Abstract
Aim:
The pathogenetic mechanisms and predictors of the development of polyposis and hypertrophy of the sinonasal mucosa (SM) in patients with chronic allergic airway inflammation have not been clearly established. The concentration of inflammatory biomarkers in nasal secretions was determined in children and adolescents with a combined course of bronchial asthma (BA) and allergic rhinitis (AR) in the absence or presence of polyposis and hypertrophy of the SM.
Methods:
A single-centre observational cross-sectional pilot study was conducted. 93 patients with BA aged 8 to 17 years were studied. Total Nasal Symptom Score (TNSS), sinonasal symptoms (SNOT-22), and peak nasal inspiratory flow (PNIF) were assessed. Concentrations of eosinophil cationic protein (ECP), interleukin 4 (IL-4), IL-1, total immunoglobulin E (IgE), and vascular endothelial growth factor (VEGF) in nasal secretions were determined.
Results:
The levels of ECP, IL-4, and IL-1 in nasal secretions were statistically significantly higher in patients with the presence of polyposis and hypertrophic SM than in those without, amounting to 83.1 [31.4; 166.8] ng/mL for ECP vs. 29.5 [5.3; 49.9] ng/mL, P < 0.001, for IL-4 174.6 [68.6; 325.5] pg/mL vs. 79.5 [42.8; 146.01] pg/mL, P = 0.004, for IL-1 98.7 [33.7; 267.5] pg/mL and 48.8 [9.01; 108.2] pg/mL, P = 0.025. There were no statistically significant differences in IgE and VEGF levels in nasal secretions, all P > 0.05. Parameters such as ECP, IL-4, and IL-1 were found to be significant predictors of polyposis and hypertrophy in the formation of SM.
Conclusions:
In patients with a combined course of BA and AR, the presence of polyposis and hypertrophy of SM is associated with higher levels of ECP, IL-4, and IL-1 in nasal secretion. This may indicate that pathological remodelling of SM is associated with both the intensity of allergic inflammation and its relationship with local activation of innate immunity.
Keywords
Bronchial asthma, allergic rhinitis, children, adolescents, polyps, biomarkersIntroduction
It is well known that the respiratory tract, from the nasal passages to the terminal bronchi, is a single functional anatomical unit [1]. In allergic diseases of the airways, both the upper airways (UA) and lower airways are involved in the process of allergic inflammation in most patients. This explains the comorbid course of bronchial asthma (BA) and allergic rhinitis/allergic rhinosinusitis (AR/ARS) [2–5]. The course of AR in a significant proportion of these patients can be accompanied by pathological remodelling of the sinonasal mucosa (SM) and the development of chronic rhinosinusitis (CRS) with or without polyps, which reduces both the patient’s quality of life and the level of control of BA [6–9]. In this regard, recent consensus documents recommend mandatory assessment of UA in patients with asthma at every stage of therapy [5, 10, 11].
There is a view that CRS with polyps is the province of people over the age of 40 [12]. However, our own studies and literature data suggest that the onset of polyposis and hypertrophic SM can be observed in childhood and adolescence [8, 13, 14].
So far, it is not known exactly why the course of allergic inflammation in UA is accompanied by the formation of polyposis and hypertrophic SM in some patients [12, 15, 16]. In this regard, considerable attention has been paid to the study of the different endotypes of inflammation that form the pathogenetic basis of BA and AR, as well as their intensity and the characteristics of the reparative processes. It has been suggested that the characteristics of the inflammation and its severity may be important in the pathogenesis of the formation of pathological remodelling of the SM in these patients [12, 15, 17, 18]. Furthermore, according to Meng et al. [19], remodelling is likely to be initiated and progress in parallel with inflammation, rather than a consequence of it.
In children and adolescents with comorbid BA and AR, the predominant endotypic variant is T2-dependent inflammation. Interleukin 4 (IL-4), immunoglobulin E (IgE), and eosinophil cationic protein (ECP) are important biomarkers that characterise the T2-dependent inflammatory process [20–26]. At the same time, there are no comprehensive data on the expression of local T2 markers in patients with and without polyposis and hypertrophy SM. It should also be noted that in recent years there has been increasing information on the combination of T2 inflammation with “classical” inflammation induced by activation of innate immunity and associated with IL-1 [27–29] in patients with polyposis and SM hypertrophy. It is also important to study the role of inducers of angiogenesis and remodelling of the SM, including vascular endothelial growth factor (VEGF), which may play an important role in both polyp formation and the induction of severe tissue edema [30, 31].
Therefore, the heterogeneity of the inflammatory mechanisms involved in the development of CRS in patients with BA and AR dictates the need to search for a spectrum of biomarkers reflecting different aspects of the pathogenesis of this pathology, which is reflected in a number of recent studies [20, 21, 32–35]. However, most of the available work focuses on the study of the already-formed polyposis process using invasive histopathological techniques, mainly in the context of surgical treatment. In this regard, studies using nasal secretions are of particular interest due to the non-invasiveness of these methods, which are carried out at the stages of the debut of pathological remodelling of SM, in childhood and adolescence.
Therefore, the study of inflammatory biomarkers in nasal secretions in children and adolescents with a combined course of BA and AR and with the formation of polyposis and hypertrophy SM is relevant. It may allow clarifying the immunogenesis of these SM alterations in this category of patients for the purpose of their timely diagnosis, treatment, and prevention. Effective endotyping of patients with polyposis may be useful in the selection of targeted therapy, as suggested by international recommendations [36, 37].
Study objective
To determine the levels of inflammatory biomarkers in nasal secretions in children and adolescents with BA and AR with and without polyposis and hypertrophic changes of the SM.
Materials and methods
Study design
A single-center, observational, cross-sectional study was conducted. Conditions of the study fulfillment: the study was conducted at the Children’s City Clinical Hospital No. 1 in Nizhny Novgorod, Russia, from 2021 to 2024.
Study participants
Patients with atopic BA between the ages of 8 and 17 years who were being treated for this disease participated in the study. Family history of atopy (asthma, AR, conjunctivitis, atopic dermatitis, urticaria) was assessed. Sensitisation to major aeroallergens (house dust mite, cat, dog, pollen allergens) was assessed by in vivo (prick test) or in vitro (with the determination of specific IgE) methods.
Inclusion criteria
Diagnosis of BA established according to current international consensus documents (GINA, 2016–2021).
The age of patients is from 8 to 17 years.
Exclusion criteria
Presence of acute infectious diseases and fever.
Presence of diabetes mellitus, autoimmune diseases, primary immunodeficiencies, cancer, atopic dermatitis, parasitic diseases, epilepsy.
Severe BA.
Systemic use of glucocorticoids and non-steroidal anti-inflammatory medications at least one month prior to the study.
Ethical review
The study was approved by the Ethical Committee of the Volga Region Research Medical University (protocol No13, dated 10.10.2016). All participants and all primary caregivers gave written informed consent.
Data source
Anthropometric indices
Basic anthropometric parameters were assessed in all patients. Anthropometric parameters [height, body weight, and body mass index (BMI)] were estimated using tables developed by the WHO, taking into account the sex and age of the patients (https://www.who.int/tools/child-growth-standards).
Spirometry
Spirometric studies were performed using Mastercreen pneumospirometer (Jaeger, Germany). The z-criterion of forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) was used to diagnose obstructive disorders, with z-criterion values < –1.645 [11]. In addition, zFVC, zFEV1, and zFEV1/FVC were calculated using the Global Lung Function Initiative calculator (http://gli-calculator.ersnet.org/index.html) developed with the support of the European Respiratory Society (ERS, https://www.ersnet.org).
All patients underwent an otorhinolaryngological examination: routine otorhinolaryngological examination, endoscopic examination and computed tomography of the sinuses, peak nasal inspiratory (PNIF) flow measurement, quantitative assessment of sinonasal symptoms using the Sino-Nasal Outcome Test-22 (SNOT-22) [28] and the Total Nasal Symptom Score (TNSS) [29].
Determination of inflammatory mediators
The collection of nasal secretion for the subsequent determination of the concentration of ECP, IL-4, IgE, IL-1, and VEGF was performed using sponge strips “Merozel” manufactured by Medtronic (USA). Sponge strips of predetermined mass were placed under the middle nasal cavity of each half of the nose for 2 min. The strips with absorbed nasal secretion were then reweighed, the mass of secretion obtained was determined, and 1.5 mL of physiological solution was added to the tube containing the sponge strips. The tube was placed on a shaker for 10 min and centrifuged at 1,500 rpm for 10 min. The selected supernatant was stored at –20°C for a maximum of two weeks. The concentration of IL-1β, IL-4, and IgE in nasal secretion was determined by enzyme-linked immunosorbent assay on an automated analyser ALISEI Quality System (Italy) using Vector Best test systems (Russia) and ECP using Aviscera Bioscience kit (USA). Patients did not receive any topical endonasal medication for at least 24 h prior to the study.
Nasal airflow
This was assessed noninvasively by measuring the PNIF (L/min) during forced inspiration using a portable PNIF meter (Incheck DIAL, Clement Clarke International, Harlow, UK). The mean value of 3 satisfactory maximal nasal inspirations was calculated.
Statistical analysis
The study was a pilot study and no sample size was calculated. Analyses were performed using Statgraphics Centurion v.16 (Statgraphics Technologies, Inc., The Plains, Virginia, USA). Quantitative measures were assessed for conformity to a normal distribution. Data are presented as Me [Q1; Q3], where Me is the median, [Q1; Q3] are the 1st and 3rd quartiles due to a distribution other than normal. The Mann-Whitney test was used to compare quantitative variables in two independent groups. Correlation analysis was performed using Spearman’s correlation coefficient. Differences were considered statistically significant at P < 0.05. The sensitivity and specificity of changes in the studied parameters were evaluated by linear regression method with receiver operator characteristic (ROC) curves construction.
Results
A total of 93 patients aged 8–17 years were studied, of whom 71% (66/93) were boys. The median age of the examined patients was 13.0 [10.0; 16.0] years, whereby patients with polyposis and hypertrophy SM were statistically significantly older, P < 0.001 (Table 1). Patients in the compared groups did not show statistical differences in z-height, P = 0.258, and zFEV1/FVC, P = 0.385. The zBMI values were statistically significantly lower in the group of patients with polyposis and hypertrophy SM, P = 0.023, and the same patients showed statistically significantly lower zFVC and zFEV1 values, P = 0.008 and P = 0.007, respectively.
Clinical characteristics of patients
| Data | All patients | Without polyposis and hypertrophy SM | With polyposis and hypertrophy SM | P-value |
|---|---|---|---|---|
| Patients, N (%) | 93 (100%) | 50 (53.8%) | 43 (46.2%) | 0.382 |
| Age | 13.0 [10.0; 16.0] | 11.0 [9.0; 13.0] | 16.0 [14.0; 17.0] | < 0.001 |
| z-height | 0.59 [–0.19; 1.68] | 0.59 [0.03; 1.86] | 0.56 [–0.26; 1.05] | 0.258 |
| zBMI | –0.03 [–0.81; 1.15] | –0.01 [–0.46; 1.3] | –0.54 [–1.1; 0.31] | 0.023 |
| zFVC | 1.2 [0.4; 2.0] | 1.4 [0.7; 2.2] | 0.6 [–0.3; 1.9] | 0.008 |
| zFEV1/FVC | –1.3 [–2.1; –0.6] | –1.4 [–2.1; –0.8] | –1.2 [–2.3; –0.04] | 0.385 |
| zFEV1 | 0.2 [–0.9; 1.01] | 0.5 [–0.5; 1.1] | –0.5 [–1.4; 0.4] | 0.007 |
| RPF (L/min) | 40.0 [30.2; 49.8] | 41.7 [33.3; 56.0] | 38.3 [28.0; 45.7] | 0.047 |
| TNSS (points) | 6.0 [4.0; 8.0] | 5.0 [3.5; 7.5] | 8.0 [5.0; 8.0] | 0.012 |
| SNOT-22 (points) | 18.0 [12.0; 25.0] | 16.0 [10.0; 21.0] | 22.0 [15.0; 31.0] | 0.001 |
| Eosinophils (109/L) | 0.3 [0.2; 0.6] | 0.4 [0.2; 0.7] | 0.3 [0.1; 0.5] | 0.378 |
| Total IgЕ (ME/mL) | 183.3 [80.2; 399.7] | 178.4 [92.6; 378.8] | 201.6 [80.2; 399.7] | 0.811 |
Data are presented as Me [Q1; Q3], where Me is the median, [Q1; Q3] are the 1st and 3rd quartiles due to a distribution other than normal. SNOT-22: Sino-Nasal Outcome Test-22; SM: sinonasal mucosa; BMI: body mass index; FEV1/FVC: forced expiratory volume in 1 s/forced vital capacity; RPF: renal plasma flow; TNSS: Total Nasal Symptom Score
As expected, patients with SM hypertrophy were characterised by statistically significantly lower nasal respiratory function scores, P = 0.047, and had statistically significantly higher TNSS and SNOT-22 scores, P = 0.012 and P = 0.001, respectively.
The levels of ECP, IL-4, and IL-1 in nasal secretion were considered statistically significantly higher in patients with the presence of polyposis and hypertrophy SM than in those without, amounting to ECP 83.1 [31.4; 166.8] ng/mL vs. 29.5 [5.3; 49.9] ng/mL, P < 0.001; for IL-4 174.6 [68.6; 325.5] pg/mL, vs. 79.5 [42.8; 146.01] pg/mL, P = 0.004; for IL-1 98.7 [33.7; 267.5] pg/mL and 48.8 [9.01; 108.2] pg/mL, P = 0.025 (Figure 1). Concentrations of both IgE and VEGF were comparable in the absence and presence of hypertrophy SM, with IgE 26.1 [5.6; 54.2] ME/mL and 16.8 [0; 62.9] ME/mL, P = 0.444, for VEGF 1,871.6 [1,102.6; 3,790.8] ME/mL and 2,624.2 [956.9; 6,448.57] ME/mL, P = 0.429.
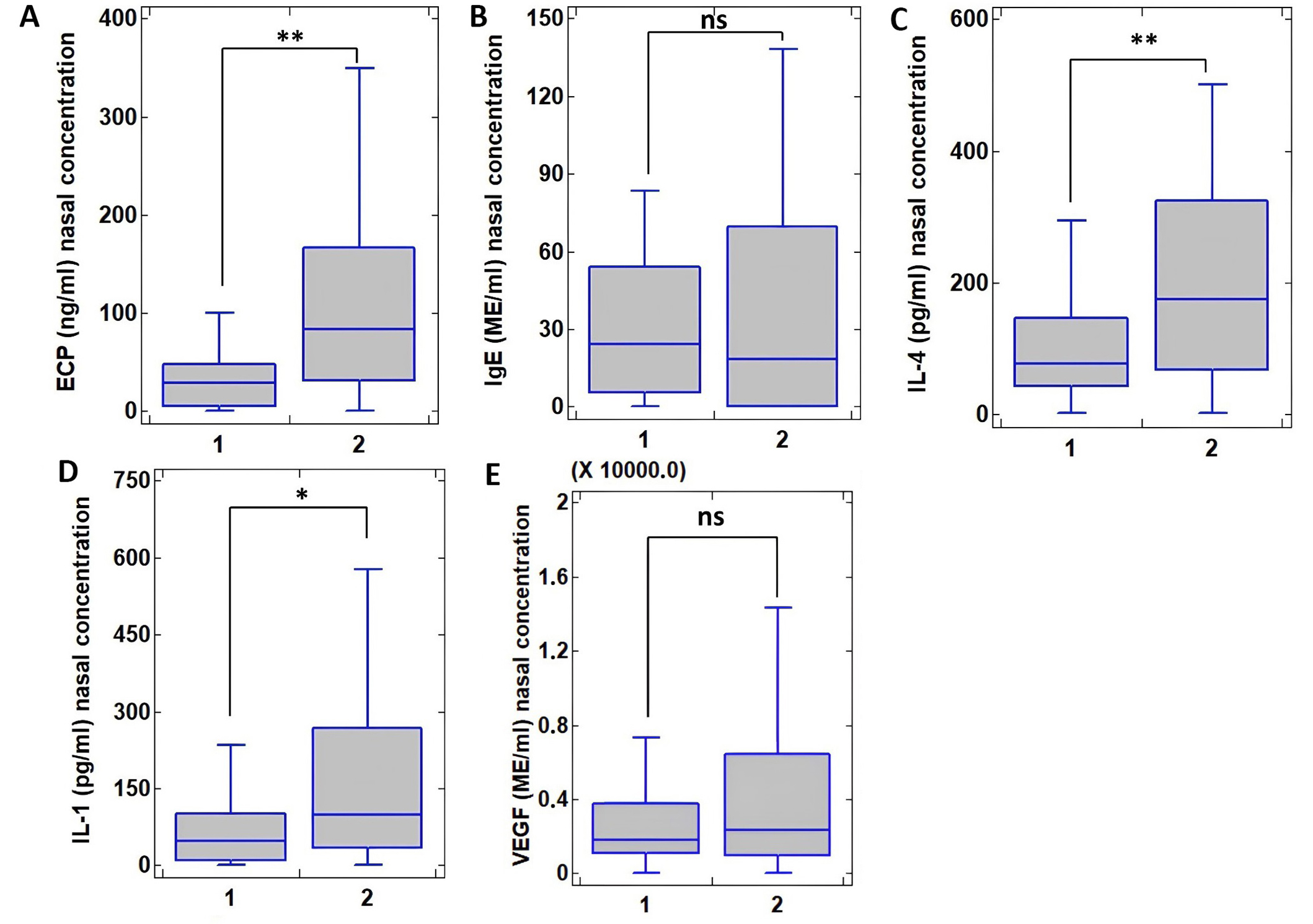
Concentration of cytokines in nasal secretion in patients with bronchial asthma and allergic rhinitis without polyposis/hypertrophic sinonasal mucosal changes (1, N = 50) and with polyposis/hypertrophic sinonasal mucosal changes (2, N = 43) evaluated by paired Wilcoxon test. (A) Concentration of eosinophil cationic protein (ECP), ng/mL; (B) Concentration of immunoglobulin E (IgE), ME/mL; (C) Concentration of interleukin 4 (IL-4), pg/mL; (D) Concentration of IL-1, pg/mL; (E) Concentration of vascular endothelial growth factor (VEGF), ME/mL. Values at P < 0.05 were considered statistically significant and are shown as * (P < 0.05) and ** (P < 0.01), ns: not significant (P > 0.05)
ROC curves were used to assess the predictive potential of the nasal secretion biomarkers studied for the development of polyposis and hypertrophy SM in children and adolescents with BA and AR (Figure 2).
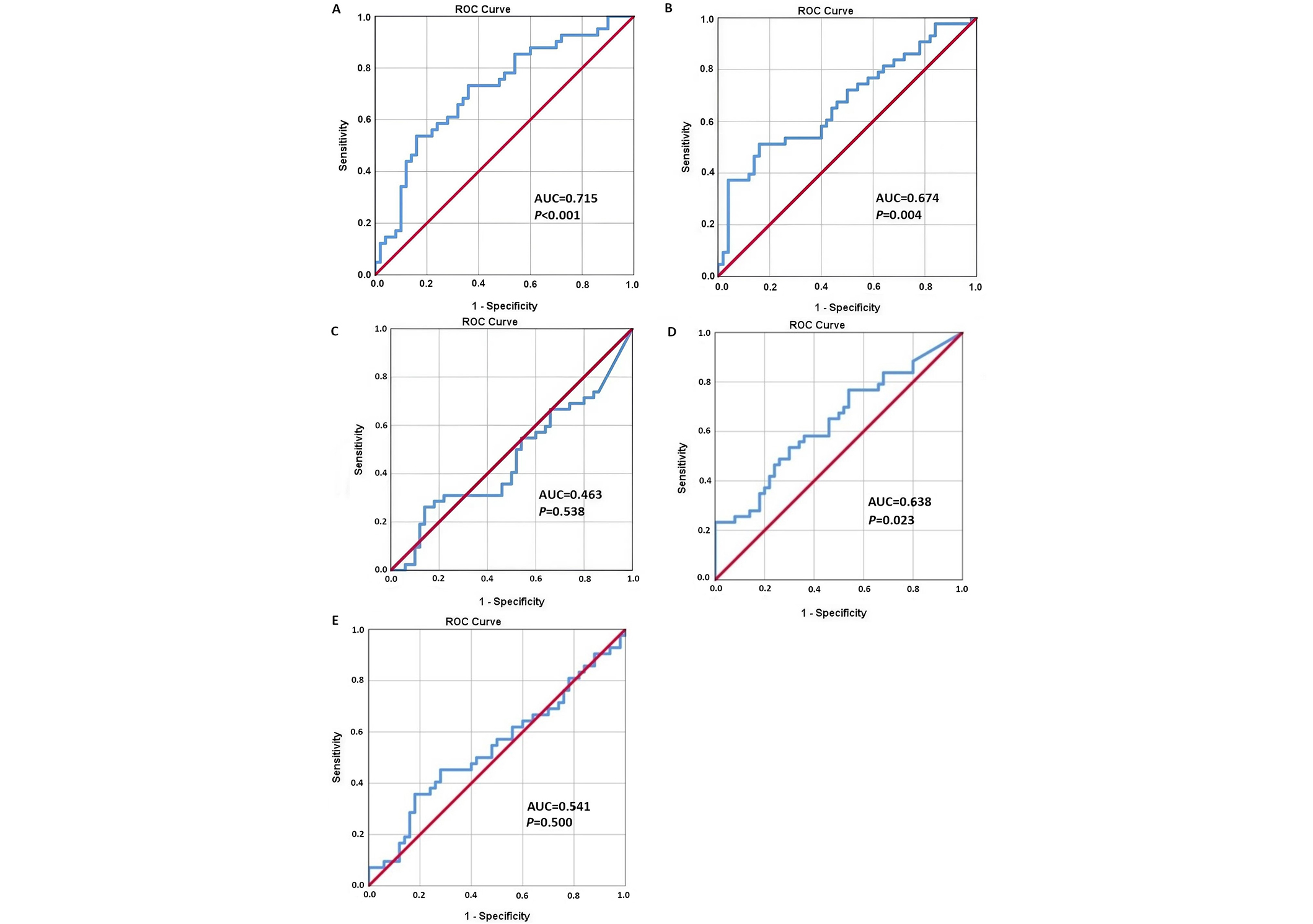
ROC curves of the analysis of prognostic factors for polyposis and hypertrophy of the sinonasal mucosa in children and adolescents with asthma and allergic rhinitis. (A) Concentration of eosinophil cationic protein (ECP), ng/mL; (B) Concentration of interleukin 4 (IL-4), pg/mL; (C) Concentration of immunoglobulin E (IgE), ME/mL; (D) Concentration of IL-1, pg/mL; (E) Concentration of vascular endothelial growth factor (VEGF), ME/mL. ROC: receiver operator characteristic; AUC: area under the curve
The concentration of ECP in nasal secretion showed good prognostic value (AUC = 0.715) (Table 2). The ROC curve of IL-4 and IL-1 levels showed an influence of these cytokines on the formation of polyposis and hypertrophy SM (AUC was 0.674 and 0.638, respectively). While the ROC curve of IgE level did not show the significance of this cytokine in predicting the development of sinonasal hypertrophy (AUC = 0.463). The ROC analysis also showed no significant effect of VEGF levels on the development of polyposis and hypertrophic SM in the study cohort (AUC = 0.541).
Regression analysis of prognostic factors for polyposis and hypertrophic changes of sinonasal mucosa in children and adolescents with asthma and allergic rhinitis
| Data | AUC | 95% CI | Sensitivity (%) | Specificity (%) | OR [95% CI] | P-value |
|---|---|---|---|---|---|---|
| ECP | 0.715 | 0.715 ± 0.055 | 78.0 | 48.0 | 1.008 [1.002–1.013] | < 0.001 |
| IL-4 | 0.674 | 0.674 ± 0.057 | 74.4 | 46.0 | 1.005 [1.001–1.008] | 0.004 |
| IL-1 | 0.638 | 0.638 ± 0.058 | 76.7 | 46.0 | 1.003 [1.000–1.006] | 0.023 |
| VEGF | 0.541 | 0.541 ± 0.062 | 71.4 | 24.0 | 0.840 [0.332–0.939] | 0.500 |
| IgЕ | 0.463 | 0.463 ± 0.062 | 69.0 | 22.0 | 0.694 [0.193–0.840] | 0.538 |
AUC: area under the curve; OR: odds ratio; ECP: eosinophil cationic protein; IL-4: interleukin 4; IgE: immunoglobulin E; VEGF: vascular endothelial growth factor
Discussion
The present study investigated the concentration of ECP, IL-4, total IgE, IL-1, and VEGF in nasal secretion in children and adolescents with a combined course of BA and AR/ARS, taking into account the absence or presence of polyposis and hypertrophy SM. To our knowledge, this is the first such study in children and adolescents with BA.
Patients with polyposis and SM hypertrophy were statistically significantly older (P < 0.001) and, as expected, had lower nasal respiratory function scores (P = 0.047) and higher nasal (TNSS) and sinonasal (SNOT-22) complaints (P = 0.012 and P = 0.001, respectively).
According to the results of our study, the level of ECP, IL-4, and IL-1 in nasal secretion was statistically significantly higher in patients with the presence of polyposis and hypertrophy SM than in patients with their absence. This may indicate that in children and adolescents with the combined course of BA and AR, the formation of polyposis and SM hypertrophy is associated with greater expression of both T2-dependent inflammatory processes (higher levels of ECP and IL-4) and primary immune response (higher IL-1 concentration).
In the literature available to us, we found no studies devoted to analysing the concentration of inflammatory biomarkers in nasal secretions in children and adolescents with a combined course of BA and AR/ARS, taking into account the presence or absence of polyposis SM. Nevertheless, our data are generally consistent with the results of studies in adult patients with formed polyps who have undergone surgical treatment, in which an increase in biomarkers reflecting the expression of different inflammatory endotypes has been demonstrated [32, 38–41]. For example, Perić et al. [32] found higher levels of ECP not only in nasal polyp tissue but also in nasal secretions in adult patients with atopy. At the same time, according to Turner et al. [42], IL-5 and IL-13 levels in nasal mucus were elevated in subjects with CRS compared to controls, whereas no significant difference was observed for IL-4. The data we have obtained generally support the view that inflammation of the T2-endotype is of fundamental importance in the formation of the polyposis process in patients with a combined course of BA and AR, which, in addition to the increase in IL-4 production and ECP concentration that we have shown, is accompanied by an increase in the production of IL-5 and IL-13, which were not studied in this work. Stimulation of Th2 cells, type 2 innate lymphoid cells, epithelial cell injury, Staphylococcus aureus enterotoxins and autoimmune antibodies play an important role in enhancing T2 inflammation and the formation of CRS with polyps [43].
The efficacy of modern therapies in the treatment of nasal polyposis using genetically engineered immunobiological drugs (GIIBPs) targeting components of the T2-dependent immune response, including IL-4 and eosinophil infiltration, can also be considered as an indirect agreement with our results [44, 45]. In our work, we found increased IL-1 levels in patients with polyposis and hypertrophic CM. These results again emphasise that CRS with polyps in children and adolescents with BA and AR is a multifactorial and heterogeneous pathological process that may be associated with the activation of innate immunity [46, 47]. Our data on the potential involvement of IL-1 in the pathogenesis of polyposis CRS formation in patients with the combined course of BA and AR are consistent with the studies [29, 48].
Although total IgE is considered an important biomarker of Th2-dependent immune responses, we found no differences in its levels in nasal secretions between patients with and without polyposis and hypertrophic SM, in contrast to, for example, ECP levels. This may be due to the fact that ECP can be considered as an integral biomarker of eosinophilic inflammation, which, in turn, can be initiated without the involvement of IgE, through the initiation of CCL-2 cells [49]. Our findings are in partial agreement with the results of Vlaykov et al. [50], who found no differences in the concentration of IgE in nasal lavage between healthy subjects and patients with AR (P = 0.312), whereas IL-4 was statistically higher in patients with AR.
We also found no differences in VEGF levels in nasal secretions between patients with and without polyposis and hypertrophy SM, P = 0.429. This is in contrast to previous studies of increased concentration of this protein in polyp tissue [30, 51]. However, we were unable to find studies on VEGF levels in nasal secretion in children and adolescents with BA and nasal polyps.
Therefore, our findings are consistent with the view that CRS with polyps is a multifactorial and heterogeneous inflammatory disease of SM, including patients with a predominance of T2-dependent mechanisms [12, 15, 52]. The inflammatory pattern of SM in patients with combined BA and AR with hypertrophic and polyposis SM is associated not only with increased expression of the T2 inflammatory cytokines ECP and IL4, but also with activation of the primary immunity, which is probably evidenced by elevated IL-1 levels. Understanding the immunogenesis of polyposis SM is important for selecting the most effective therapies. Without this knowledge, it is difficult to develop personalised treatment approaches based on individual patient characteristics.
Limitations of this study were: no control group of healthy participants to compare the results, no stratification of the sample by age groups, sex, and puberty, the relationship between clinical and functional parameters and biomarkers was not analysed.
Abbreviations
| AR: | allergic rhinitis |
| BA: | bronchial asthma |
| BMI: | body mass index |
| CRS: | chronic rhinosinusitis |
| ECP: | eosinophil cationic protein |
| FEV1/FVC: | forced expiratory volume in 1 s/forced vital capacity |
| IgE: | immunoglobulin E |
| IL-4: | interleukin 4 |
| ROC: | receiver operator characteristic |
| SM: | sinonasal mucosa |
| SNOT-22: | Sino-Nasal Outcome Test-22 |
| TNSS: | Total Nasal Symptom Score |
| UA: | upper airways |
| VEGF: | vascular endothelial growth factor |
Declarations
Author contributions
SVK: Conceptualization, Resources, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. TIE: Conceptualization, Investigation, Formal analysis, Supervision, Resources, Writing—original draft, Writing—review & editing. EIK and EAL: Resources, Investigation, Methodology. RNK: Data curation, Software. KVG: Resources, Data curation. DYO, NAG, and NIK: Writing—review & editing. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethical Committee of the Volga Region Research Medical University (protocol No13, dated 10.10.2016).
Consent to participate
All participants and all primary caregivers gave written informed consent.
Consent to publication
Not applicable.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author (Svetlana Viktorovna Krasilnikova, mashkovasv@mail.ru) upon request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.