Abstract
Aim:
This study aimed to investigate the susceptibility patterns of Pseudomonas aeruginosa strains, examine infection characteristics, and evaluate the appropriateness of empiric antibiotic therapy. Additionally, the study sought to identify factors influencing 30-day all-cause mortality in patients with Pseudomonas aeruginosa infections.
Methods:
This was a retrospective study conducted at Jordan University Hospital from January 2018 to March 2024. Adult patients (≥ 18 years) with confirmed Pseudomonas aeruginosa infections were included. Data were collected from medical records, focusing on demographics, infection characteristics, antibiotic treatment, and outcomes. The susceptibility patterns of Pseudomonas aeruginosa isolates were classified as multidrug-resistant (MDR) or non-MDR. Logistic regression was used to identify factors associated with 30-day mortality.
Results:
A total of 210 patients were included in the study, with 106 males (50.5%) and 104 females (49.5%). The majority of infections were community-acquired (n = 178, 84.8%), with the respiratory tract being the most common infection site (n = 81, 38.6%). Nearly half of the Pseudomonas aeruginosa isolates were MDR (n = 99, 47.1%). Empiric antibiotic therapy was administered to all patients, with imipenem-cilastatin (55.7%), vancomycin (35.7%), and piperacillin-tazobactam (26.7%) being the most commonly used antibiotics. Of the 210 patients, 32.4% (n = 68) received inappropriate empiric therapy. The 30-day all-cause mortality rate was 4.9% (n = 10). Multivariate analysis revealed that non-localized infections, such as bacteremia and sepsis, were strongly associated with increased mortality [adjusted odds ratio (AOR) = 17.455, P < 0.001].
Conclusions:
This study highlights the high prevalence of MDR Pseudomonas aeruginosa infections, especially in community-acquired cases, and emphasizes the need for improved antimicrobial stewardship. The significant proportion of patients (32.4%) receiving inappropriate empiric therapy calls for better guidance in antibiotic prescribing practices. The key predictor of mortality was infection localization, indicating the importance of early intervention for systemic infections to reduce mortality rates.
Keywords
Pseudomonas aeruginosa, multidrug-resistant, antibiotic resistance, mortality predictors, JordanIntroduction
Multidrug-resistant (MDR) bacteria present a significant global health threat, complicating the treatment of infections and leading to increased healthcare costs and patient risks [1, 2]. Among these, Pseudomonas aeruginosa is particularly concerning due to its ability to thrive in healthcare settings and its natural resistance to many antibiotics [3]. The overuse and misuse of antibiotics are major contributors to the rise of resistant strains [4], highlighting the need for urgent action in antibiotic stewardship and infection control [5].
Healthcare environments, especially hospitals, are particularly vulnerable to MDR bacteria. High patient turnover, invasive procedures, and the frequent use of medical devices create conditions conducive to the spread of these pathogens [6]. Healthcare-associated infections (HAIs) are especially dangerous for immunocompromised patients, worsening an already critical situation [7]. Effective infection control practices, such as maintaining hygiene and sterilization, are crucial for preventing the spread of these harmful bacteria [8].
In the Middle East, the prevalence of P. aeruginosa infections is alarming, driven by factors such as high rates of chronic diseases and inadequate infection control measures [9]. The misuse of antibiotics further exacerbates the issue, promoting the development of resistant strains [10, 11]. As treatment options for P. aeruginosa are limited by the rise of MDR strains, healthcare providers are increasingly resorting to combination therapy, which involves using multiple antibiotics to enhance effectiveness [12].
Despite the significant impact of P. aeruginosa infections, there is a notable lack of research focused on susceptibility patterns and treatment practices in Jordanian hospitals. This gap in knowledge makes it difficult to develop effective infection control strategies tailored to the region. Therefore, this study aimed to investigate the susceptibility patterns of P. aeruginosa strains, examine infection characteristics, and evaluate the appropriateness of empiric antibiotic therapy. Additionally, the study sought to identify factors influencing 30-day all-cause mortality in patients with P. aeruginosa infections.
Materials and methods
Study design and setting
This retrospective study was conducted at Jordan University Hospital (JUH), the first academic teaching hospital in Jordan, with a 550-bed capacity. The study aimed to investigate adult patients (aged 18 years and older) hospitalized with P. aeruginosa infections. It involved a review of electronic medical records for patients who tested positive for P. aeruginosa from January 2018 to March 2024.
Inclusion and exclusion criteria
The study included adults aged 18 years and older who were diagnosed with P. aeruginosa infections and admitted to the hospital during the study period. Exclusion criteria were applied to ensure the accuracy of the analysis. Patients with incomplete medical records, those lacking susceptibility testing data, individuals with polymicrobial infections, and patients who were transferred out of the hospital before receiving empirical antibiotic therapy were excluded from the study. This approach enabled us to assess the appropriateness of empiric antibiotic therapy initiated during the hospital stay.
Data collection
Data for all eligible patients were collected through a review of their medical records. The collected data included demographic information such as age, gender, admission date, and the total duration of hospitalization. Clinical details were also gathered, including the department of admission, site of infection acquisition, the season of infection acquisition, admitting diagnosis, comorbid conditions, previous colonization, any recent hospitalizations within the past three months, antimicrobial use, and the use of corticosteroids or immunosuppressants prior to infection acquisition. Additionally, information on invasive medical procedures before infection was obtained. The study also collected details about the primary infection site, specimen retrieval, empirical antibiotic therapy (including initiation date, specific antibiotics used, dosage, and duration), susceptibility testing results, adjustments to therapy based on culture outcomes, and the 30-day all-cause mortality rate.
Study outcomes
The study aimed to assess several key outcomes related to P. aeruginosa infections. One of the primary outcomes was the susceptibility patterns of the P. aeruginosa strains, which were classified into four categories based on resistance: non-MDR strains, which are sensitive to all tested antibiotics or resistant to only one agent in one or two antimicrobial drug classes; and MDR strains, which exhibit resistance to antibiotics in at least one agent in three or more antimicrobial drug classes. These classes are aminoglycosides, monobactams, cephalosporins, cephalosporin with beta-lactamase inhibitors, penicillins, fluoroquinolones, polymyxins, carbapenems, and glycylcyclines. Another key outcome was the evaluation of management practices, particularly the appropriateness of empirical antibiotic therapy. This included an assessment of the timely initiation of treatment, the choice of antibiotics, dosages, and the duration of therapy. The study also focused on 30-day all-cause mortality, aiming to identify factors that influence mortality within 30 days of infection.
Ethical considerations
The study adhered to the ethical guidelines established by the World Medical Association’s Declaration of Helsinki. The research protocol was approved by the Institutional Review Board at JUH (Approval number R023/28293). Patient confidentiality was ensured by anonymizing all medical records, and as the study was retrospective, direct patient contact was not involved. Consequently, informed consent was not required for this study.
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to summarize categorical variables with frequencies and percentages, and continuous variables with means and standard deviations. Continuous variables were tested for normality using the Shapiro-Wilk test before performing statistical comparisons. Chi-square test was used to evaluate the relationship between previous medication use (immunosuppressant/chemotherapy, antibiotics, and corticosteroids) and the presence of MDR versus non-MDR P. aeruginosa. Moreover, logistic regression analysis was conducted to identify independent factors associated with 30-day all-cause mortality. Variables with a P-value less than 0.250 in simple logistic regression were included in multiple logistic regression analysis. Prior to this, potential multicollinearity among variables was assessed, ensuring that the Pearson correlation coefficient between any two variables was less than 0.9. A P-value of < 0.05 was considered statistically significant.
Results
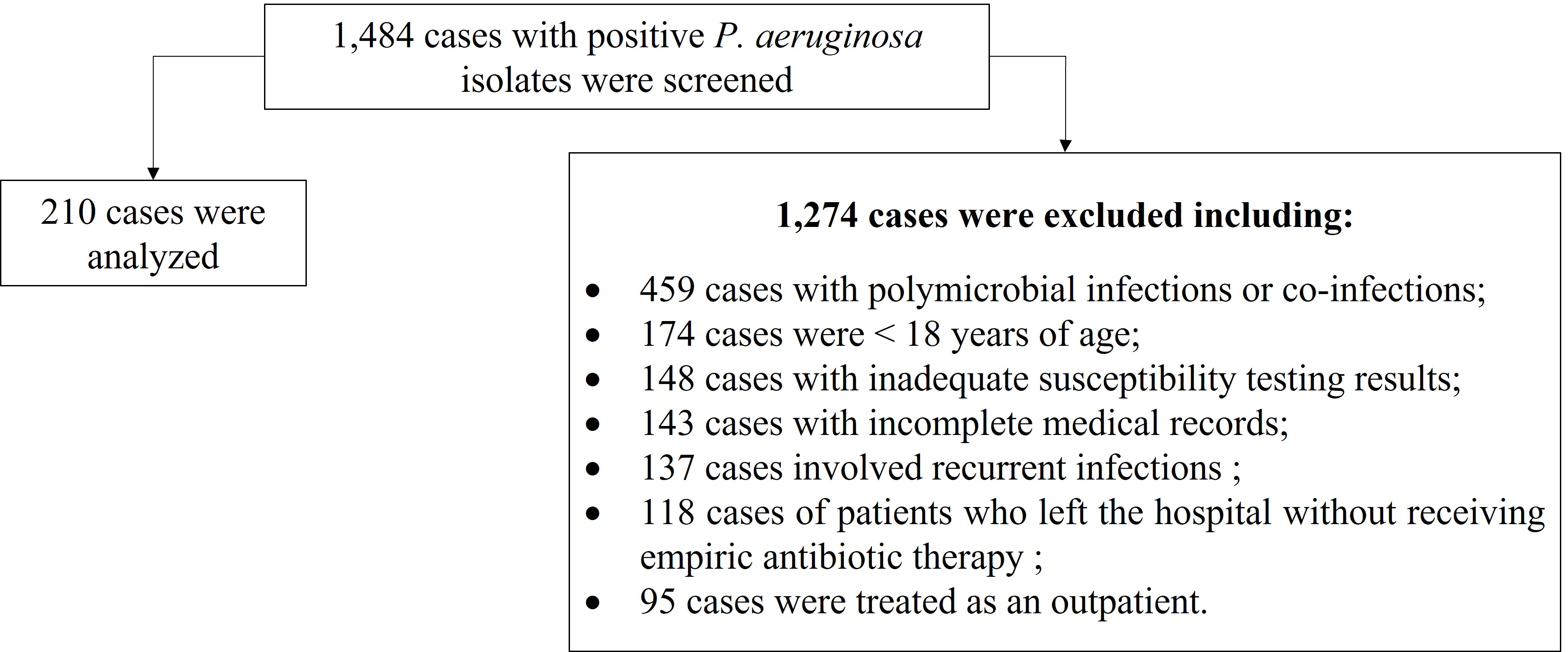
A total of 1,484 cases with positive P. aeruginosa isolates were screened, of which 1,274 cases were excluded as they did not meet the inclusion criteria. These include: 459 cases with polymicrobial infections or co-infections, 174 cases were < 18 years of age, 148 cases with inadequate susceptibility testing results, 143 cases with incomplete medical records, 137 cases involved recurrent infections, 118 cases of patients who left the hospital without receiving empiric antibiotic therapy, and 95 cases were treated as an outpatient. As such, a total of 210 cases were included and analyzed (14.1%) (Figure 1).
The study cohort, as shown in Table 1, consisted of 210 patients, with a nearly balanced gender distribution: 106 males (n = 106, 50.5%) and 104 females (n = 104, 49.5%). Approximately one-quarter of the patients were aged 65 years or older (n = 54, 25.7%). Regarding smoking status, 31.9% (n = 67) were current smokers, 7.1% (n = 15) were ex-smokers, and the majority, 61.0% (n = 128) were non-smokers. The majority of patients had public insurance coverage (n = 202, 96.2%), while only a small minority (n = 8, 3.8%) had private insurance.
Patient demographics and medical characteristics (n = 210)
| Parameter | Frequency (%) |
|---|---|
| Gender | |
| Male | 106 (50.5) |
| Female | 104 (49.5) |
| Age categories (years) | |
| < 35 | 41 (19.5) |
| 35−44 | 43 (20.5) |
| 45−54 | 29 (13.8) |
| 55−64 | 43 (20.5) |
| ≥ 65 | 54 (25.7) |
| Smoking status | |
| Non-smoker | 128 (61.0) |
| Ex-smoker | 15 (7.1) |
| Smoker | 67 (31.9) |
| Health status | |
| Healthy | 36 (17.1) |
| With chronic illnesses | 174 (82.9) |
| Most common chronic illnesses | |
| Diabetes mellitus | 75 (35.7) |
| Hypertension | 74 (35.2) |
| Bronchiectasis | 25 (11.9) |
| Myocardial infarction | 21 (10.0) |
| Insurance type | |
| Public | 202 (96.2) |
| Private | 8 (3.8) |
In terms of health status, 82.9% (n = 174) of the cohort had chronic medical conditions. The most common chronic conditions were diabetes mellitus type II (n = 75, 35.7%), hypertension (n = 74, 35.2%), bronchiectasis (n = 25, 11.9%), and myocardial infarction (n = 21, 10.0%).
The majority of infections were community-acquired (n = 178, 84.8%), while 15.2% (n = 32) were hospital-acquired infections. The primary sites of infection were the respiratory tract (n = 81, 38.6%), skin and soft tissue (n = 48, 22.9%), and urinary tract (n = 40, 19.0%).
Infections occurred most frequently during the winter and fall seasons (n = 61 each, 29.0%). Within the three months prior to infection acquisition, 39.5% of patients (n = 83) had been hospitalized, and 39.5% (n = 83) had received antibiotics. Approximately 6.2% (n = 13) of patients had been exposed to major surgery, and 5.2% (n = 11) had undergone urethral stenting before infection acquisition.
Among the tested P. aeruginosa isolates, nearly half (47.1%, n = 99) were MDR pathogens. Regarding treatment outcomes, 4 patients (out of 210) were lost to follow-up during the 30-day period (discharged against medical advice). Of the remaining 206 patients, the 30-day all-cause mortality rate was 4.9% (n = 10), while 95.1% (n = 196) survived. Detailed information is presented in Table 2.
Medical information related to P. aeruginosa infections (n = 210)
| Parameter | Frequency (%) |
|---|---|
| Source of infection acquisition# | |
| Community-acquired | 178 (84.8) |
| Hospital-acquired | 32 (15.2) |
| Primary site of infection | |
| Respiratory tract | 81 (38.6) |
| Skin and soft tissue | 48 (22.9) |
| Urinary tract | 40 (19.0) |
| Surgical site | 15 (7.1) |
| Bloodstream | 7 (3.3) |
| Bone | 5 (2.4) |
| Others | 14 (6.7) |
| Season of infection acquisition | |
| Winter | 61 (29.0) |
| Spring | 45 (21.4) |
| Summer | 43 (20.5) |
| Fall | 61 (29.0) |
| Hospitalization within the last three months | |
| No | 127 (60.5) |
| Yes | 83 (39.5) |
| Use of antibiotics within the last three months | |
| No | 127 (60.5) |
| Yes | 83 (39.5) |
| Use of corticosteroids within the last three months | |
| No | 177 (84.3) |
| Yes | 33 (15.7) |
| Previous immunosuppressant/chemotherapy use within the last three months | |
| No | 198 (94.3) |
| Yes | 12 (5.7) |
| Invasive procedures prior to infection acquisition | |
| No | 167 (79.5) |
| Major surgery | 13 (6.2) |
| Double J stent | 11 (5.2) |
| Othersa | 19 (9.0) |
| Infection localization | |
| Localized | 193 (91.9) |
| Sepsis | 12 (5.7) |
| Bacteremia | 5 (2.4) |
| Resistance pattern of P. aeruginosa | |
| Non-MDR bacteria | 111 (52.9) |
| MDR bacteria | 99 (47.1) |
| 30-days, all-cause mortality^ | |
| Survived | 196 (95.1) |
| Died | 10 (4.9) |
a Foley, central venous line, endoscopy, venous access port, dialysis/acute renal replacement therapy, cystoscopy, ureteroscopy. # Community-acquired infections are those acquired within 48 hours of hospital admission, while hospital-acquired infections are those that become evident after 48 hours of hospitalization. ^ The percentages were calculated based on 206 individuals after excluding patients who were discharged against medical advice. MDR: multidrug-resistant
All patients in the study received empiric antibiotics (n = 210, 100.0%). The number of empiric antibiotics administered varied among patients: 21.0% (n = 44) received one antibiotic, while 44.3% (n = 93) received two antibiotics. In terms of the route of administration, the majority of patients were treated with intravenous antibiotics (n = 206, 98.1%). These findings are summarized in Table 3.
Empirical antimicrobial therapy received (n = 210)
| Parameter | Frequency (%) |
|---|---|
| Empiric antibiotics received | |
| Yes | 210 (100.0) |
| No | 0 (0) |
| Number of empiric antibiotics received | |
| 1 | 44 (21.0) |
| 2 | 93 (44.3) |
| 3 | 49 (23.3) |
| ≥ 4 | 24 (11.4) |
| Route of administration# | |
| Intravenous | 206 (98.1) |
| Oral | 166 (79.0) |
| Topical | 4 (1.9) |
# Patients could receive antibiotics via more than one route of administration, which is why the percentages for each route do not necessarily sum to 100%
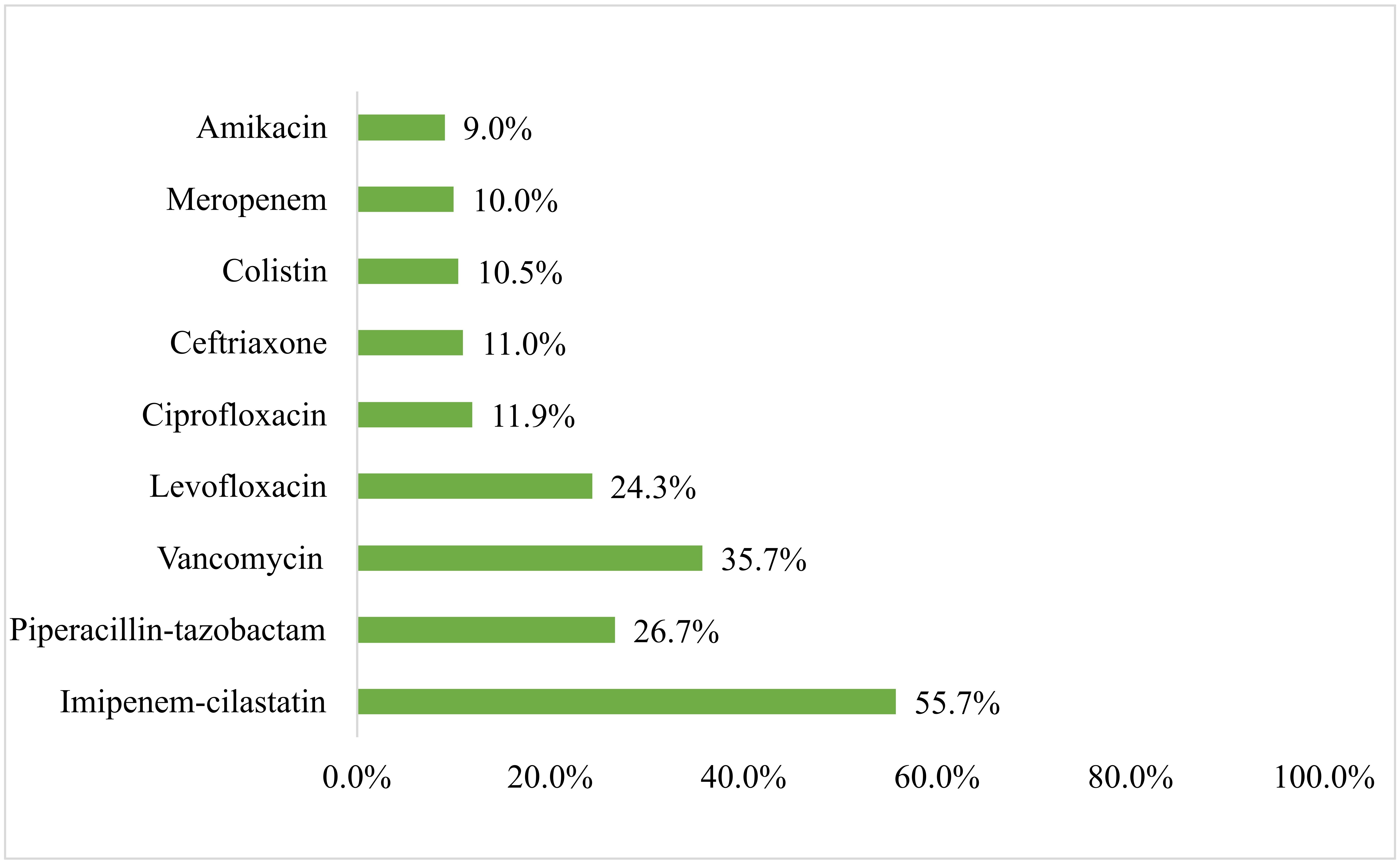
The most frequently used empiric antibiotics were imipenem-cilastatin (55.7%), followed by vancomycin (35.7%), piperacillin-tazobactam (26.7%), and levofloxacin (24.3%) (Figure 2). When evaluating the appropriateness of the prescribed antibiotics, almost one-third of the patients (n = 68, 32.4%) received inappropriate initial antimicrobial therapy. Antibiotic prescriptions by infection type are presented in Table S1.
When evaluating predictors of mortality in patients with P. aeruginosa infection (Table 4), infection localization was significantly associated with 30-day all-cause mortality. Patients with non-localized infections, such as bacteremia and sepsis, were 17.45 times more likely to experience mortality compared to those with localized infections [adjusted odds ratio (AOR) = 17.455, P < 0.001].
Assessment of predictors associated with 30-days all-cause mortality in patients with P. aeruginosa infection (n = 206)
| Parameter | 30-days all-cause mortality (0: Survived, 1: Died) | |||
|---|---|---|---|---|
| COR | P-value# | AOR | P-value$ | |
| Age | ||||
| < 65 years | Reference | 0.02^ | Reference | 0.12 |
| ≥ 65 years | 4.625 | 3.406 | ||
| Gender | ||||
| Males | Reference | 0.48 | -- | -- |
| Females | 1.595 | |||
| Health status | ||||
| Healthy | Reference | 0.99 | -- | -- |
| With chronic illnesses | 0.000 | |||
| Source of infection acquisition | ||||
| Community-acquired | Reference | 0.04^ | Reference | 0.36 |
| Hospital-acquired | 4.000 | 2.136 | ||
| Site of infection | ||||
| Respiratory tract | Reference | 0.91 | -- | -- |
| Others | 0.930 | |||
| Hospitalization within the last three months | ||||
| No | Reference | 0.52 | -- | -- |
| Yes | 1.531 | |||
| Use of antibiotics within the last three months | ||||
| No | Reference | 0.99 | -- | -- |
| Yes | 1.009 | |||
| Use of corticosteroids within the last three months | ||||
| No | Reference | 0.18^ | Reference | 0.36 |
| Yes | 2.571 | 2.416 | ||
| Previous immunosuppressant/chemotherapy use within the last three months | ||||
| No | Reference | 0.04^ | Reference | 0.74 |
| Yes | 5.873 | 1.504 | ||
| Invasive procedures prior to infection acquisition | ||||
| No | Reference | 0.40 | -- | -- |
| Yes | 0.407 | |||
| Infection localization | ||||
| Localized | Reference | < 0.001^ | Reference | < 0.001* |
| None localized | 25.227 | 17.455 | ||
| Appropriateness of empiric therapy | ||||
| Not appropriate | Reference | 0.60 | -- | -- |
| Appropriate | 0.709 | |||
| Type of antimicrobial resistance | ||||
| Non-MDR | Reference | 0.39 | -- | -- |
| MDR | 1.985 | |||
# Using simple logistic regression; $ Using multiple logistic regression; ^ Eligible for entry in multiple logistic regression; * Significant at 0.05 significance level. MDR: multidrug-resistant; COR: crude odds ratio; AOR: adjusted odds ratio
When evaluating the relationship between previous medication use and the presence of MDR versus non-MDR P. aeruginosa infections (Table 5), recent antibiotic use is significantly associated with MDR infections (P < 0.001). In contrast, corticosteroid use does not show a significant correlation with MDR infections (P = 0.091). Furthermore, the use of immunosuppressants or chemotherapy is significantly linked to non-MDR infections (P = 0.029).
The association between previous medication use and antimicrobial resistance
| Parameters | Non-MDR bacteria (n = 111) | MDR bacteria (n = 99) | P-value$ |
|---|---|---|---|
| Use of antibiotics within the last three months | |||
| Yes (n = 83) | 31 (37.3) | 52 (62.7) | < 0.001* |
| No (n = 127) | 80 (63.0) | 47 (37.0) | |
| Use of corticosteroids within the last three months | |||
| Yes (n = 33) | 13 (39.4) | 20 (60.6) | 0.091 |
| No (n = 177) | 98 (55.4) | 79 (44.6) | |
| Previous immunosuppressant/chemotherapy use within the last three months | |||
| Yes (n = 12) | 10 (83.8) | 2 (16.7) | 0.029* |
| No (n = 198) | 110 (51.0) | 97 (49.0) | |
$ Using Chi-square test. * Significant at 0.05 significance level. MDR: multidrug-resistant
Discussion
This study offers an examination of P. aeruginosa infections at a tertiary teaching hospital in Jordan, focusing on clinical profiles, infection sources, antibiotic susceptibility patterns, and management strategies. The study found that the majority of P. aeruginosa infections (84.8%) were community-acquired, which contrasts with the traditional belief that P. aeruginosa is predominantly a hospital-acquired pathogen [13]. The primary infection sites were the respiratory tract (38.6%), skin and soft tissue (22.9%), and urinary tract (19.0%), which is consistent with P. aeruginosa’s preference for moist environments and areas with impaired defense mechanisms [14]. P. aeruginosa can colonize and multiply in hospital water systems such as sinks, showers, and water distribution, leading to biofilm formation. Inhalation of these droplets or direct contact with contaminated water can result in respiratory infections or colonization of the skin and soft tissues [15].
Early empiric therapy for high-risk patients was crucial, with most patients receiving combination antibiotic therapy. Clinicians favored imipenem-cilastatin and vancomycin, reflecting the need to provide broad-spectrum coverage for both Gram-negative and Gram-positive pathogens, especially MDR strains common in Jordan [14]. The study also highlighted concerning resistance patterns, particularly to ciprofloxacin (48.6%) and meropenem (49.8%), which are critical antibiotics for managing severe infections. Notably, colistin demonstrated the highest sensitivity, with only 3.4% resistance, highlighting its role as a last-resort therapy for MDR infections. However, the emerging resistance to colistin, even at low levels, signals a growing concern for its long-term efficacy [16].
Antibiotic resistance in P. aeruginosa was widespread, with 48% of isolates being MDR. These findings align with global trends and mirror those observed in Qatar, where similar resistance patterns were reported, emphasizing the increasing challenge of treating P. aeruginosa infections [17]. This highlights the need for effective antimicrobial stewardship programs to manage and limit the spread of resistant strains. Our study finds that recent antibiotic use is significantly associated with the presence of MDR P. aeruginosa infections. This can be explained by selective pressure, where frequent antibiotic use eliminates non-resistant bacteria, allowing resistant strains to survive and multiply [18]. Additionally, immunosuppressive therapies weaken the immune system, making patients more vulnerable to infections caused by non-resistant bacteria.
Despite the high resistance rates, the study found no significant difference in the 30-day mortality rate between patients infected with non-MDR strains and those infected with MDR strains. This is consistent with other research, which has indicated that MDR strains do not always lead to worse outcomes if treated promptly and effectively [19]. The key predictor of mortality in this study was infection localization, with non-localized infections such as bacteremia and septicemia significantly increasing the likelihood of death. This finding emphasizes the importance of early detection and treatment of systemic infections to improve patient outcomes [20].
The study also found that 32.4% of patients received inappropriate initial therapy, likely due to inadequate coverage of P. aeruginosa or resistance to prescribed antibiotics. This highlights the need for better utilization of antibiograms and rapid diagnostic tools to guide empirical therapy and optimize patient outcomes [21].
This study was conducted within a single tertiary teaching hospital in Jordan, which may limit the generalizability of the results to other healthcare settings. The retrospective design and exclusion of newer antibiotics, such as ceftazidime-avibactam and imipenem-cilastatin-relebactam, from the susceptibility testing may affect the completeness of the resistance patterns observed. Moreover, by restricting the study to hospitalized patients who received empirical antibiotic therapy during their hospital stay, outpatients were excluded. This exclusion may have led to an overestimation of resistance patterns. Consequently, the observed trends likely represented more severe infections requiring hospitalization, making it difficult to generalize the findings to milder cases managed in outpatient settings. Additionally, due to the retrospective nature of the study and incomplete documentation in the medical records, we did not evaluate the effect of empiric antibiotics on patient health conditions, focusing instead on the appropriateness of empiric therapy and 30-day mortality. Furthermore, the exclusion of polymicrobial or co-infection cases limits the understanding of the complexity and severity of infections involving multiple microbes. Future research should include these cases to provide a more comprehensive understanding and improve treatment strategies.
Conclusions
In conclusion, this study highlights the rising challenge of P. aeruginosa infections, particularly with increasing MDR. The high rate of community-acquired infections and widespread resistance emphasize the need for improved antimicrobial stewardship, early empiric therapy, and better use of rapid diagnostics. While no significant mortality difference was found between MDR and non-MDR strains, infection localization was a key predictor of death. The study emphasizes the importance of early intervention, the need for newer antibiotics in susceptibility testing, and further research to optimize treatment strategies for P. aeruginosa infections.
Abbreviations
| MDR: | multidrug-resistant |
Supplementary materials
The supplementary table for this article are available at: https://www.explorationpub.com/uploads/Article/file/1001312_sup_1.pdf.
Declarations
Author contributions
SS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. RI: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. KAH: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. RKAF: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The research protocol was approved by the Institutional Review Board at JUH (Approval number R023/28293).
Consent to participate
Due to the retrospective nature of the study, direct patient contact was not involved. Consequently, informed consent was not required for this study.
Consent to publication
Not applicable.
Availability of data and materials
Required information can be available upon request from the corresponding author (Rana Abu-Farha, r_abufarha@asu.edu.jo).
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.