Abstract
Aim:
COVID-19, a multisystemic syndrome caused by SARS-CoV-2, often results in long-term complications collectively referred to as long COVID. This study explores the persistence of neurological and otolaryngological symptoms in patients two years after acute infection, with a focus on gender differences and variant-specific effects.
Methods:
A retrospective follow-up was conducted in January 2024 on 112 patients who had been hospitalized for COVID-19. Patients completed a questionnaire assessing the persistence of neuropsychiatric, otolaryngological, and systemic symptoms.
Results:
Findings reveal that 18.3% of women reported persistent neuropsychiatric symptoms, such as memory deficits, depression, and concentration issues, compared to 5.7% of men. Otolaryngological symptoms, including anosmia and ageusia, largely resolved, with only 4.5% reporting persistent issues. Symptom persistence was more common in older individuals, women, smokers, and those with severe acute-phase illness. Neuropsychiatric symptoms remain prominent, underscoring the need for targeted long-term care.
Conclusions:
Vaccination significantly reduces the risk and severity of long COVID, particularly neuropsychiatric symptoms, emphasizing its role in mitigating the long-term burden of SARS-CoV-2. Future research should explore biomolecular markers and imaging techniques to better understand and address these long-term sequelae.
Keywords
NeuroCOVID, SARS-CoV-2, COVID-19 variants, neurological, otolaryngologicalIntroduction
The coronavirus disease 2019 (COVID-19) is a multisystemic viral septic syndrome that can affect various organs, with symptoms ranging from mild to potentially life-threatening. Neurological complications are frequently reported and may arise as direct or indirect consequences of the viral infection, medical treatments, systemic inflammation triggered by immune activation, or hypoxia [1]. In some cases, these complications may also occur as incidental associations. However, it is known that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has neuroinvasive potential. The term long COVID refers to the persistence of at least one symptom, such as fatigue, shortness of breath, cognitive decline or brain fog, post-exertional malaise, memory problems, musculoskeletal pain or spasms, coughing, sleep disturbances, rapid heartbeat or palpitations, altered sense of smell or taste, headaches, chest pain, and depression, in individuals with a prior diagnosis of COVID-19. Key demographic and clinical predictors identified so far include being female, older age, smoking, pre-existing medical conditions, lack of COVID-19 vaccination, infection with pre-Omicron SARS-CoV-2 variants, the number of acute-phase symptoms, high viral load, severe or critical COVID-19 illness, and the need for invasive mechanical ventilation.
Growing evidence suggests that our microbiota plays a crucial role in regulating the immune system and controlling inflammation. When its balance is disrupted, it could contribute to the lingering symptoms of long COVID. Recent studies have even found that changes in the nasal microbiota might be linked to the persistence of post-COVID symptoms. This sheds new light on how our microbiota isn’t just involved in the initial infection but may also influence long-term recovery, paving the way for potential new treatments [2]. In particular, recent research has described how changes in the nasal microbiota may be linked to the chronic persistence of post-COVID-19 symptoms [3]. The microbiota of the upper airways plays a crucial role in defending against respiratory infections by influencing both local immune responses and inflammation regulation. Changes in its composition could contribute to the persistence of symptoms like fatigue, shortness of breath, brain fog, and olfactory disturbances, which are common in long COVID. Recent studies suggest that imbalances in the nasal microbiota may promote chronic inflammation and hinder tissue repair, offering new insights into the pathophysiology of long COVID and potential therapeutic strategies.
While we still know little about the upper respiratory tract microbiota in long COVID patients, significant research has focused on its alterations in COVID-19 patients, including the microbiomes of the mouth, nose, oropharynx, and especially the nasopharynx. In many cases, this dysbiosis persists even after the SARS-CoV-2 virus and the acute symptoms of the disease have disappeared. It is particularly noteworthy that changes in the oral microbiota of long COVID patients have been observed, potentially contributing to ongoing inflammation, strengthening the idea that the upper respiratory tract microbiota plays a key role in the development of long COVID.
In addition, more research will be needed to understand whether dysbiosis can lead to the release of bacterial toxins, which could impact mitochondrial function and contribute to the chronic fatigue many long COVID patients experience. What’s even more important, though, is that we still don’t fully understand the roles of factors like the virome, microbiome, metabolome, and meta-transcriptome in long COVID patients. These elements may also play a significant role in the disease’s development, so they definitely need more attention in future studies [4].
A retrospective study [5] offers a detailed look at the connection between the microbiota and COVID-19. The respiratory, intestinal, and oral systems are all susceptible to SARS-CoV-2 infection, and the microorganisms in these areas undergo significant changes. These disruptions can affect tissues and organs throughout the body, triggering cytokine storms, damaging immune barriers, and suppressing immune responses. Additionally, metabolites produced by the microbiota may influence immunity in various ways; for instance, short-chain fatty acids could help reduce viral infection by lowering the expression of angiotensin-converting enzyme 2 (ACE2) and TMPRSS2 genes. The microbiota can also interact with ACE2, the primary receptor that allows the virus to enter human cells. It’s been recognized as a potential diagnostic biomarker for a range of diseases. For instance, intestinal dysbiosis can persist long after the virus is gone, leading to lasting effects on the body. As a result, clinical care shouldn’t just focus on removing the virus but should also aim at restoring the balance of the intestinal microbiota to support long-term health [6, 7].
However, this argument does have its limitations: it’s challenging to say with certainty whether the changes in the microbiota are directly caused by COVID-19 or are the result of treatments, individual factors, diet, or other variables. Additionally, we still don’t fully understand the exact mechanisms through which the microbiota affects the severity and susceptibility to COVID-19. By studying how the microbiota interacts with the body’s immune system, we may be able to develop targeted interventions to improve the effectiveness of vaccines.
Based on this universally agreed-upon definition, long COVID can be summarized as a clinical syndrome characterized by the persistence of at least one typical COVID-19 symptom that has not resolved three months after recovering from an acute SARS-CoV-2 infection.
Accurately estimating the epidemiological burden of long COVID, as well as identifying its predictors, remains challenging. This is largely due to the use of varying definitions and follow-up durations, as well as the inclusion of diverse populations with differing demographic characteristics (such as age, gender, and ethnicity) and clinical profiles (including disease severity, comorbidities, and vaccination status).
What sets long COVID apart from other post-viral syndromes is its significantly higher epidemiological burden, with prevalence estimates up to six times greater than similar conditions following other viral infections (up to 63% compared to approximately 10%) [8]. Notably, when these figures are considered alongside official WHO statistics (650 million diagnosed SARS-CoV-2 infections by the end of 2022), it suggests that up to 400 million people worldwide (a conservative estimate) could potentially seek care for long COVID in the near future. This would place unprecedented strain on an already overburdened and depleted healthcare system.
Materials and methods
In January 2024, two years after the initial hospitalization, we conducted a follow-up to monitor the health conditions of the 112 patients examined, divided into 52 men and 60 women (Figure 1). These patients were interviewed via phone and given a questionnaire about the current state of their clinical symptoms in both the otolaryngological and neurological areas. The Sapienza University of Rome Hospital Ethical Committee approved this retrospective study (Ref. 6536), and all the study procedures followed the Helsinki Declaration of 2013, for human rights and experimentation. This retrospective study was conducted at the University Hospital “Policlinico Umberto I” in Rome.
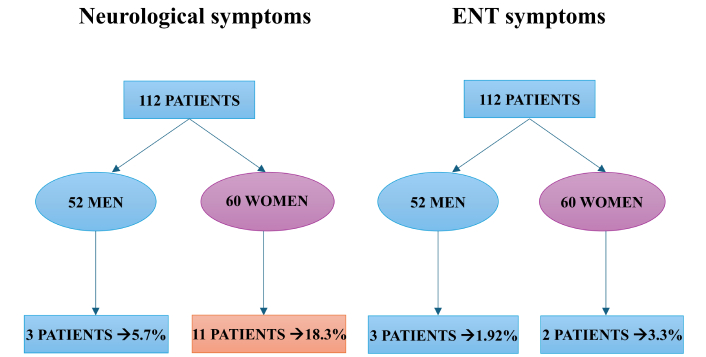
Follow-up analysis of neurological and otolaryngological symptoms in 112 patients. The figure indicates that neurological symptoms at 24 months are more frequent than other symptoms, especially in women
Results
The retrospective study conducted in January 2024 reveals that, despite the Omicron variant causing a reduction in otolaryngological symptoms (anosmia, ageusia, dizziness) both during the acute phase and over time, neuropsychological symptoms remain more prominent. The findings indicate a higher incidence of neuropsychiatric alterations compared to the non-hospitalized post-COVID-19 population during the acute phase.
The study significantly highlights the persistence of neurological symptoms in a large proportion of patients hospitalized during the acute phase. This clinical-epidemiological observation must be carefully evaluated and further investigated. This will involve expanding our analysis with a larger sample and using targeted questionnaires, imaging techniques, and the identification of biomolecular markers to explore the long-term neurological consequences of SARS-CoV-2 infection, also known as NeuroCOVID.
The questionnaire results showed that 18.3% of the women reported neuropsychiatric alterations 24 months later, such as memory deficits, periods of depression or anxiety, and concentration problems. Among the 52 men, only 5.7% reported cognitive performance changes. In contrast to the variants before Omicron, otolaryngological symptoms like anosmia, ageusia, sudden hearing loss, and dizziness have mostly decreased or fully resolved in nearly all patients. Only 4.5% of the sample reported persistent symptoms in the otolaryngological domain.
Based on our study, combined with findings from other meta-analyses, we can conclude that the most significant predictors of symptom persistence two years after recovery were advanced age (most of the articles examined found that long COVID symptoms were associated with older age [9]), female sex, cigarette smoking, and disease severity.
Discussion
It is crucial to highlight the ongoing presence of neurological symptoms in COVID-19 patients even 24 months after infection. Several studies have shown that these neurological issues can persist over time, significantly affecting patients’ overall well-being. Common symptoms include cognitive difficulties, such as problems with concentration and mental fatigue, as well as motor impairments. These issues, often referred to as “brain fog”, have been among the most disabling for those recovering from COVID-19, limiting their ability to perform daily tasks and diminishing their quality of life [10]. Neurological symptoms, with their various patterns of presentation, are among the main symptoms observed in the acute phase in patients who were hospitalized during the acute phase of COVID-19. A study involving 214 COVID-19 patients revealed that 36.4% experienced neurological symptoms, with a notably higher incidence (45.5%) in patients who had a severe form of the infection [11]. These neurological symptoms included acute cerebrovascular events, altered consciousness, and muscle damage. This data suggests that the severity of the respiratory infection is a key factor in the development and persistence of neurological symptoms, possibly indicating direct damage to the nervous system caused by the virus. Given the continued presence of neurological symptoms even after 24 months, it is essential to monitor patients who experienced severe COVID-19, paying close attention to their neurological health. Such long-term monitoring can deepen our understanding of the underlying mechanisms of these complications and help develop more effective treatment strategies to improve patients’ quality of life and reduce the long-term impact of these persistent symptoms. The emergence of new COVID-19 variants may impact both the severity and the persistence of neurological symptoms. Variants such as B.1.1.7 (Alpha), B.1.526, and B.1.351 (Beta) have shown different transmission patterns, immune evasion strategies, and possibly more serious clinical outcomes. For example, the B.1.1.7 variant, first identified in the carries mutations that enhance the virus’s ability to bind to the ACE2 receptor, which may lead to more severe infections and a higher likelihood of neurological symptoms. Similarly, the B.1.526 variant, discovered in New York, includes mutations that could alter the virus’s interaction with the immune system, potentially resulting in prolonged symptoms and complications, particularly neurological ones [12]. Given these differences, it is vital to understand how these variants might influence the long-term effects of COVID-19. They may contribute to more persistent or severe neurological issues. As such, ongoing monitoring of patients infected with various variants is essential to track emerging trends in symptom duration and to adjust treatment strategies accordingly. The rapid development and rollout of COVID-19 vaccines began with the goal of protecting the population from the original SARS-CoV-2 strain, which was driving a surge in infections, particularly in densely populated areas [13]. Since the introduction of vaccines in 2021, numerous new variants of the virus have emerged, raising fresh public health concerns (Table 1). To simplify the naming and communication about these variants, on May 31, 2021, the WHO introduced a classification system based on the Greek alphabet, assigning names like Alpha, Beta, Gamma, and Delta. These new variants include, among others, D614G, B.1.1.7 (also known as Alpha, VOC-202012/01 or 201/501Y.V1), B.1.526, B.1.351 (also known as Beta or 501Y.V2), B.1.1.28.1 (including P1, also known as Gamma), and B.1.617. These strains exhibit various mutations, many of which are located in the spike protein, leading to changes in the virus’s behavior and pathogenicity. Notably, alterations in the receptor-binding domain (RBD) have enhanced the virus’s ability to evade natural immunity. The emergence of these variants undoubtedly poses a serious threat to controlling the COVID-19 pandemic. One of the first significant mutations in the original SARS-CoV-2 genome was the D614G point mutation in the spike protein, which led to the G614 variant. This mutation emerged early in the pandemic, first identified in Germany and China in late January 2020, before spreading globally [14]. Alongside this genetic change, three additional mutations were commonly observed: a C-to-T mutation in the 5' UTR at position 241, another similar C-to-T mutation at position 3,037, and a C-to-T mutation at position 14,408 in the RNA-dependent RNA polymerase (RdRp) gene [15, 16]. It has been found that this mutation increases both infectivity and viral replication in human tissues compared to the original D614 virus, although this increase in infectivity did not result in higher lethality [15, 17, 18]. The enhanced infectivity is linked to a greater affinity of the RBD due to the acquired mutation, as the glycine (G) replacing aspartic acid (D) likely provides more flexibility to the trimeric structure of the spike protein, improving its binding affinity [15].
SARS-CoV-2 variants and associated symptoms, emergence periods, and vaccination
| Variant | Neurological symptoms | Vestibular symptoms | Othorhinolaryngologicalsymptoms | Vaccine introduction and effectiveness | Period of emergence |
|---|---|---|---|---|---|
| Alpha (B.1.1.7) | HeadacheBrain fog | Dizziness | Loss of taste/smellSore throat | December 2020 (first detected in the UK) | Vaccines were introduced in December 2020. Moderate reduction in neutralization for Moderna and Novavax vaccines compared to the original strain. |
| Beta (B.1.351) | HeadacheFatigueCognitive issues | Dizziness (rare) | Nasal congestionSore throat | Late 2020 (first detected in South Africa) | Pfizer vaccine showed reduced neutralization. Covaxin demonstrated significant neutralization. |
| Gamma (P.1) | AnxietyDepressionHeadache | Dizziness | Prolonged loss of taste/smellNasal congestion | Late 2020 (first detected in travelers from Brazil in Japan) | Reduced neutralization observed with Pfizer-BioNTech and Moderna vaccines. |
| Delta (B.1.617.2) | Severe headacheConfusion | Dizziness (rare) | Severe sore throatEar painReduced loss of smell | Late 2021 (first detected in India) | Pfizer and Moderna vaccines showed reduced immune response due to L452R mutation. Improved transmissibility linked to spike protein mutations. |
| Omicron (BA.1, BA.2, BA.4, BA.5) | Mental fatigueBrain fog | Dizziness (less common) | Predominant sore throatLess frequent loss of taste/smell | Late 2021 (first detected in multiple regions) | Vaccines and prior immunity appear to mitigate the severe effects. Less anosmia due to differences. In cellular tropism and reduced inflammatory dysregulation. |
| Omicron (XBB, BQ.1) | HeadacheBrain fogPeripheral neuropathy | Dizziness (less common) | Dominant nasal symptomsDry cough | Late 2022 (first detected in multiple regions) | Updated booster vaccines were introduced in mid-2022 to target Omicron subvariants. |
In terms of therapeutic effects on the mutated variant, studies have shown that the Pfizer-BioNTech BNT162b2 vaccine, based on the original D614 sequence, exhibited a 1.7- to 2.0-fold reduction in neutralization, making it less effective [18]. On the other hand, the Moderna mRNA-1273 vaccine demonstrated similar neutralization levels compared to the original strain [19]. The pathogenic B.1.1.7 variant (also known as Alpha, VOC-202012/01 or 201/501Y.V1) was first detected in the UK in December 2020 and is now present in over 40 countries. This variant carries 17 non-synonymous mutations, including 8 in the spike protein and the D614G mutation. Three of the 8 mutations in the spike protein are particularly notable: a two-amino-acid deletion at positions 69-70, N501Y, and P681H [20]. The N501Y mutation has been shown to increase the affinity of the RBD for ACE2, similar to the D614G mutation. Both the Moderna and Novavax vaccines showed only a moderate reduction in neutralization of the B.1.1.7 variant in vitro compared to the original strain [20, 21]. The B.1.526 variant was first identified in New York in November 2020, and its prevalence has since grown exponentially in the state and surrounding areas. Notable mutations in B.1.526 include E484K (the most significant) and S477N in the spike protein, along with five other common mutations: L5F, T95I, D253G, D614G, and A701V [22]. The B.1.351 variant (also known as Beta or 501Y.V2) was first identified in South Africa at the end of 2020 and was the predominant variant in the region at that time. It has shown reduced neutralization both with convalescent serum and serum from individuals vaccinated with the Pfizer vaccine (Table 1) [23]. The B.1.1.28 variant was first identified in Rio de Janeiro, Brazil, in February 2020. This variant includes the E484K mutation. Studies have found that the Covaxin vaccine (developed in India) significantly increased neutralization against this variant [24]. However, serum from individuals vaccinated with Pfizer-BioNTech and Moderna showed reduced neutralization of the variant. The P1 variant (also known as Gamma or 20J/501Y.V3) belongs to the B.1.1.28 lineage and was initially identified in travelers from Brazil who arrived in Japan. It carries several mutations that make it more severe, increasing infectivity and reducing neutralization by antibodies. As with the B.1.351 variant, serum from individuals vaccinated with Pfizer-BioNTech and Moderna showed reduced neutralization of the P1 variant. The most recent variant of concern is B.1.617, identified in India at the end of 2020. This variant has evolved into three subvariants, the most prominent being B.1.617.2 (also known as Delta). It carries characteristic mutations in the spike protein, including D111D (a synonymous substitution), G142D, L452R, E484Q, D614G, and P681R. Three concerning mutations in the RBD—L452R, E484Q, and P681R—are located at the furin cleavage site [25]. These mutations may increase binding with ACE2, as seen in other variants with similar mutations, and enhance the cleavage between S1 and S2, improving transmissibility. The L452R mutation has also been shown to reduce the immune response to Pfizer-BioNTech and Moderna vaccines compared to the original strain [26]. The Omicron variant appears to be highly transmissible, exhibiting numerous substitutions in the spike glycoprotein, and emerged at a time when much of the global population had received the SARS-CoV-2 vaccine. It is possible that the characteristics of the Omicron variant, or previously acquired immunity, either from prior infection or vaccination, may explain the lower incidence of olfactory disturbances. Omicron might lead to less anosmia due to differences in cellular tropism, mechanisms of cell entry, and producing less inflammatory dysregulation [27]. It is important to note that not all variants are associated with the persistence of symptoms months after the initial infection (Table 1). Our study aimed to assess, over a period of 24 months following hospitalization, the persistence, reduction, or disappearance of clinical symptoms related to neurological and otolaryngological conditions. The sample of patients, who were interviewed by phone, was focused on identifying, based on their own subjective reports, the presence of the following symptoms: anosmia, ageusia, dizziness, sudden hearing loss (in the otolaryngological domain); memory deficits, attention problems, depressive symptoms, and concentration issues (in the neurological domain); as well as fatigue or muscle weakness (as systemic symptoms). The questionnaire revealed that 18.3% of women reported neuropsychiatric issues, including memory deficits, depression, anxiety, and concentration problems, 24 months post-infection. In comparison, only 5.7% of men experienced cognitive changes. Unlike earlier variants, Omicron-related otolaryngological symptoms-anosmia, ageusia, hearing loss, and dizziness-largely resolved, with only 4.5% reporting persistent issues. This percentage aligns with the data in the literature regarding the persistence of neurological symptoms in long COVID, which is typically around 20% [28]. Notably, in the male cohort, these values correspond to 5.7%. Moving forward, it will be important to evaluate the potential presence of co-factors that may contribute to a higher incidence of neurological symptoms in the female population compared to the male population. Several reasons have been reported in the literature to explain the differences in the incidence of neurological symptoms between men and women. However, when considering COVID-19 specifically, we must also take into account many other factors, and this will be one of the future objectives of our work. It is also noteworthy that the otolaryngological symptoms have almost completely disappeared, suggesting that the Omicron variant, as highlighted by several molecular studies, likely had a reduced impact on the pathophysiological effects on the neurosensory systems compared to previous variants. It is important to remember that this evaluation could also factor in vaccination, as the Omicron variant, unlike previous variants, was the first one to emerge following the global mass vaccination campaign against SARS-CoV-2. Epidemiological studies are still ongoing to assess the effectiveness of vaccines, both in terms of infectious and immune responses. In addition, although it is challenging to estimate the persistence of symptoms over time across different ethnic groups, it is notable that the Caucasian population appears to be the most affected by long-term symptoms. One possible explanation for this could be that Caucasians more frequently carry a variant of the ACE2 expressed in the olfactory epithelium [29, 30]. The persistence of COVID-19 symptoms, commonly referred to as long COVID, is thought to be driven by several hypotheses.
For example, some studies suggest potential biomarkers associated with the persistence of symptoms [31]. Soluble CD163 (sCD163) (the soluble form of CD163, a specific receptor for monocytes/macrophages that binds hemoglobin-haptoglobin complexes) is likely released in the central nervous system (CNS) by activated macrophages and microglia through complex immunomodulatory mechanisms in the microenvironment [32]. It has been suggested that this biomarker could be used to assess the risk of disease progression, as increased plasma levels of sCD163 have been observed upon admission in COVID-19 patients, particularly those who develop acute respiratory distress syndrome (ARDS). The potential role of sCD163 as a biomarker of CNS damage is of interest. It is known that sCD163 is upregulated during the pro-inflammatory response, and the release of matrix metalloproteinases (MMPs) plays a key role in driving this process. A positive correlation has been observed between levels of CSF (cerebrospinal fluid) sCD163 and MMP-9, suggesting that elevated levels of sCD163 and MMP-9 in the CSF may contribute to the infiltration of monocytes into the CSF in COVID-19 patients. Plasma levels of sCD163 were found to be elevated compared to those seen in cognitively normal individuals or those with mild, asymptomatic neurocognitive damage [33]. Additionally, in neurodegenerative diseases like Parkinson’s disease, sCD163 is considered a potential biomarker related to cognition, highlighting the role of monocytes in both peripheral and brain immune responses [34]. Regardless of the underlying mechanisms causing symptom persistence, vaccination has proven essential not only in reducing the transmission of SARS-CoV-2 but also in protecting the population from the long-term effects of the infection. Recent studies indicate that vaccinated individuals are significantly less likely to develop long COVID compared to those who are unvaccinated. Moreover, among those who contract the infection despite being vaccinated, persistent symptoms tend to be less severe and of shorter duration. Therefore, vaccination is a crucial tool in preventing both acute COVID-19 infection and its potential long-term consequences. Emerging evidence suggests that COVID-19 vaccines may not only efficiently reduce the risk of developing severe or critical COVID-19, but they could also play a role in preventing long COVID. A recent meta-analysis published by Notarte et al. [35], which included a total of 11 peer-reviewed studies and 6 preprints (up to June 20, 2022) with 17,256,654 participants, concluded that COVID-19 vaccination was globally associated with a lower risk of long COVID. In particular, two doses of the vaccine showed more favorable results compared to a single dose. Specifically, among the 11 studies that examined changes in long COVID symptoms following vaccination, 7 concluded that long COVID symptoms could improve after vaccination against COVID-19. Moreover, compared to vaccinated individuals who received at least one booster dose, unvaccinated individuals had a 40% higher risk of developing long COVID (OR: 1.41; 95% CI: 1.05–1.91).
Although, as we have repeatedly emphasized in this discussion, vaccines have played a key role in limiting the spread of the virus and improving the prognosis of patients with COVID-19, it is also important to consider the safety profile of these drugs. In particular, some studies have highlighted the occurrence of psychiatric adverse events (AEs) following COVID-19 vaccination, raising questions about the impact of the vaccine not only on the immune system but also on neurological and psychological levels.
Symptoms such as anxiety, insomnia, mood disturbances, and, in rare cases, psychotic episodes have sparked scientific debate about the potential link between vaccination and side effects on the CNS. While the exact mechanism is not yet fully understood, it is suggested that the inflammatory response triggered by the vaccine could affect neurotransmitters and brain circuits involved in mood and stress regulation. Additionally, the pandemic context itself, marked by high levels of psychological stress, may have contributed to exacerbating or making these symptoms more noticeable.
However, it’s important to view these findings in the broader context of the overall benefits of vaccination. That being said, research should continue to explore these AEs, aiming to better understand their causes, identify any risk factors, and improve how we manage patients who are affected by them.
The study examined the psychiatric AEs following COVID-19 vaccination in a large cohort from Seoul, South Korea. Over 2 million participants were included, divided into two groups: vaccinated and unvaccinated [36].
The results showed an increased incidence of depression, anxiety, dissociative, stress-related, and somatoform disorders, sleep and sexual disorders in the vaccinated group compared to the unvaccinated group. However, vaccination was associated with a lower risk of schizophrenia and bipolar disorder. Statistical analyses revealed that the risk increased for depression (HR = 1.683), anxiety and related disorders (HR = 1.439), sleep disorders (HR = 1.934), while it decreased for schizophrenia (HR = 0.231) and bipolar disorder (HR = 0.672) [36].
In conclusion, while our retrospective study on the persistence of post-COVID symptoms at 24 months has provided valuable insights, there are some limitations that affect the interpretation of the results.
Limitations of the study
One of the main limitations of this study is the sample size, which, with only 112 patients, may affect the generalizability of the results. A small number of participants limits the ability to apply the findings to a broader population, highlighting the need for a larger sample to obtain more robust and representative data.
In addition to the sample size, another limitation is the method of data collection, which was carried out through telephone interviews. Telephone interviews represent a practical and accessible methodology, particularly useful in longitudinal studies where long-term follow-up can be challenging. This approach allows for efficient patient outreach, reducing costs and dropout rates compared to more invasive methods or those requiring in-person visits. Moreover, it enables the collection of subjective information about persistent symptoms directly from the patients, without the need for additional clinical exams or diagnostic tests.
However, a key limitation of this approach is that it relies on self-reported data, which can be influenced by memory bias and personal interpretation. Since our study looked at symptoms up to 24 months after COVID-19 infection, some participants might have trouble recalling the exact duration, frequency, or severity of their symptoms. This is known as recall bias, and it could lead to either an overestimation or an underestimation of how long neurological symptoms actually persisted.
Moreover, the way symptoms are perceived can vary from person to person, influenced by factors like emotional state, mental health, and overall well-being. For instance, symptoms like brain fog, fatigue, or sleep issues might feel different to each individual and could be reported unevenly. Without objective tests (like neurocognitive assessments or other diagnostic tools), our study relies solely on how patients perceive their symptoms, which could be a limitation.
Finally, the lack of direct clinical evaluation limits the ability to confirm or quantify certain symptoms, especially those of a neurological nature. While the telephone interviews were conducted using structured and standardized questions to minimize these biases, there remains the risk of variability in the responses, which could affect the results.
Implications for the future studies
To improve upon the limitations of this study, future research will aim to increase the sample size in order to make the results more representative and statistically reliable. A larger sample will allow for a deeper look at how gender, age, and comorbidities may affect the persistence of post-COVID symptoms, giving us a fuller picture of how these factors contribute to long-term health outcomes.
In addition, it will be crucial to complement the telephone interviews with more objective clinical assessments, such as neuropsychological tests or diagnostic exams, to better understand and confirm the severity of neurological symptoms. Using a mixed-methods approach, which combines self-reported data with standardized clinical measurements, could reduce the impact of personal biases and provide more accurate results.
To minimize memory bias, future studies might include more frequent follow-ups and digital tools like electronic symptom diaries. This would enable researchers to track symptom progression in real-time, improving the reliability of the data. Along with regular follow-ups, there’s a growing interest in identifying biomarkers. It would be beneficial to explore non-invasive or minimally invasive biomarkers, such as those that can be measured from routine blood tests. These biomarkers could serve as useful indicators of the patients’ overall health and the severity of the disease.
One study found that patients with long COVID had higher levels of inflammatory biomarkers long after the initial infection. Our own research also revealed significant links between certain biomarkers and long COVID symptoms. For example, patients with long COVID showed higher levels of IL-6 (30%), CRP (15%), and TNF-α (15%) compared to those who had fully recovered, along with lower hemoglobin levels (10%). Among the biomarkers analyzed, cytokines/chemokines and biochemical markers made up 23.9% and 39.1%, respectively. In particular, 44.2% of the biomarkers were cytokines/chemokines, while 20.9% were vascular markers [37], suggesting that these biomarkers could be important indicators of long COVID syndrome.
These approaches would help solidify our understanding of the persistence of post-COVID-19 symptoms and long-term cognitive dysfunction [38] and pave the way for more focused strategies to support the long-term care of these patients. In this direction, it is clear that new explorations on the therapeutic aspects of ENT disorders in long COVID-19 patients would be useful [39]. To date, active clinical trials on the medical therapy of anosmia, focusing on the efficacy of neuro-protective and anti-inflammatory agents as palmitoylethanolamide (PEA), luteolin (LUT), and cerebrolysin [39]. The protective effects on olfactory epithelium are restorative on post-COVID-19 anosmia, and also biologics, olfactory training, and electrical stimulation are suggested methods for the treatments of olfactory impairment in post-COVID-19 patients. The potential therapeutic approaches, pharmacological, biological, molecular, and genetic, are under investigations [39].
Abbreviations
| ACE2: | angiotensin-converting enzyme 2 |
| AEs: | adverse events |
| CNS: | central nervous system |
| COVID-19: | coronavirus disease 2019 |
| CSF: | cerebrospinal fluid |
| MMPs: | matrix metalloproteinases |
| RBD: | receptor-binding domain |
| SARS-CoV-2: | severe acute respiratory syndrome coronavirus 2 |
| sCD163: | soluble CD163 |
Declarations
Author contributions
WAR: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. CB: Conceptualization, Writing—original draft, Writing—review & editing, Supervision. AM: Conceptualization, Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Sapienza University of Rome Hospital Ethical Committee (Ref. 6536), and all the study procedures followed the Helsinki Declaration of 2013, for human rights and experimentation.
Consent to participate
Informed consent was not needed due to the retrospective nature of this study.
Consent to publication
Not required.
Availability of data and materials
The datasets that support the findings of this study are available from the corresponding author on reasonable request.
Funding
We acknowledge financial support under the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.1, Call for tender No. 104 published on 2.2.2022 by the Italian Ministry of University and Research (MUR), funded by the European Union—NextGenerationEU—Project Title—Mapping NEUROCOVID via neurobiology and neurovolatilome in Post-COVID-19 patients—CUP B53D23018450006—Grant Assignment Decree No. 1110 adopted on 20 July 2023 by the Italian Ministry of Ministry of University and Research (MUR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.