry mouth, and also all of them reported significant improvement after treatment in both groups; a burning sensation was reported by more than half of the participants in both groups, and the majority of them reported significant improvement after treatment. None of the participants in both groups reported any psychological/personal dysfunction. Regarding social functioning, only two or one participant in group A reported the effect of dry mouth on speaking to people or getting out of the home, which was also improved after treatment. All of the participants reported dry mouth at night in both groups; also, more than 95% reported significant improvement. None of the participants wore dentures.
Other secondary outcomes, including the Challacombe score and the candida load, are presented in Table 5. 37.5% of the participants in group A and 45.8% of the participants in group B had an insignificant candidal concentration at baseline.
Inter- and intra-group comparisons of the Challacombe scale and log candidal concentrations
| Intervals | Challacombe scale for clinical oral dryness (Mean ± SD) | p-value | Log candidal concentrations (Mean ± SD) | p-value | ||
|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |||
| Baseline | 5.96 ± 2.33 | 5.92 ± 2.38 | 0.954ns | 1.23 ± 0.66 | 1.29 ± 0.69 | 0.679ns |
| After 2 weeks | 2.78 ± 1.86 | 2.96 ± 1.65 | 0.733ns | 1.06 ± 0.36 | 1.17 ± 0.31 | 0.254ns |
| p-value | < 0.001* | < 0.001* | - | 0.414ns | 0.284ns | - |
* significant (p < 0.05). ns: non-significant (p > 0.05); SD: standard deviation. -: no data
Additionally, there was a strong positive correlation between subjective and clinical oral dryness that was statistically significant (rs = 0.760, p < 0.001). Regarding treatment side effects, two participants in group B reported an intense bitter taste sensation, while only one in group A reported an unpalatable taste.
Discussion
Saliva has an important role in oral health; it protects oral tissues by keeping them moist and providing a lubricating mucoid secretion. It plays an essential role during speech, mastication, digestion, and swallowing, as well as in taste perception by transportation of the dissolved substances to stimulate taste chemoreceptors [27]. It takes part in the remineralization of dental enamel and maintaining the hypotonic environment with high calcium and phosphate concentrations to regulate pH levels, also controlling the composition of oral microflora against extrinsic factors with its antibacterial, antifungal, and antiviral properties. Qualitative and quantitative salivary dysfunctions cause alterations in oral mucosa with a negative impact on the quality of life-related to all the previous aspects [28]. Xerostomia is the chief complaint of patients with SS. However, it is not pathognomonic to SS [29].
The lack of on-shelf salivary substitutes in some countries such as Egypt, and the scarcity of local products in pharmacies, we aimed to provide an alternative that is cost-effective, safe, and acceptable for the patients to resemble the effect of natural saliva. In the present study, we evaluated the efficacy of a salivary substitute containing a mixture of aloe vera gel and coconut oil (study treatment A-Gel) in comparison to the conventional CMC substitute (control B-Gel) for the management of xerostomia in patients with SS.
The design of the study was a crossover randomized controlled trial, reminiscent of other studies on salivary substitutes [15, 23, 30, 31]. We used a crossover design to eliminate the inter-subject variability from group comparisons and can lessen the impact of variables [32]. The study included 24 females, classified as SS, either primary or secondary.
In the present study, both subjective and objective methods were used to evaluate the effectiveness of the salivary substitutes. Subjective oral dryness was measured using the BI1. Additionally, we used the modified XeQoLS as a subjective measure. While objective oral dryness was measured using the COD score. Our findings collectively revealed a significant improvement in subjective and objective outcomes in both groups; however, there was no significant difference between the intervention and control groups.
All similar previous studies evaluating salivary substitutes in patients with xerostomia measured either subjective or objective oral dryness or both but with different scales. Subjective oral dryness was measured by VAS scores of oral dryness in many studies [17, 23, 30, 31, 33–35]. While it was measured, using the xerostomia survey developed by Fox et al. [36], 1987, in other studies [15, 17, 23].
Similar to our results, all of the previous studies reported a statistically significant improvement in the symptoms of xerostomia after treatment compared to the baseline status within each group except for those groups using mineral water [31] or normal saline [17]. Moreover, in the same context, a systematic review by Assery et al. [37] concluded that all salivary substitute products tested in the included studies reduced symptoms of xerostomia.
Regarding our findings according to the modified XeQoLS in part 1, there was an improvement in the quality of life scale in all domains (physical functioning, pain, and clinical acceptance) in both groups with no significant difference between them.
This improvement in quality of life is consistent with other studies that reported the same outcome but with different questionnaires to evaluate the effect of different salivary substitutes in the treatment of xerostomia [30, 33, 35].
As for part 2 in the modified XeQoLs, there was significant improvement in chewing in both groups. Salivary substitutes increase moisture in the oral cavity and pharynx, which makes oral manipulation easier and less painful [8]. Regarding the properties of A-Gel, CMC is a hydrophilic component [38]. Aloe vera gel has a high water content with other lubrication components such as glucomannans, amino acids, and lipids [39]. This combination simulates the natural effect of saliva that contains a high water content with multiple lubrication molecules, such as mucin and proline-rich glycoprotein albumin complex, that provide a lubricating interface between teeth and facilitate chewing [27].
Our results revealed that about two-thirds of the participants in each group complained of difficulty in swallowing. It is similar to the prevalence of self-reported swallowing dysfunction in SS which is approximately 60% [40].
Regarding the difficulty in speech, the majority of the participants in both the study and control groups reported an improvement in speech. This may be due to the lubricant effect of both salivary substitutes on the lips and the entire oral mucosa.
Regarding taste impairment, about one-third of the participants in both groups reported improvement in taste affection. This is similar to the effect of aloe vera and ginger mouthwashes seen in a study by Badooei and his co-authors who compared the effect of ginger, aloe vera, and normal saline mouthwashes on xerostomia in patients with type 2 diabetes [17].
Decreased salivation leads to tongue papillary atrophy, which is correlated to the state and number of taste receptors. The reduction of saliva secretion also limits the direct short-term contact of food substances dissolved in saliva to the taste buds, reducing the flavor’s perception, and also reduces the regeneration of receptor sites and their ability to defend against bacterial infection, thermal, and mechanical stress [41].
Besides its lubricant properties, aloe vera’s regenerative properties are due to the compound glucomannan, a mannose-rich polysaccharide that interacts with fibroblast growth factor receptors and stimulates their activity and proliferation, which in turn increases collagen production and cross-linking, leading to improvement in taste perception [42].
Interestingly, in our study, more than half of the participants in both groups reported an improvement in burning sensation after treatment, which is inconsistent with previous similar studies [17, 31]. There is a strong correlation between SS and the presence of BMS. Moreover, some SS patients may present a co-existence of dysgeusia, halitosis, and BMS, described as burning sensations in the tongue, which is reported in about half of patients [43]. The relationship between dysgeusia associated with BMS and peripheral neuropathies is related to damage or hypofunction of the chorda tympani. The pathomechanism of nervous system impairment in SS includes epineural infiltration by inflammatory cells, vascular injury mediated by autoantibodies, and ischemia due to small vessel vasculitis [41].
Aloe vera has a neuroprotective effect, which could be attributed to its anti-inflammatory properties [44]. Aloe vera downregulates pro-inflammatory tumor necrosis factor alpha (TNF-α) and inhibits the cyclo-oxygenase pathway, which downregulates the generation of prostaglandin E2 with its’ consequences of pain increase and swelling at the site of inflammation [45]. Also, topical use of virgin coconut oil inhibits anti-inflammatory activity by inhibiting different concentrations of cytokines, including TNF-α, interferon gamma (IFNg), interleukin-6 (IL-6), IL-5, and IL-8 [46]. This combination may improve peripheral neuropathy and the related burning sensation in patients with SS.
Regarding the clinical acceptance section, our results revealed that most of the participants reported a decrease in waking up at night to sip water due to the lubricant effect of the products on the oral mucosa. This is consistent with the effect of oil pulling seen in the study by Ludwar and co-authors [31].
We measured COD using the Challacombe score. To our knowledge, the only other study that used the Challacombe score was reported by Atashi et al. [16]. Other studies used salivary flow rate, either stimulated or unstimulated, to assess objective oral dryness [15, 30, 31, 35]. However, the salivary flow rate is inappropriate to be used in patients with SS due to the permanent dysfunction in salivary glands.
In the present study, there was an improvement in CODs in both groups after treatment, while there was no statistically significant difference between groups despite the previously reported promising properties of aloe vera and coconut oil. These findings are inconsistent with those published in 2018 by Atashi and his co-authors, who found a statistically significant difference between the aloe vera-peppermint group and the CMC group [16]. The proposed explanation is the variation in the study design, as our study was a crossover to eliminate personal variability. Additionally, the short-term follow-up of his sample of ICU intubated patients.
Despite the previously reported antifungal effect of both aloe vera and coconut oil that are used in the study group, there was no statistically significant difference between both groups regarding candidal load. This was not surprising, as the candidal load of participants at baseline was close to normal. We recommend using the products in symptomatic patients with a high countable candidal load.
A natural mixture of aloe vera gel, coconut oil, and CMC could resemble the natural effect of saliva; it should be considered when recommending appropriate and cost-effective ways for the relief of xerostomia and its consequences in patients with moderate to severe oral dryness.
From our point of view, the small sample size and the short follow-up period are considered limitations in our study because they only allow the evaluation of the short-term effect of the products in SS patients. Thus, high-quality, long-term studies are recommended to assess the efficacy of the CMC-based natural mixture of aloe vera gel and coconut oil in oral health improvement regarding antifungal, anti-cariogenic, and antiplaque effects in patients with complications of oral dryness.
Conclusions
Topical administration of moisturizing CMC-based gel containing 10% aloe vera and 10% coconut oil had a positive effect on quality of life and oral signs and symptoms of xerostomia similar to that of traditional CMC in patients with Sjogren’s syndrome, making it an alternative option to satisfy the patient’s individual preference with negligible side effect.
Abbreviations
| BI1: | Bother 1’ xerostomia index |
| BMS: | burning mouth syndrome |
| CMC: | carboxymethyl cellulose |
| CODs: | clinical oral dryness |
| IL: | interleukin |
| PG: | propylene glycol |
| SS: | Sjogren’s syndrome |
| TNF-α: | tumor necrosis factor alpha |
| VAS: | visual analog scale |
| XeQoLS: | Xerostomia Quality of Life Scale |
Declarations
Acknowledgments
The authors would like to thank Dr. Mohamed Zaki, lecturer of pharmaceuticals in the Faculty of Pharmacy at Al Azhar University, Cairo, Egypt, for preparing both the study and control treatment.
Author contributions
AM: Conceptualization, Formal analysis, Investigation, Resources, Writing—original draft. BE: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—review & editing. ME: Methodology, Project administration, Supervision, Validation. WAE: Conceptualization, Data curation. ST: Methodology, Project administration, Validation, Visualization. MZ: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was performed following the Declaration of Helsinki, and the protocol was approved by the Research Ethics Committee in the Faculty of Dentistry at Cairo University [approval no 16 2 20]. The trial is registered on clinicaltrials.gov, identifier: NCT04252209.
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The datasets used and/or analyses used during the current study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.