Abstract
Aim:
This study aimed to evaluate the differential effects of active joint mobilization (AJM) versus traditional passive joint mobilization (PJM) in individuals with chronic ankle instability. We hypothesized that the integration of active components may yield superior outcomes through enhanced proprioceptive feedback and neuromuscular recruitment patterns.
Methods:
In this single-blind, parallel-group randomized controlled trial, thirty participants with chronic ankle instability were randomly assigned to either AJM (n = 15) or PJM (n = 15) groups. Interventions were administered by certified physical therapists three times per week for four weeks, with each session lasting 10 minutes. Primary outcome measures included the Cumberland Ankle Instability Tool (CAIT) and dorsiflexion range of motion (DFROM). Secondary outcomes focused on neuromuscular control parameters including joint position sense (JPS) and static balance, assessed at baseline and post-intervention.
Results:
Both groups demonstrated significant within-group improvements in multiple parameters. The PJM group showed significant improvements in CAIT (p < 0.001), DFROM (p < 0.001), and JPS (p < 0.01). Similarly, the AJM group exhibited significant improvements in CAIT (p < 0.01), DFROM (p < 0.001), and JPS (p < 0.001). Between-group comparison revealed no significant differences in any outcome measures (p > 0.05).
Conclusions:
Both AJM and PJM demonstrated effectiveness in improving functional ankle stability, range of motion, and proprioceptive function in individuals with chronic ankle instability. While both techniques can serve as viable therapeutic approaches, the slightly larger effect sizes observed in AJM for DFROM and proprioceptive function suggest potential additional benefits of active components (ClinicalTrials.gov identifier: NCT04630899).
Keywords
Ankle joint, joint instability, musculoskeletal manipulations, physical therapyIntroduction
The escalating incidence of ankle sprains in athletic populations has emerged as a significant public health concern, with recent epidemiological data indicating these injuries account for up to 60% of all sports-related musculoskeletal trauma [1]. While acute lateral ankle sprains are often perceived as minor injuries, approximately 40% of cases progress to chronic ankle instability (CAI), which represents a pathological condition characterized by mechanical joint laxity and impaired neuromuscular control, resulting in recurrent ankle sprains and persistent symptoms of giving way [2, 3]. In understanding CAI, it is essential to distinguish between mechanical instability, defined as the inability of a joint to withstand loads, and clinical instability, characterized by the clinical consequences of neurological deficit and/or pain [4, 5]. Modern biomechanical analysis has revealed that approximately 70% of affected individuals exhibit complex neuromuscular deficits extending beyond simple mechanical instability [3], highlighting the need for a comprehensive assessment approach that considers both aspects of instability.
Recent advances in biomechanical analysis have revolutionized our understanding of CAI pathomechanics, demonstrating that approximately 70% of affected individuals exhibit complex neuromuscular deficits extending beyond simple mechanical instability [3]. Modern imaging studies have revealed significant alterations in sensorimotor integration and cortical activation patterns, suggesting a more sophisticated pathophysiological model than previously recognized [6]. Furthermore, advanced motion analysis has identified specific biomechanical signatures, including altered center of pressure trajectories and compromised dynamic postural control strategies [7].
The contemporary therapeutic landscape for CAI has evolved significantly, with emerging evidence supporting various intervention strategies. Recent systematic reviews and meta-analyses have demonstrated the efficacy of targeted neuromuscular training [8], novel manual therapy techniques [9], and advanced rehabilitation protocols [10]. Particularly noteworthy is the growing evidence supporting active therapeutic approaches, which have shown promising results in enhancing both mechanical stability and neuromuscular control [11, 12].
While traditional passive joint mobilization (PJM) techniques have historically dominated clinical practice, recent research has highlighted the potential advantages of active, weight-bearing mobilization strategies [13–16]. These emerging approaches theoretically provide enhanced proprioceptive input and more functionally relevant neuromuscular activation patterns. However, robust comparative analyses of these contrasting therapeutic paradigms remain limited.
Therefore, this investigation aims to evaluate the differential effects of active joint mobilization (AJM) versus traditional PJM in individuals with CAI. We hypothesize that the integration of active components may yield superior outcomes through enhanced proprioceptive feedback and neuromuscular recruitment patterns.
Materials and methods
Study design and participants
This study was designed as a single-blind, parallel-group randomized controlled trial comparing the effects of AJM and PJM in patients with CAI. The study protocol was registered at ClinicalTrials.gov (NCT04630899/Sep 30, 2021) and approved by the Korea National Institute for Bioethics Policy (KoNIBP), a public institutional review board (P01-202105-11-003/May 27, 2021). All participants provided written informed consent before participation.
Participants were recruited from Gwangju Health University and The Better Hospital in October 2021. Eligible participants were individuals who had their initial ankle sprain at least one year before the study commenced and had experienced a minimum of two subsequent ankle sprains within the past 12 months. Additional inclusion criteria were a Cumberland Ankle Instability Tool (CAIT) score below 24 and no incidence of ankle sprain within six weeks of trial commencement. Participants were excluded if they had a history of lower-extremity surgery, received therapy for the affected lower extremity within the previous month, or had psychiatric disorders [15, 17, 18].
Sample size calculation
The sample size was calculated based on joint position sense (JPS) as the primary outcome measure [17], with an anticipated effect size of d = 1.07. Using a power of 0.80 and an alpha level of 0.05, we determined that 24 participants would be required. Accounting for an expected dropout rate of 20%, we aimed to recruit a total of 30 participants.
Interventions
All therapeutic interventions were administered by certified physical therapists with extensive clinical experience. All therapists underwent standardized training in both AJM and PJM techniques prior to the study commencement. Inter-rater reliability testing among the therapists showed excellent agreement [intraclass correlation coefficient (ICC) > 0.85] for both mobilization techniques. Treatment sessions were conducted three times per week for four weeks, with each session lasting 10 minutes, totaling 12 interventions.
The AJM protocol incorporated a novel biomechanical approach [19–21]. The initial proprioceptive awareness stage (weeks 1–2) employed specific positioning and manual techniques. With participants in prone position and knees flexed, the practitioner secured the patient’s foot against their sternum for stabilization. The therapist placed the thumb of one hand on the talus while gently encompassing the foot with the remaining fingers. On the other hand, the therapist stabilized the lateral malleolus of the fibula using the thenar eminence. As the therapist shifted their body weight forward, the patient’s ankle moved into dorsiflexion, creating an anterior to posterior gliding of the talus and lateral malleolus of the fibula. This technique facilitated a coordinated mobilization movement along the joint’s anatomical axis.
Static holds at neutral position were maintained for precisely 30 seconds per set, with 15-second rest intervals between each of the three sets. Small-amplitude oscillations were performed at a controlled frequency of 1 Hz for 30 seconds, guided by a metronome to ensure consistency. Progression criteria from the initial stage to the active engagement phase required consistent position matching with less than 5° error over three consecutive sessions and stable maintenance without compensatory movements.
The active engagement phase (weeks 3–4) incorporated patient-driven ankle movements through available range with verbal and tactile feedback from the practitioner. Progressive weight-bearing exercises were implemented using a calibrated pressure biofeedback unit, beginning at 20% body weight and advancing by 10% increments when patient.
For PJM, we implemented a modified Maitland technique [22]. This approach utilized grade III mobilization characterized by oscillatory movements spanning the middle to end ranges of available joint motion. With participants supine, the practitioner executed posterior talar mobilization while maintaining tibial stabilization, incorporating one-second rhythmic oscillations at the point of tissue resistance.
Outcomes
Outcome measures were assessed during pre-intervention testing (10–15 minutes) immediately before the first treatment session and post-intervention testing (10–15 minutes) following the completion of the final session.
Our assessment protocol incorporated both primary and secondary outcome measures. Primary outcomes encompassed two key parameters: functional ankle stability and dorsiflexion range of motion (DFROM). Ankle stability was quantified using the CAIT [23, 24], a validated instrument comprising nine weighted items. The CAIT’s psychometric properties demonstrate robust test-retest reliability (ICC = 0.96), with a validated minimal clinically important difference threshold of three points [25]. DFROM assessment utilized smartphone-based clinometer technology [clinometer 1.2 dynamometer (TKK-5401, Japan), SHIGETO TAKAGI] with standardized positioning protocols [26, 27].
Our assessment protocol incorporated both primary and secondary outcome measures based on their relevance to CAI pathomechanics. While primary outcomes evaluated functional stability and range of motion, secondary outcomes were specifically selected to assess key aspects of neuromuscular control that are frequently compromised in CAI. Static balance and JPS were chosen as they represent fundamental components of postural stability and proprioceptive function, which Mihcin [4] identified as crucial factors in joint stability assessment. Other potential parameters such as dynamic balance tests, strength measurements, and functional performance assessments were considered but not included to maintain focus on the basic mechanisms of postural control and proprioceptive accuracy, which directly influence both mechanical and clinical aspects of ankle stability.
Secondary outcomes focused on neuromuscular control parameters. We employed the APP-Coo-Test system (version 2.1, Pentawire, Italy) for both static balance and JPS assessment, which has demonstrated high intra-rater reliability (ICC = 0.89–0.94) in previous validation studies [28, 29]. For static balance evaluation, the smartphone was secured to the participant’s sternum while maintaining a bilateral stance position. Following the selection of the static balance test module, participants maintained their position for 10 seconds while the system tracked postural sway. For JPS assessment, the smartphone was attached to the plantar surface of the participant’s foot. Participants started in a plantarflexed position and were instructed to move into neutral dorsiflexion and maintain this position for the 10-second testing duration. Both measurements generated percentage-based scores, with higher values indicating better neuromuscular performance.
Randomization and blinding
The enrolled participants were randomly allocated to either AJM or PJM groups in equal numbers using allocation software (Random Allocation Software 2.0, Isfahan University, Iran). Each participant was assigned a unique two-digit identification code to maintain anonymity throughout the study. To ensure single-blinding, treatment sessions for each group were scheduled at different times to prevent participants from identifying their group allocation or interacting with participants from the other group. All outcome measurements were conducted by a single assessor at baseline and post-intervention time points.
Statistical analysis
Data analysis was conducted using statistical software (IBM SPSS v25.0). The Shapiro-Wilk test was performed to assess the normality of data distribution. Our analytical approach encompassed descriptive statistics for demographic characterization, with between-group comparisons utilizing chi-squared analyses for categorical variables and independent t-tests for continuous measures. Treatment effects were evaluated through both between-group and within-group analyses, with statistical significance established at p < 0.05. Cohen’s d effect sizes were calculated to determine the magnitude of treatment effects, with values of 0.2, 0.5, and 0.8 representing small, medium, and large effects, respectively [30].
Results
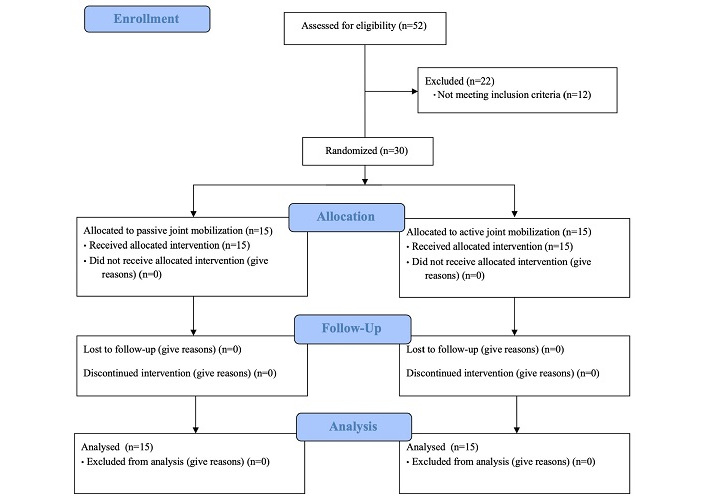
Figure 1 shows a flowchart of this study based on the Consolidated Standards of Reporting Trials (CONSORT) guidelines. Fifty-two potential participants were screened, and twenty-two participants were excluded, with twelve not meeting inclusion criteria. All 30 enrolled participants were analyzed without any dropouts.
Characteristics of the participants
Table 1 presents the participants’ general characteristics. There were no significant differences between the groups in terms of sex, affected side, age, height, weight, body mass index (BMI), foot size, and CAIT (p > 0.05).
Characteristics of the participants
| Variables | PJM (n = 15) | AJM (n = 15) | x2/t |
|---|---|---|---|
| Sex (female, n) | 11 | 9 | 0.600 |
| Age (years) | 20.80 ± 1.82 | 20.33 ± 1.23 | 0.822 |
| Height (cm) | 166.67 ± 6.38 | 168.33 ± 5.30 | 0.449 |
| Mass (kg) | 62.00 ± 14.18 | 59.27 ± 9.31 | 0.624 |
| BMI (kg/m2) | 22.26 ± 4.70 | 20.87 ± 2.70 | 0.992 |
| Foot size (cm) | 247.33 ± 12.37 | 245.33 ± 13.82 | 0.418 |
| Affected side (left, n) | 6 | 3 | 1.429 |
| CAIT (point) | 19.67 ± 4.03 | 20.73 ± 3.92 | –0.735 |
AJM: active joint mobilization; BMI: body mass index; CAIT: Cumberland ankle instability tool; PJM: passive joint mobilization
Results of outcome measures
Table 2 presents differences between and within groups for ankle outcome measures. The analysis found no significant differences between PJM and AJM groups for any outcome measures [CAIT: t = 1.659, 95% confidence interval (CI) = –0.61 to 5.81; DFROM: t = –0.839, 95% CI = –8.72 to 3.65; JPS: t = –0.513, 95% CI = –11.11 to 6.66; Static balance: t = –0.179, 95% CI = –9.72 to 8.16].
Differences between groups by measurement time point for ankle primary and secondary outcome measures
| Variables | PJM (n = 15) | AJM (n = 15) | ||
|---|---|---|---|---|
| Baselines (A) | Post-test (B) | Baselines (A) | Post-test (B) | |
| Cumberland ankle instability tool | ||||
| Mean ± SD (point) | 19.67 ± 4.03 | 25.87 ± 5.76 | 20.73 ± 3.92 | 24.33 ± 4.69 |
| B–A (Cohen’s d) | 6.20** (1.248) | 3.60* (0.834) | ||
| t (95% CI) | 1.659 (–0.61 to 5.81) | |||
| Dorsiflexion range of motion | ||||
| Mean ± SD (°) | 21.73 ± 7.81 | 35.13 ± 7.42 | 24.13 ± 10.43 | 40.07 ± 4.95 |
| B–A (Cohen’s d) | 13.40** (1.758) | 15.93** (1.951) | ||
| t (95% CI) | –0.839 (–8.72 to 3.65) | |||
| Joint position sense | ||||
| Mean ± SD (%) | 38.79 ± 10.38 | 52.54 ± 15.32 | 42.44 ± 11.31 | 58.41 ± 13.20 |
| B–A (Cohen’s d) | 13.75* (1.051) | 15.97** (1.300) | ||
| t (95% CI) | –0.513 (–11.11 to 6.66) | |||
| Static balance | ||||
| Mean ± SD (%) | 77.63 ± 14.77 | 82.63 ± 10.86 | 77.10 ± 12.60 | 82.89 ± 9.76 |
| B–A (Cohen’s d) | 5.01 (0.386) | 5.79 (0.514) | ||
| t (95% CI) | –0.179 (–9.72 to 8.16) | |||
* p < 0.01; ** p < 0.001. AJM: active joint mobilization; CI: confidence interval; PJM: passive joint mobilization
Within-group analysis revealed significant improvements in both groups. The PJM group showed significant improvements in CAIT (6.20, p < 0.001), DFROM (13.40, p < 0.001), and JPS (13.75, p < 0.01). Similarly, the AJM group demonstrated significant improvements in CAIT (3.60, p < 0.01), DFROM (15.93, p < 0.001), and JPS (15.97, p < 0.001).
Cohen’s d effect sizes indicated large effects for most within-group improvements, particularly in DFROM (PJM: d = 1.758; AJM: d = 1.951) and JPS (PJM: d = 1.051; AJM: d = 1.300).
Discussion
This study investigated the comparative effects of active versus PJM in individuals with CAI. Our findings contribute to the growing body of evidence regarding therapeutic approaches for CAI management while highlighting several important insights.
Research on joint mobilization for CAI has traditionally focused on passive techniques, with recent systematic reviews indicating their effectiveness in improving ankle dorsiflexion and dynamic postural control [31]. Our study extends this understanding by directly comparing active and passive approaches, addressing a significant gap in the literature.
Both AJM and PJM groups demonstrated significant within-group improvements across multiple parameters. The PJM group showed substantial improvements in CAIT scores (p < 0.001), DFROM (p < 0.001), and JPS (p < 0.01), consistent with previous findings [2]. The AJM group exhibited comparable improvements, with slightly larger effect sizes in DFROM (d = 1.951) and proprioceptive function (d = 1.300), though between-group differences were not statistically significant. The relatively modest effect sizes observed in static balance measures (PJM d = 0.386; AJM d = 0.514) compared to other outcome parameters reflect the complexity of postural control mechanisms. While both interventions demonstrated improvements in balance, these moderate effects suggest that static balance may require additional targeted interventions beyond joint mobilization alone. The slightly larger effect size in the AJM group may indicate that active engagement facilitates better integration of proprioceptive input into postural control strategies, although this difference did not reach statistical significance.
The mechanisms underlying these improvements likely involve multiple pathways. First, joint mobilization techniques may enhance mechanoreceptor activation and sensorimotor integration, as suggested by recent neuroimaging studies showing altered cortical activation patterns in CAI patients [32, 33]. Second, the mechanical effects on joint arthrokinematics could improve talocrural joint mobility, addressing the restricted posterior talar glide commonly observed in CAI [11].
The slightly enhanced outcomes in the AJM group, particularly in proprioceptive measures, may be attributed to increased neuromuscular recruitment during active engagement. This aligns with contemporary motor learning principles emphasizing task-specific training [6]. Recent research has demonstrated that active, weight-bearing exercises can enhance both mechanical stability and neuromuscular control in CAI patients [14].
However, our findings contrast with some previous studies suggesting superior outcomes with active mobilization techniques [34]. This discrepancy might be explained by differences in intervention protocols, patient populations, or outcome measures. Furthermore, the similar effectiveness of both approaches suggests that the mechanical stimulus provided by joint mobilization, whether active or passive, may be the primary driver of therapeutic benefits.
These results have important clinical implications. While both techniques prove effective, the choice between AJM and PJM might be influenced by individual patient factors such as pain tolerance, activity level, and rehabilitation stage. The comparable outcomes suggest clinicians can confidently employ either approach, potentially selecting based on patient preference or specific clinical circumstances.
Clinical decision-making for intervention selection should consider individual patient characteristics. AJM may be more appropriate for patients demonstrating good pain tolerance and capability for active engagement, particularly those requiring enhanced proprioceptive training. Conversely, PJM might be better suited for patients in acute phases or those with pain limitations. Treatment selection should be guided by factors including activity level, rehabilitation stage, and specific functional deficits identified during initial assessment.
Study limitations include the relatively short intervention period and focus on immediate outcomes. Future research should investigate long-term effects and potential differences in return-to-sport outcomes, while also considering the development of patient-specific treatment algorithms based on individual functional deficits and recovery patterns. The protocol could be optimized by adjusting treatment parameters (e.g., mobilization intensity, duration) according to patient’s baseline characteristics and their response to intervention [31]. Furthermore, our methodology was limited to clinical and functional outcome measures without incorporating structural imaging or comprehensive biomechanical analyses. The inclusion of magnetic resonance imaging (MRI) or ultrasound evaluation could provide valuable insights into structural adaptations of ligamentous and soft tissue components following different mobilization techniques, while gait analysis could quantify functional movement pattern changes resulting from treatment. Studies incorporating such advanced imaging and neurophysiological measurements could better elucidate the underlying mechanisms of improvement, potentially helping identify patient subgroups who respond better to specific interventions. Such an evidence-based personalization approach could enhance treatment efficiency and lead to more robust outcomes in managing CAI.
In conclusion, both AJM and PJM demonstrated effectiveness in improving functional ankle stability, range of motion, and proprioceptive function in individuals with CAI. While the active approach showed slightly larger effect sizes in certain parameters, both techniques can serve as viable therapeutic options. For clinical practice, we recommend that therapists consider incorporating either approach based on individual patient characteristics and treatment phase, with AJM potentially offering additional benefits for patients requiring enhanced proprioceptive training. Future research investigating the long-term effects and combining these approaches with other rehabilitation strategies may further optimize treatment outcomes for CAI patients.
Abbreviations
| AJM: | active joint mobilization |
| CAI: | chronic ankle instability |
| CAIT: | Cumberland Ankle Instability Tool |
| CI: | confidence interval |
| DFROM: | dorsiflexion range of motion |
| ICC: | intraclass correlation coefficient |
| JPS: | joint position sense |
| PJM: | passive joint mobilization |
Declarations
Author contributions
HK: Conceptualization, Methodology, Investigation, Writing—original draft, Writing—review & editing, Supervision. EL: Conceptualization, Methodology, Formal analysis, Visualization, Writing—review & editing. MK: Conceptualization, Methodology, Resources, Data curation, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Korea National Institute for Bioethics Policy (KoNIBP) institutional review board (P01-202105-11-003).
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.