Affiliation:
1Pediatrics Section, Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, 84081 Baronissi (Salerno), Italy
†The authors contributed equally to the work.
ORCID: https://orcid.org/0000-0001-7152-7254
Affiliation:
1Pediatrics Section, Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, 84081 Baronissi (Salerno), Italy
†The authors contributed equally to the work.
ORCID: https://orcid.org/0000-0002-9216-7881
Affiliation:
2Pediatrics, AOU San Giovanni di Dio e Ruggi d’Aragona, 84131 Salerno, Italy
ORCID: https://orcid.org/0000-0001-8248-9740
Affiliation:
2Pediatrics, AOU San Giovanni di Dio e Ruggi d’Aragona, 84131 Salerno, Italy
ORCID: https://orcid.org/0000-0002-3263-4046
Affiliation:
1Pediatrics Section, Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, 84081 Baronissi (Salerno), Italy
Email: pvajro@unisa.it
ORCID: https://orcid.org/0000-0002-9418-5880
Affiliation:
3Department of Pediatrics, Santobono-Pausilipon Children’s Hospital, 80129 Naples, Italy
ORCID: https://orcid.org/0000-0002-2991-1102
Explor Med. 2021;2:333–342 DOI: https://doi.org/10.37349/emed.2021.00051
Received: May 13, 2021 Accepted: June 28, 2021 Published: August 31, 2021
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero Universitaria di Modena, Italy
The article belongs to the special issue Exploring NAFLD/NASH
The term non-alcoholic fatty liver disease (NAFLD) appears unfitting both in adults and in children. As obesity and metabolic syndrome play a relevant pathogenic role, an international group of adults’ liver disease experts has proposed to rename this condition metabolic (dysfunction)-associated fatty liver disease (MAFLD). While this new more appropriate and useful definition has mostly been met with good reactions in adults, it may present a tangled path in pediatrics. Here we further stress the recommendations of the North American and the European Societies for Pediatric Gastroenterology, Hepatology and Nutrition that a hyperechogenic liver in a child affected by obesity or overweight with chronically elevated liver enzymes should not be assumed to have NAFLD only. Especially in those patients who are not in the classic age range or who have particularly severe laboratory anomalies, other genetic, metabolic (inborn errors of metabolism, IEM), endocrine, intestinal and hepatic pediatric-onset conditions, should in fact be excluded, particularly when response to a weight loss trial is not available. The term pediatric fatty liver disease (PeFLD) with three subtypes [1. contextual diagnosis of an IEM; 2. metabolic (dysfunction)-associated fatty liver; 3. unknown cause of fatty liver] has recently been proposed aiming to separate true MAFLD from IEM and/or the other above mentioned conditions, which may be rare when considered individually but represent a large group when considered collectively. Although the cost-effectiveness ratio of this attitude is still indeterminate, it is likely that the advantage of the early identification of a specifically treatable pediatric-onset liver disease associated to/mimicking MAFLD would be rewarding.
Owing to the relentless increase in pediatric overweight and obesity, fatty liver is nowadays the most frequent cause of liver disease also in childhood [1]. Based on data derived from autopsies carried out in the United States, its prevalence in pediatric age ranges from 9.6% in normal weight individuals to 38% in obese individuals. In other series, tested with the same or different (imaging) tools these figures in the obese population may reach also higher percentages [2, 3]. This condition has hitherto almost universally been termed “non-alcoholic fatty liver disease” (NAFLD) because the hepatic histological picture is quite identical to that observed in patients abusing alcohol consumption. According to both the European [4] and North American [5] Societies for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN and NASPGHAN, respectively), as in adults also pediatric NAFLD refers to a spectrum of histological liver lesions that range from (usually macro-vesicular) simple steatosis, which may be complicated by fibrosis and inflammation [i.e., “non-alcoholic steatohepatitis” (NASH)] up to cirrhosis and hepatocellular carcinoma. A histopathological difference of preadolescent children vs. adolescents/adults however does exist and regards lack of ballooning, predominant portal rather than lobular inflammation, and lesser rapid cirrhotic evolution. In the transition from childhood to adulthood, pediatric NAFLD tends to progress, worsen and initiate/go hand in hand with a series of obesity related comorbidities that reduce overall survival and quality of life. Remarkably, as in adult population, pediatric NAFLD per se has been acknowledged to represent an independent risk factor of more adverse cardiovascular (and, maybe, renal) outcome, above and beyond that associated with individual or collective components of accompanying metabolic syndrome (MetS) [6, 7]. For these reasons, early diagnosis, and treatment not only of the liver disease itself, but also of the entire spectrum of related comorbidities, are important public health issues [4].
Provided that the definitions and clinical/prognostic implications of at least two obesity phenotypes emerged in recent years (metabolically healthy and metabolically unhealthy obesity), it is still unknown also why only some people with obesity will develop NAFLD [8]. Indeed, NAFLD appears to be generated by several multiple parallel (and not sequential) offenders acting with different combinations, at times synergistically. Together with insulin resistance (IR) and oxidative stress there are several other offenders including hormones released from adipose tissue, gut-liver axis perturbation, environmental agents (e.g., endocrine disruptors and particulate matter interacting among themselves). Differences in NAFLD prevalence amongst different ethnic/racial groups suggest that genetic background also plays a role. Sequence variations in genetic loci (e.g., GCKR, HSD17B13, MBOAT7, PNPLA3, TM6SF2, genetic variants involved in regulation of oxidative stress), the deregulation of microRNAs over the course of time have been shown to confer susceptibility to NAFLD in children and adults [9]. Whether these susceptible genes can be clinically used for risk stratification and personalized care is however still under study. Similar to the European Association for the Study of the Liver (EASL) [10] and the American Association for the Study of Liver Diseases (AASLD) [11], also ESPGHAN [4] and NASPGHAN [5] do not support their use in the routine clinical care [12].
The term NAFLD has appropriately been felt unfitting in adults because it is an “exclusion definition” which does not accurately reflect pathogenesis and does not help in patient stratification for management [13]. As the coexistence of visceral obesity, subclinical inflammation, and IR represent crucial elements in both MetS and NAFLD, the latter has repeatedly been suggested to be considered the liver manifestation of MetS at all ages, including pediatrics [14]. It is therefore not surprising that to better and positively define the hepatic disease nature, the more appropriate term metabolic (dysfunction)-associated fatty liver disease (MAFLD) instead of NAFLD has recently been proposed by an international group of adult liver disease experts [13]. This new term is not meaningless because it lies beneath the strict association between liver disease and metabolic dysregulation. According to this group [13], diagnosis of MAFLD in adults is based on the simple evidence of hepatic steatosis and the presence of one of the following three criteria:
overweight or obesity;
type 2 diabetes;
evidence (in the lean/normal weight patient) of metabolic dysregulation, defined as the presence of > 2 of the following seven conditions:
waist circumference > 95th percentile for age and sex;
blood pressure > 95th percentile for age, sex, and height;
triglycerides > 150 mg/dL;
high-density lipoprotein (HDL) < 40 mg/dL;
prediabetes;
homeostasis model assessment-insulin resistance (HOMA-IR) score > 2.5;
C-reactive protein (CRP) levels > 2 mg/L.
The possibility of a “dual” etiology occurrence has also been appropriately considered by mentioning a limited number of additional or alternative causes of liver function tests abnormalities possibly observed in clinical practice [13].
Apart from some hesitations hitherto published in adults [15] and pediatrics [6], the above concept of MAFLD appeared overall convincing.
In pediatrics, however, such a straightforward change of terminology will pose an age specific problem because of the existence of a plethora of pediatric-onset inborn errors of metabolism (IEM) which can mimic MAFLD in an obese individual and can be the main cause or a contributory cause of fatty liver disease at this age. As IEM may present in deceitful ways, it is prudent to continue to have a high suspicious index before assigning a MAFLD/NAFLD label to childhood patients with fatty liver. In view of this hindrance, the King’s College Pediatric liver unit in the UK has recently proposed to use an umbrella term which may probably be more appropriate to describe these children, i.e., pediatric fatty liver disease (PeFLD) [2, 16].
The sub-classification of PeFLD into three groups proposed by Hegarty et al. [16] introduces:
patients with the contextual diagnosis of an IEM (proposed to be classified as type 1);
children with metabolic (dysfunction)-associated fatty liver disease (i.e., MAFLD sensu stricto, or type 2);
cases where the cause of fatty liver is not known based on current knowledge (type 3).
This classification requires that before making a definitive diagnosis of presumed NAFLD/MAFLD in children, several other causes of liver steatosis and hypertransaminasemia must be carefully excluded. Importantly, some of these conditions are rare when considered individually but represent a large group when considered collectively. Notably, one should also consider that fatty livers both in lean and obese subjects may be due to causes other than IEM and likely benefitting from early specific pharmacological or dietary therapies that can modify the quality of life and the course of the disease [1, 4, 5, 17, 18].
For this reason, already in the past, the ESPGHAN experts’ committee [4] in addition to recommending that the diagnosis should be made by combining clinical features, ultrasound, laboratory tests, including liver biopsy in ambiguous cases, warned about possible suspicious red flags suggesting a diagnosis other than NAFLD. Namely these were:
not classic age range for NAFLD (NAFLD does not usually occur in children < 3 years old and is infrequent in children < 10 years old);
classical age group but with laboratory/ultrasound anomalies unresponsive to weight loss: early onset fatty liver disease, the presence of acute liver failure, cholestasis or large organomegaly, are indicative of other liver diseases rather than pediatric NAFLD sensu stricto.
Similarly, a few years later, NASPGHAN recommended their own algorithm based on a slightly different red flag age group and on levels of serum transaminase rather than their combined association with ultrasonographic imaging. Presence of risk factors such as severe obesity, family history of severe liver disease and a shorter list of conditions to be considered in the differential diagnosis for pediatric hepatic steatosis were also underlined [5]. For both societies, in any case, hyperechogenic liver in an obese or overweight child presenting with chronically elevated liver enzymes should not be assumed to have NAFLD/MAFLD only.
In 2018, quite contemporaneously, Hegarty et al. [2] and Alfani et al. [1] warned once more against the high risk of NAFLD misdiagnosis by reviewing the list of those conditions which ESPGHAN and NASPGHAN had indicated as possibly presenting with fatty liver and/or hypertransaminasemia, in both normal weight and overweight/obese pediatric patients. The list (Table 1) is very broad since it includes not only the pediatric onset-genetic and metabolic causes but also toxic and pharmacologic causes, and gastrointestinal/nutritional/endocrine/hepatic conditions. Although most of them can present already in the neonatal period, they can occur also later. As summarized in Figure 1, the known causes of IEM based hepatic steatosis in children are much insidious as may have clinical presentations encompassing many scenarios making difficult to correctly address the differential diagnosis [2]. In this regard, as shown in Table 2, the busy diagnostic path amongst the plethora of suspected rare genetic-metabolic causes might be guided by their prevalence and frequency of liver involvement [19].
| Genetic and metabolic causes | Toxics and drugs | Nutritional/GI and hepatic causes |
|---|---|---|
| Urea cycle disorders | Ethanol | Celiac disease |
| Hereditary fructose intolerance/galactosemia/tyrosinemia | Ecstasy | Inflammatory bowel disease |
| Glycogenosis I and VI and IX | Nifedipine | Intestinal failure |
| Bile acids synthesis defects | Diltiazem | Dysbiosis |
| Citrin deficiency | Cocaine | Viral hepatitis (HCV and HBV) |
| Cystic fibrosis | Solvents | Autoimmune hepatitis |
| Schwachman-Diamond syndrome | Pesticides | Kwashiorkor/malnutrition |
| Wilson’s disease | Glucocorticoids | Anorexia nervosa |
| Lipid storage diseaseNiemann-Pick disease type C | Estrogens | Rapid weight loss |
| Abeta/hypobetalipoproteinemia | Sodium valproate | Parenteral nutrition |
| Mitochondrial, fatty acids | Methotrexate | Obesity |
| ER function (e.g., CDG) or protein metabolism disorders | Amiodarone | PCOS |
| Alpha 1-AT deficiency | Tetracycline | OSAS |
| NBAS | L-asparaginase | Endocrine causes |
| Turner syndrome | Aspirin | Hypothyroidism |
| Lipodystrophies | Antipsychotics | Hypothalamic-pituitary disorders |
| Porphyria cutanea tarda | Antidepressants | Diabetes mellitus type 1 (Mauriac syndrome) |
| Familial hyperlipoproteinemia | Antiretroviral drugs | Sepsis |
| Porto-systemic shunt | Vitamin E | Others |
| Some myopathic disorders |
MCADD, LCHAD, VLCHAD, MADD; *mitochondria and other organelle dysfunction leading to the accumulation of fat droplets, and peroxisomal defects, frequently microvacuolar; Alpha 1-AT: alpha 1-antitrypsin; CDG: congenital disorders of glycosylation; ER: endoplasmic reticulum; NBAS: neuroblastoma amplified sequence; OSAS: obstructive sleep apnea syndrome; PCOS: polycystic ovarian syndrome; MCADD: medium-chain acyl-CoA dehydrogenase deficiency; LCHAD: long-chain 3-hydroxyacyl-CoA dehydrogenase; VLCHAD: very long-chain 3-hydroxyacyl-CoA dehydrogenase; MADD: multiple acyl-CoA dehydrogenase deficiency
Clinical and laboratory findings useful for orienting diagnosis of some genetic metabolic liver diseases with hepatic steatosis [19]
| Clinical/laboratory findings | Possible genetic-metabolic causes | Prevalence | Liver involvement |
|---|---|---|---|
| Pancreatic failure, hematological disorders | Schwachman syndrome | 1:50000 | +++ |
| Asymptomatic, hemolysis | Wilson disease | 1.30000 | +++ |
| Previous neonatal cholestasis, hepatomegaly | Alfa 1 antitrypsin deficiency | 1:7000 | +++ |
| Hypoglycemia, hepatomegaly | Glycogen storage disease (I, VI, IX) | From 1:00000 to 1:1000000 | +++ |
| Fructose refusal, hepatomegaly | Hereditary fructose intolerance | 1:20000 | +++ |
| Lethargy, increased serum ammonia levels | Urea cycle defects | 1:30000 (all disorders) | ++ |
| Chubby face, fatty liver, specific serum amino acids pattern | Citrin deficiency | 1:20000 (east Asia) | ++ |
| Failure to thrive, lactic acidosis | Mitochondrial disease | 1:8500 | + |
| Failure to thrive, ketoacidosis, hypoglycemia | Organic acidosis | 1:1000 (all disorders) | + |
| Mild coagulopathy, clinical phenotype | Congenital disorders of glycosylation | From 1.10000 to 1.100000 | + |
| Short stature, female gender, karyotype | Turner syndrome | 1:2000 | + |
| Failure to thrive, positive sweat test | Cystic fibrosis | 1:2500 | + |
Possible in 1–2 years of life; + Possible; ++ Frequent; +++ Almost always
Putting together the European and American pediatric societies recommendations, the first line work-up of MAFLD should:
include careful clinical, anthropometric, ultrasound and laboratory evaluation;
consider anamnestic data (e.g., ethnicity, high dietary fructose intake);
consider clinical risk predictors.
The anthropometric parameters [body mass index, waist circumference, blood pressure and clinical signs of IR (i.e., acanthosis nigricans)] and standard liver function tests, particularly transaminases, are generally assessed. According to NASPGHAN [5], transaminases must be preferred to imaging, but this has been partly questioned because they may be found normal in a subgroup of patients [18, 20, 21]. The first-line imaging evaluation is liver ultrasound, which has a sensitivity of 60% to 96% compared to biopsy and a specificity of 84% to 100%, depending on the degree of severity of the steatosis, eventually combined with elastography for the evaluation of fibrosis [22, 23]. Magnetic resonance imaging (MRI) and MRI with spectroscopy (MRS) can more accurately assess the fat content in the analyzed tissue, but they are scarcely available [22, 23]. A pediatric stepwise approach, as that summarized in the proposed algorithm of Figure 2 which takes into consideration the age range and the response to weight reduction, may help to evaluate when to exclude the most common etiologies of hepatic steatosis and hypertransaminasemia unrelated to NAFLD/MAFLD. Liver biopsy is necessary in cases with uncertain diagnosis and/or in the context of controlled studies, and it remains the diagnostic gold standard to distinguish some of the different etiologies of a steatotic liver [24]. The problems related to the procedure (costs, invasiveness, complications, variability in sampling with possible false negatives) do not make it possible to formulate precise guidelines regarding liver biopsy indications. Less invasive alternatives to biopsy are increasingly being studied and being validated (e.g., biological markers, scoring systems, imaging scores and the so-called “liquid biopsies”) [25].
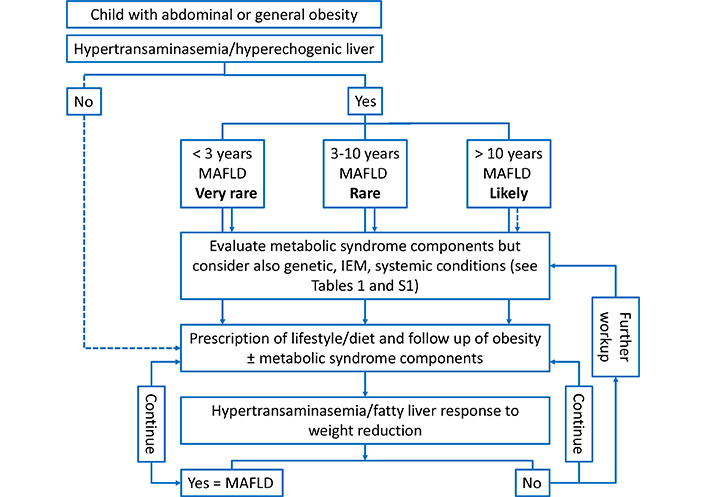
Suggested algorithm for the assessment of liver disease in a child with abdominal/general obesity and hypertransaminasemia/hyperechogenic liver according to age range and to response to weight reduction. Notes: Further work-up for genetic-IEM causes, according to Hegarty et al [2]. Blood: 1) glucose, ketones, lactate, uric acid, creatine kinase, ammonia, thyroid function test, lipid profile, hepatitis serology and molecular testing; 2) acylcarnitine and amino acids profile, carnitine (free and total); 3) alpha 1-antitrypsin phenotype; 4) glucose tolerance test; 5) copper studies; 6) sweat test; 7) galactose-1-uridyl transferase enzymology; 8) lysosomal acid lipase activity; 9) glycogenosis enzymology; 10) transferrin isoform analysis. Urine: 1) organic acids; 2) bile acids. Imaging: 1) liver ultrasonography; 2) magnetic resonance imaging of the brain. Other: 1) liver/muscle/skin biopsy; 2) bone marrow aspirate; 3) targeted next generation sequencing
The relentless diffusion of obesity in all social categories requires a more and more extraordinary effort to address the awareness of the association MetS/fatty liver not only in adults but also in the pediatric age [26]. The new welcome term MAFLD proposed in adults and extended also to childhood, however, may raise concerns on possible collateral diagnostic errors/delays of a possible associated pediatric-onset liver condition.
Regardless of the body mass index and metabolic dysfunction, pediatric liver abnormalities with/without hepatic hyperechogenicity require therefore careful evaluation by clinicians. Differential diagnosis should be much broader than that proposed in adults especially in those cases who have been initially classified as affected by NAFLD, but for whom a response to the loss of weight trial is not easily available. Finally, one should also consider that the definition of metabolic syndrome itself in pediatric age is still challenging with various definitions proposed by different authors and scientific societies over the years [27–30] (Table S1).
Pending more specific markers of metabolic syndrome and MAFLD, new non-invasive methods which could help to screen these conditions are necessary. In our hands, for example, levels of salivary uric acid and insulin do increase in parallel with the number of components of the MetS. When combined with selected anthropometric parameters, through a z-logit function, they allow an easy prediction of hepatic steatosis with high sensitivity, specificity, and accuracy [31]. Also in saliva are obtainable metabolomic patterns of obesity and MAFLD which reflect changes of metabolites involved in energy metabolism, amino acids, and organic acids, as well as the chemical processes that occur within intestinal bacteria [32]. This novel information is of interest and requires further studies investigating in that direction also in terms of the appealing field of personalized therapies and diets.
Pending new guidelines endorsed by the pediatric societies and/or the pediatric adaptation of those released by the international adults’ experts [13], in the meanwhile, the term PeFLD with three subtypes could help in raising the awareness of the necessity to separate true MAFLD from fatty liver due to an ever-growing number of pediatric-onset IEM and other affine conditions [33]. Though the cost-effectiveness ratio of this attitude is still indeterminate, it is likely that the advantage of the early identification of another specifically treatable pediatric-onset liver disease associated to/mimicking MAFLD would be rewarding.
ESPGHAN: European Society for Pediatric Gastroenterology Hepatology and Nutrition
IEM: inborn errors of metabolism
IR: insulin resistance
MAFLD: metabolic (dysfunction)-associated fatty liver disease
MetS: metabolic syndrome
NAFLD: nonalcoholic fatty liver disease
NASPGHAN: North American Society for Pediatric Gastroenterology, Hepatology and Nutrition
PeFLD: pediatric fatty liver disease
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100151_sup_1.pdf.
MCR, AC and PV contributed conception and design of the study and reviewed the literature; CM wrote the first draft of the manuscript; MCR, AC, AGEDA, LN, PV and CM wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Noel C. Salvoza ... Natalia Rosso
Amedeo Lonardo, Stefano Ballestri
Marvin Ryou ... Gyorgy Baffy
Michael Doulberis ... Stergios A. Polyzos
Marica Meroni ... Paola Dongiovanni
Ivana Mikolasevic ... Sandra Milic
Valerio Rosato ... Marcello Persico
Carlo Acierno ... Ferdinando Carlo Sasso
Rosa Lombardi ... Anna Ludovica Fracanzani
Angelo Di Vincenzo ... Marco Rossato
