Abstract
Rational government of patient fluxes from primary care to hepatology clinic is a priority of nonalcoholic fatty liver disease (NAFLD) research. Estimating pre-test probability of disease, risk of fibrosis progression, and exclusion of competing causes of liver disease must be addressed. Here we propose a novel taxonomic classification of NAFLD based on hepatic, pathogenic and systemic features of disease in the individual patient. The variable course of disease in any given patient remains a clinical enigma. Therefore, future studies will have to better characterize the role of genetic polymorphisms, family and personal history, diet, alcohol, physical activity and drugs as modifiers of the course of disease and clues to the early diagnosis of hepatocellular carcinoma. A better understanding of these, together with a taxonomic diagnosis, may prompt a more accurate personalization of care. For example, understanding the putative role of psycho-depression in NAFLD promises to revolutionize disease management in a proportion of cases. Similarly, sex differences in outcome and response to treatment are insufficiently characterized. More studies are awaited regarding those forms of NAFLD which occur secondary to endocrine derangements. The intersections between NAFLD and the lung must better be defined. These include the bi-directional associations of NAFLD and chronic obstructive pulmonary disease and sleep apnoea syndrome, as well as the totally unexplored chapter of NAFLD and coronavirus disease 2019 (COVID-19). Finally, the therapeutic roles of intermittent fasting and anticoagulation must be assessed. In conclusion, over the last 20 years, NAFLD has taught us a lot regarding the pathogenic importance of insulin resistance, the limitations of correcting this in the treatment of NAFLD, the root causes of diabetes and the metabolic syndrome, sex differences in disease and the role of nuclear receptors. However, the overwhelming COVID-19 pandemic is now expected to reset the priorities of public health.
Keywords
Alcoholic fatty liver disease, coronavirus disease 2019, chronic obstructive pulmonary disease, endocrine nonalcoholic fatty liver disease, genetic risk score, liver biopsy, natural history, sex differences“The history of a science is that science itself.”
-Johann Wolfgang Goethe
Introduction
Definitions and history
Nonalcoholic fatty liver disease (NAFLD) is a clinico-pathological syndrome which covers a large gamut of hepatic and extra-hepatic manifestations and complications associated with and predisposing to either individual features of the metabolic syndrome (MetS) [typically type 2 diabetes (T2D) and obesity] or to the full-blown MetS [1–7]. The epidemiological surge of NAFLD is, at least in part, spurious, [i.e. fuelled by our ever increasing ability to discover this common condition in asymptomatic individuals as well as in cases of otherwise “cryptogenic” cirrhosis and hepatocellular carcinoma (HCC)] [8, 9] and, to a larger extent, due to (western-type) lifestyle and lifestyle changes (in developing countries) [10]. Irrespective of this academic contention (spurious phenomenon versus true increase), hard data support the notion that NAFLD accounts for > 30% of deaths due to liver disease and diabetes and ~8% of all-cause mortality in the USA [11].
In 1980, Ludwig from the Mayo Clinic, USA, in reporting on 20 patients whose liver biopsies exhibited fatty and necro-inflammatory changes, Mallory bodies, fibrosis and cirrhosis, coined a novel definition consisting of a double composite word: “nonalcoholic steatohepatitis” (NASH) [12]. However, liver disease in the obese had been addressed by Zelman in 1952; in 1977, Haller had already understood that “hepatic steatosis” was a feature of the MetS; what we now call “NASH” was first reported by Thaler from Wien in 1962; what we would now call “NASH-cirrhosis” was first reported in 1979 by Itoh in Japan; in 1988, Diehl defined NAFLD as an “alcohol-like liver disease in the non-alcoholic” and, in 1995, Lonardo et al, in reporting on what they had defined the “bright liver syndrome” highlighted the associations with gallstones and atherosclerosis [13, 14]. Analysis of the history of NAFLD research has shown that early progress in our understanding of the disease was fostered by individual researchers/groups. However, from 2000 onward, contributions of symposia and scientific societies became prominent [13]. Additional aspects of NAFLD’s history have been focused in their excellent article by Fouad et al. [15].
Aims
The idea behind this narrative review article, based on a 25-year experience in the study of this disease, is to provide a personal point of view regarding some selected topics of research on NAFLD. To this end, rather than following the “canonical pathway” in writing, which leads to diagnosis and management starting from epidemiology and pathogenesis, specific research questions will be addressed here. This article is not intended to be exhaustive of all the possible topics: to this end, reference should be made to excellent, more systematic reviews [16–19] and scientific society guidelines, which have been compared and annotated elsewhere [20, 21]. The present article is principally addressed to clinical and translational investigators but Health Authorities, Ph.D. students, nurses and pharmaceutical industries involved in the active research of innovative drug therapies may also find hints of potential interest here.
Burden and referral patterns
Mention has previously been made to the true and artefactual determinants underlying the growing clinical and epidemiological importance of NAFLD. Alarming data on the incidence and prevalence of NAFLD pinpoint the necessity of regulating large fluxes of patients from primary practice to consultants and return. The challenges are to allocate the sufficient amount of resources to a condition which is strategically located at the intersection of preventive medicine with clinical medicine. NAFLD fully belongs to the domain of internal medicine given that it is positioned at the crossroad of several medical specialities spanning from hepatology to endocrinology and metabolism and from oncology and cardiovascular medicine to gastroenterology and endoscopy, just to cite some. Active search and identification of individuals at a high risk for NAFLD and regulation of patient fluxes will maintain their key role for proper allocation of resources and maximization of efficacy irrespective of the resources destined to this chapter of public health expenses by each country’s Health Authorities. On this background, the most logical pathway to follow is to stratify the risk in primary care, with subsequent referrals of those requiring specialist cares [22].
In conclusion, rational regulation and strict government of fluxes from primary (and specialistic such as diabetology) care to hepatology care is a priority of NAFLD research.
Personalized diagnosis
The key questions in evaluating the individual patient with suspected NAFLD pertain to a) the a priori risk of NAFLD (i.e. pre-test probability of disease) [23, 24]; b) the risk of either advanced or progressive disease (i.e. probability of fibrosis and fibrosis progression) [25]; and c) the exclusion of competing causes of liver disease.
Pre-test probability of disease
Novel genetic risk scores (GRS) are increasingly being proposed and validated [26–28] to gauge the pre-test probability of NAFLD. However, the use of GRS has a much wider scope spanning further and beyond the simple pre-test assessment of disease [26–31] (Table 1).
Gamut of applications of GRS in NAFLD
| Author, year [ref] | Method | Finding | Conclusion |
|---|---|---|---|
| Vespasiani-Gentilucci, 2018 [26] | 107 individuals with NASH-cirrhosis, 93 with non-cirrhotic NAFLD, and 90 controls were submitted to genotyping. | Compared to a GRS = 0, a GRS of 1–2 was associated with a 4-fold increased risk, and a score of 3–4 was associated with a 20-fold increased risk of having non-cirrhotic NAFLD. A GRS = 3–4 was associated with a four-fold increased risk of NASH-cirrhosis. | A dose-response relationship was found between increasing GRS and risk of severe liver disease. |
| A risk score based on SNPs for the PNPLA3, TM6SF2, and KLF6 variants was developed. | |||
| Kawaguchi-Suzuki, 2018 [29] | 55 participants of an RCT on long-term pioglitazone treatment in NASH were enrolled. | The genetic response score was significantly associated with achievement of the primary outcome. | Genetic factors account for a fraction of the inter-individual variability in response to pioglitazone administration in NASH patients. |
| Primary outcome defined as ≥ 2-point reduction of NAS. | |||
| SNPs in putative candidate genes were evaluated. | |||
| A genetic response score was developed based on the sum of response alleles for selected genes. | |||
| Ma, 2018 [30] | 1521 participants of the 3rd-generation cohort of the Framingham Heart Study were enrolled. | Higher GRS were associated with increased steatosis in individuals who had decreased MDS or AHEI scores, but not in those with stable or improved diet scores. | Dietary improvements are particularly recommendable to those who are at a high genetic risk for developing NAFLD. |
| Dietary intake was assessed with the self-administered semi-quantitative 126-item Harvard food frequency questionnaire. Diets were scored based on either the MDS or the AHEI. | |||
| The extent of steatosis was assessed using CT images. | |||
| Weighted GRS for NAFLD was determined based on multiple SNPs identified in GWAS of NAFLD. | |||
| Danford, 2018 [31] | 177 individuals with biopsy-proven NAFLD were recruited. | The combination of eLP-IR with the genetic score and age accurately predicted advanced stages of fibrosis (stages 3–4 liver) with an AUROC = 0.82. | A study supporting the notion that genetic and metabolic drivers dictate the severity of NAFLD as well as indicating a novel risk stratification based on pathogenic determinants of disease. |
| The eLP-IR index was calculated based on serum biomarkers using MRS. | |||
| Genetic score - Individuals who had neither allele of PNPLA3 and TM6SF2 received a 0 score. 1 point was assigned for either heterozygotes or homozygotes of NPLA3 and TM6SF2 minor alleles. A score of 2 was assigned to those who had ≥ 1 allele of both PNPLA3 and TM6SF2 minor alleles. | |||
| Di Costanzo, 2019 [27] | 230 obese Italian children underwent metabolic assessment and evaluation of gene polymorphisms (PNPLA3, TM6SF2, GCKR, and MBOAT7). HFF% was assessed with MR. | HFF% was accounted for by anthropometric and metabolic variables (BMI, HOMA-IR, MetS, transaminases, GGT and albumin) for 8.7%. And by genetic factors for 16.1%. | Genetic factors play a key role in the determinism of intra-hepatic fat content in obese Italian children. |
| A weighted-GRS (combining PNPLA3, GCKR, and TM6SF2 risk alleles) was associated with an approximately eight-fold increased NAFLD risk. | |||
| Zusi, 2019 [28] | A GRS was developed taking into account the SNPs of GCKR, MBOAT7, GPR120, SOD2, PNPLA3, TM6SF2, LPIN1, ELOVL2, FADS2, MTTP and KLF6 as well as clinical risk factors in a cohort of 514 obese children and adolescents. | By adding a 11-polymorphism GRS, the accuracy of the statistical model for predicting the risk of NAFLD was significantly (albeit modestly) improved as compared to a model evaluating established clinical risk factors alone. | NAFLD was strongly associated with three genetic variants, TM6SF2 rs58542926, PNPLA3 rs738409 and GCKR rs1260326 and, more slightly, with ELOVL2 rs2236212, in obese children and adolescents. |
| NAFLD was diagnosed with US. |
AHEI: alternative healthy eating index; AUROC: area under the receiver operating characteristic curve; BMI: body mass index; CT: computed tomography; ELOVL2: ELOVL fatty acid elongase 2; eLP-IR: enhanced lipoprotein IR index; FADS2: Fatty Acid Desaturase 2; GCKR: glucokinase regulator; GGT: gamma-glutamyl-transferase; GPR120: G-protein coupled receptor 120; GWAS: genome-wide association studies of NAFLD; HFF%: hepatic fat fraction; HOMA-IR: homeostasis model assessment of insulin resistance; KLF6: Kruppel like factor 6; LPIN1: Lipin 1; MBOAT7: Membrane Bound O-Acyltransferase Domain Containing 7; MDS: mediterranean-style diet score; MR: magnetic resonance; MRS: nuclear magnetic resonance spectroscopy; MTTP: microsomal triglyceride transfer protein; NAS: NAFLD activity score; PNPLA3: Patatin-like phospholipase domain-containing protein 3; RCT: randomized controlled trial; SNPs: single nucleotide polymorphisms; SOD2: superoxide dismutase 2; TM6SF2: Transmembrane 6 Superfamily Member 2; US: ultrasound
This research pathway is predicted to gain increasing popularity, not only in paediatric practice, but also in adults, thanks to a growing availability, at cheaper costs, of commercial kits including genetic polymorphisms useful in evaluating the predisposition to developing NAFLD. Besides these tests, which will contribute to expanding our knowledge of the so called “genetic NAFLD”, other algorithms will have to be developed and validated to identify the common forms of “metabolic NAFLD”. The prototype of such tests, the fatty liver index, originally proposed by Bedogni et al. [32], should probably be reworked through inclusion of at least sex and reproductive status, which are two key modifiers of the risk of NAFLD [33]. In any case, direct confirmation of steatosis through imaging techniques, together with the exclusion of competing causes of (steatogenic) liver disease remain a key step in the diagnosis of NAFLD. The type of imaging technique [for example conventional ultrasonography with semi-quantitative indices versus controlled attenuation parameter (CAP) versus magnetic resonance (MR)] will vary as a function of the resources allocated with the more expensive (and accurate) MR imaging predicted to be used more in affluent countries than the cheaper and globally more available ultrasonographic techniques [34].
In conclusion, the most rational (non-invasive) diagnostic approach to NAFLD and its accurate classification in the individual patient are a definite research priority.
Risk of advanced/progressive disease
Collectively, evidence suggests that NASH, rather than simple steatosis, carries an increased risk of more rapidly progressing to advanced fibrosis/cirrhosis and developing HCC [19, 35]. However, some longitudinal studies have proven that NAFLD is not simply a dichotomous disease (NASH vs. non-NASH). For example, compared to simple steatosis at baseline, which has the lowest risk of disease progression, the presence of even minor degrees of liver injury and inflammation (and perhaps severe steatosis, although in the absence of NASH), is associated with an increased risk of developing significant fibrosis, often in parallel with the deterioration of metabolic comorbidities [36].
A systematic review of ten longitudinal histological studies showed, as early as 2009, that inflammation was the key predictor of eventual histological fibrosis progression and a rational therapeutic target in NASH [37]. More recently, a European study based on an exploration cohort of 140 individuals with suspected NAFLD and a separate validation cohort of 78 patients with biopsy-proven NASH found that liver fibrosis and NASH were strongly associated with increasing BMI and HOMA-IR [38]. A recent study, by reporting that obesity, either alone or added to alcohol consumption and T2D, is strongly associated with fibrosing liver disease, extends the importance of BMI beyond NAFLD [39]. Together, these studies suggest that fibrosis is the end-result of repeated bouts of hepatic necro-inflammation which, in their turn, are fuelled by metabolic derangements principally resulting from obesity.
Consistently, data from treatment trials support a strong association between histological resolution of steatohepatitis and improvement of fibrosis in NASH patients [40]. Histopathological classification of NASH has traditionally been based on Brunt’s criteria and the NAS by Kleiner et al. [41, 42]. The NAS, being quantifiable and relatively more reproducible than the diagnosis of definite NASH by Brunt, has largely been used as an endpoint in clinical trials, allowing to semi-quantitatively monitor the severity of NAFLD over time. However, studies suggest that these histopathological classification systems may have distinct clinic-pathological correlations.
Diagnosis of definite NASH according to Brunt’s histological criteria has been reported as a stronger predictor of metabolic abnormalities than the NAS score by Kleiner [43–45]. Moreover, studies have shown that–given that it was unable to predict fibrosis progression–NAS was not associated with adverse clinical outcomes in these patients [36].
The new and recently validated histopathological ‘Fatty Liver Inhibition of Progression’ algorithm and Steatosis, Activity, and Fibrosis (SAF) score have proven to be able to identify NASH patients with distinct clinical (higher prevalence of metabolic risk factors, more severe IR, and higher levels of aminotransferases) and biological (liver fibrosis) profiles of disease severity [38].
Indeed, solid evidence suggests that liver fibrosis dictates the long-term prognosis of NAFLD patients being significantly associated with overall, liver-related and also cardiovascular mortality [46–48].
The identification of subjects with NASH and/or fibrosis is a key topic in the management of NAFLD patients allowing for adequate treatment schedules and follow-up of these patients [49]. Liver biopsy is the reference standard for the diagnosis of liver steatosis with grading/staging of concurrent necro-inflammatory/ fibrotic changes; however, this invasive procedure may be associated with major complications and patient discomfort [50, 51]. In addition, it is exposed to the risk of sampling errors and diagnostic inaccuracies as a result of NASH being unevenly distributed throughout the liver tissue [52]. Therefore, it should be reserved only to selected patients at risk of progressive liver disease and, of course, cannot be adopted in large epidemiological or interventional studies. On these grounds the research on diagnostic tools for non-invasive and accurate identification of NASH and fibrosis staging is actively ongoing [50, 51, 53, 54].
Clinical scores used for detecting NASH have shown inconsistent results [55]. Biomarkers of fibrosis, such as the NAFLD fibrosis score (NFS), may be adopted in clinical practice to non-invasively screen subjects for the absence or presence of advanced fibrosis, but all of these perform sub-optimally [36, 51]. Subjects with suspected advanced fibrosis or indeterminate scoring should either undergo non-invasive assessment of liver fibrosis with sonoelastographic techniques or be evaluated for liver biopsy [36, 49, 51].
Ultrasound semi-quantitative scores, such as ultrasonographic fatty liver indicator (US-FLI), have shown to be sensitive for liver steatosis as low as 10% and correlated with anthropometric, metabolic and histological parameters of NAFLD [53, 56, 57]. A US-FLI score ≤ 4 is quite accurate in ruling out NASH but higher cut-offs performed sub-optimally in differentiating steatosis from NASH [56, 58]. Therefore, this cannot substitute liver biopsy but rather assist in identifying subjects at risk of progressive NASH in whom liver fibrosis severity must be assessed non-invasively by ultrasound-based elastography first and with liver biopsy, when indicated [53]. Studies evaluating the diagnostic performance of ultrasound semi-quantitative scores or new ultrasound quantitative techniques, including CAP and those more recently developed (e.g., backscatter analysis, acoustic structure quantification and speed of sound), coupled with clinical laboratory parameters for detecting NASH are eagerly awaited. MR elastography has shown good accuracy for detecting NASH but it is expensive and its availability is limited [50].
Sonoelastographic techniques, such as transient elastography performed by Fibroscan or acoustic radiation force impulse (ARFI), have shown good accuracy for estimating advanced fibrosis and cirrhosis (F3 and F4 stages), especially if coupled with clinical data, but their accuracy in detecting F1-F2 fibrosis is sub-optimal [50, 51, 59].
Emerging evidence suggests that certain patterns of expression of microRNA (e.g., miR-122, miR-30c, miR-27b/30) are significantly associated with NASH and/or fibrosis, representing ideal candidates to be integrated into prediction algorithms of NAFLD severity [60–62].
In conclusion, future studies should be aimed at evaluating cost-effectiveness and cost-utility ratios of genetic tests and GRSs that also interact with endocrine and metabolic features of the individual patient. Non-invasive diagnostic algorithms based on integrated imaging, liver biomarkers and genetic signature should be devised to accurately detect NASH and, especially, hepatic fibrosis and monitor its changes over time.
Ruling out competing causes of liver disease: how far should we go?
It is obviously important to carefully exclude those causes of steatosis which are amenable to specific treatment. This is the case, for example, of alcoholic liver disease, hepatitis C virus (HCV) infection and hypothyroidism. Abstinence remains the backbone of treatment of alcoholic liver disease [63]. Effective antiviral cures are available to eradicate HCV [64]. Thyroid replacement therapy has the potential to reverse the pathogenic mechanisms of NAFLD [65]. However, complicating both research and clinical practice, multi-causality may intervene in the development of NAFLD in the individual patient, either at baseline or in the course of follow-up.
Over time, NAFLD has variably been named diabetic hepatitis, alcohol-like liver disease in the non-alcoholic, bright liver syndrome, and insulin-resistance-associated hepatic iron overload [13]. However, given that consensus must be reached on how the diagnosis of NAFLD should be pursued in the individual patient, the very name of NAFLD should give way to more precise definitions, such as proposed by multiple Authors [66–68]. Additionally, in clinical practice and in clinical trials, primary NAFLD (i.e. associated with MetS) should be differentiated from NAFLD deemed to predispose to incident MetS and, ideally, from specific forms of genetic NAFLD and from forms owing to composite etiology (i.e. genetic-and-metabolic) as well as from all the innumerable forms of secondary NAFLD [69].
The rationale for distinguishing alcoholic fatty liver disease (AFLD) from NAFLD was first challenged by Völzke in 2012 [70]. For example, the liver in the obese closely mimics alcoholic liver disease histologically [71, 72]. Moreover, the distinction of AFLD from NAFLD is mainly based on specific pathogenic mechanisms. However, alcohol abuse and metabolic disorders associated with obesity often overlap in the same individual and deceptive differences in clinical presentation and outcome are possibly accounted for by selection bias [70]. AFLD and NAFLD also share genetic factors, behavioural/social underpinnings, as well as certain contributors to mortality [73, 74]. For example, in both conditions, disease is more prevalent in males [74] and both NAFLD [2, 75] and AFLD [76] exhibit prominent extra-hepatic/systemic features. As for the differences between AFLD and NAFLD it has been pinpointed that the advanced stages NAFLD usually present more in older age than AFLD [74]. Regarding laboratory features, the aspartate aminotransferase (AST)/alanine aminotransferase (ALT) < 1 ratio is more common in NAFLD than AFLD, provided that cirrhosis is absent [74]. To sum up, commonalities between AFLD and NAFLD outnumber distinctions, which supports a shift from artificial categories to a more general approach to fatty liver syndromes as “multi-factorial disorders” [70, 74]. This mutated paradigm is predicted to contribute to improving prevention schedules and assist in exploring more updated nomenclatures and innovative treatment strategies [74].
Academic attention has focused on the risk of NAFLD occurring secondary to rare diseases such as LAL but findings have been inconclusive so far [77].
Further to its etiologic definition, histological characterization of NAFLD should be specified whenever liver biopsy is deemed to be clinically indicated. If liver biopsy is not indicated, non-invasive indices e.g., NFS and fibrosis-4 [78] should be performed, alone or, preferentially, combined with the evaluation of liver stiffness obtained through elastographic (either sono- or MR-based) techniques.
Moreover, a complete work-up of concurrent diseases should be performed based on our present understanding of extra-hepatic manifestations and complications of NAFLD [2]. A proposal for a rational classification of NAFLD based on an extensive diagnostic evaluation of hepatic and extra-hepatic disease is illustrated as follows (Figure 1).
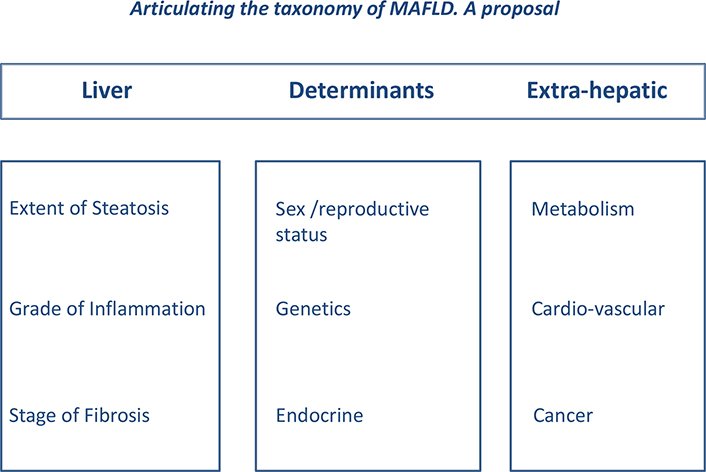
Articulating the taxonomy of MAFLD. The “LDE” system. Based on archaic suggestions [66] and earlier attempts [67], an international panel including eminent world authorities in NAFLD, none excluded, renamed NAFLD as metabolic associated fatty liver disease (MAFLD) [79]. This proposal now needs to be further articulated in order to fully explore the complexity of disease in the individual patient. The LDE system has a simple structure consisting of a prefix (L for liver), a central section (D for determinants) and a suffix (E for extra-hepatic); Liver (L) - We propose that MAFLD should include a prefix illustrating what, in the individual patient, is known about liver involvement. Information regarding this may be achieved, based on clinical judgement, either with non-invasive or biopsy evaluation [36]. Steatosis and inflammation may be classified into mild, moderate or severe and fibrosis as absent (F0), mild (F1-F2) ore advanced (F3-F4). Individuals who have overt cirrhosis and/or HCC should be declared so; Determinants (D) - The importance of sex and reproductive status cannot be overemphasized and genetic determinants, although not requested in all patients with NAFLD (reviewed in 49), must be acknowledged whenever data are available (Table 1). The minimal endocrine assessment in those with NAFLD is to ascertain whether they have either T2D or IR (assessed with HOMA-IR) and whether T2D is associated or not with obesity (i.e. so called “diabesity” [4]. Given its distinct pathogenic features and the possibility to receive specific treatment, hypothyroidism should also be evaluated in all cases [65]. Other types of NAFLD secondary to other endocrine derangements, such as polycystic ovary syndrome (PCOS), growth hormone (GH) deficiency and hypogonadism, should always be declared when the diagnosis is clinically overt [80]; Extra-hepatic (E) - Finally, the systemic nature of NAFLD must be fully illustrated by the suffix “E”, which explores and synthesizes data on extra-hepatic manifestations of disease [2]; As an example, illustrating this proposed taxonomy, patient Ms. X.Y. might be declared to have MAFLD (L steatosis mild; inflammation absent; fibrosis absent; D post-menopausal; no SNP identified; associated with hypothyroidism the full-blown MetS; E arterial hypertension; medio-intimal carotid thickening; previous colon cancer). This taxonomy lends itself to being expressed as either a descriptive diagnosis or a score
Natural course
A seminal study has clarified that liver histology is the major determinant of hepatic (as opposed to extra-hepatic) course of disease [81]. Another meta-analytical review including 13 studies, enrolling 4, 428 patients with NAFLD (2, 875 of these with NASH) found that, compared to no fibrosis (stage 0), in the subgroup of those who had NASH, the risk of all-cause mortality; liver-related mortality; liver transplantation and liver-related events increased with worsening fibrosis stage irrespective of age or sex [48]. However, while it is impossible, unnecessary [82], sometimes unethical [83] and unfeasible to propose and perform liver biopsy on all patients who have NAFLD, it is fully conceivable that so far unidentified factors may also contribute to modulate patterns of disease in the individual patient.
For example, the risk factors for the development of HCC in those with NAFLD need to be urgently identified. HCC is the principal cause of mortality in patients with cirrhosis and a leading cause of cancer death worldwide [84]. Early detection of HCC through a surveillance program (defined as screening repeated at pre-fixed intervals of time) may halt its progression via early treatment, thereby reducing mortality from this disease. Ultrasonography, with/without alpha-fetoprotein testing, is accepted as the best available tool for implementing HCC surveillance [50]. NASH-cirrhosis is the strongest risk factor for HCC, but accumulating data suggest that HCC does develop in a significant proportion of NAFLD patients (up to 15–20%) without histological evidence of cirrhosis, especially if obese/diabetics. A surveillance program is cost-effective in patients with NASH-cirrhosis showing a yearly incidence of HCC ranging between 2.4% and 12.8%, which is higher than the accepted thresholds for HCC surveillance [85, 86]. Conversely, the absolute risk of HCC in NAFLD, although higher than matched NAFLD-free controls, is too low to recommend a universal HCC surveillance in the whole NAFLD population [86]. Therefore, we need to identify those clinical, laboratory, imaging or genetic-molecular markers for detecting NAFLD patients at high-risk of developing HCC to be selectively surveilled for the early detection of HCC.
In conclusion, future studies will have to better characterize key modifiers of the natural course of NAFLD factors including family and personal history, diet, physical activity and drugs.
Personalized management
Research on novel pharmacological approaches has so far focused on the identification of a “magic bullet”, i.e. a drug which benefits all patients. This standard model of treatment (i.e. “one size fits all”), although appealing for commercial and practical reasons, conflicts with the impressive variety of NAFLD pathobiology [87]. Stated otherwise, what needs to be investigated in-depth is a (more) personalized management schedule based on the specific pathobiological features of disease in the individual patient. For example, NAFLD in the subject with either pre-diabetes or T2D should ideally be managed differently from either the non-diabetic individual [88, 89] or from the individual who has dyslipidemia alone. NAFLD in the patient who has morbid obesity may indeed be amenable to bariatric surgery [90], but not all NAFLD patients will accept this. Lifestyle modification is universally recommended as the first-line approach for the treatment of NAFLD but adherence to long-lasting calorie restriction is hard to achieve in clinical practice.
Intermittent fasting (IF)
Conflicting with the large availability of foodstuffs in many countries worldwide, evolution has selected our ability to survive and to adapt to a food-deprived/fasted state [91]. IF defines recurring periods (e.g. 16h to 48h) with little or no energy intake, with intervening periods of normal food intake [91]. IF, together with periodic fasting (i.e. IF with periods of fasting or fasting-mimicking diets from 2 to ≥ 21 days), beneficially affect health and disease processes/states both in experimental conditions and in humans. These favorable effects, which are mediated by activation of adaptive cellular stress response, improved mitochondrial health, DNA repair, autophagy, stem cell-based regeneration as well as enduring metabolic effects, translate into increased longevity and protection from degenerative disease, cardiovascular disease and cancer [91]. IF also protects from the MetS and associated cardio-metabolic disorders [91]. The only study conducted in patients with NAFLD has shown that an alternate-day calorie restriction diet followed for 8 weeks–as compared to usual habit diet–was associated with beneficial effects on BMI, ALT, grade of hepatic steatosis at ultrasonography and fibrosis scores (assessed with ARFI shear wave elastography) with a good adherence level [92]. Further studies are needed to confirm and expand these results.
Endocrine NAFLD/NASH
Other examples of personalized management protocols may regard those forms of NAFLD which occur secondary to specific endocrine derangements [80]. Furthermore, a deeper understanding of NAFLD pathophysiology in these forms of NAFLD/NASH secondary to endocrine disorders may help in identifying novel therapeutic approaches [65]. For example, a key question is how we may succeed in uncoupling NAFLD from IR [93].
Anticoagulation
A growing number of patients with NAFLD and progressive liver disease (i.e. NASH-cirrhosis) will be candidates for anticoagulant therapy in the forthcoming years. This prediction is based on the notions that NAFLD/NASH, a globally epidemic condition, is independently associated with an increased risk of abnormalities of cardiac structure/function, including cardiac rhythm disorders such as atrial fibrillation (AF), and also with idiopathic venous thromboembolism (VTE) [94–100]. Accumulating “real world” data suggest that direct oral anticoagulants can be used in AF or VTE patients with advanced chronic liver disease showing similar efficacy and reduced bleeding complications compared to warfarin [99]. An association between inflammation, activation of the coagulation system and the development of hepatic fibrosis and portal hypertension in chronic liver disease has been disclosed by experimental and clinical studies [99, 101]. A protective effect of anticoagulant therapy on the complications of portal hypertension and fibrogenesis has been observed in small sample studies performed in patients with cirrhosis mainly of viral origin [99, 101].
By highlighting that the imbalance between pro-coagulant and anticoagulant activities can also lead to thrombosis, recent evidence has subverted the antique dogma that chronic liver disease represents an acquired bleeding disorder [102]. Stine et al. [103], were first in reporting on the increased risk of portal vein thrombosis in patients with cirrhosis owing to NASH. This is a population in which a more liberal use of oral anticoagulation for preventive and therapeutic purposes will have to be considered. However, given the complex interaction between NAFLD, obesity and body composition; and based on the potential for exercise to beneficially affect all three, additional research is needed to better define the causal role of each in contributing to the pro-thrombotic state of NAFLD in order to improve patient-oriented outcomes [104, 105].
In conclusion, future studies should better define NAFLD in the individual patient (and the taxonomy proposed in Figure 1 may be an example of how this aim may be achieved) in order to guide personalized management aimed at impacting on the liver itself, the disease complications as well as on cardiovascular outcome are eagerly awaited.
The efficacy and long-term feasibility of intermittent fasting need to be evaluated. Although maintenance of life-style changes remains a challenge over the long run, it is worth remembering that even transient remissions of steatosis may metabolically be beneficial [106].
Anticoagulation strategy in patients with cirrhosis is expected to reduce the risk of portal vein thrombosis but, among the bleeding risks, it will obviously be important considering variceal and non-variceal haemorrhage. Cost-utility and risk-benefit ratios of this strategy remain to be evaluated.
A healthy liver in a healthy mind?
In general, it comes as contradictory if not frankly paradoxical that NAFLD, which results from unhealthy lifestyle habits, should be managed with pharmacological interventions rather than non-pharmacological approaches [107, 108]. In this regard, an ancient Roman aphorism states “mens sana in corpore sano” meaning that you will have a healthy mind provided that your body is healthy. This adage was intended to encourage (young) people to engage in physical activity. Further expanding and virtually overturning this concept, it is now also likely that your liver will remain healthy provided that you maintain psychically healthy. For example, poor diet, inadequate physical activity and sedentary behaviour are strongly and independently associated with NAFLD [109]. Of interest, these unhealthy lifestyle habits are also important correlates of psycho-depression [110], suggesting that the latter may be the deep root cause of NAFLD in a proportion of cases. Should this hypothesis be confirmed, it would justify the expectation that management of underlying psychological factors, e.g., through psychological and cognitive-behavioural interventions may eventually promote virtuous lifestyle changes and, in the final analysis, result in the improvement of NAFLD. This rational construct must be evaluated by appropriate studies.
In conclusion, assessing the value, if any, of psychological consultation in the management of carefully selected cases of NAFLD associated with either overt or covert psycho-depressive traits is a research priority. The potential benefits of using anti-depressant drugs in selected NAFLD cases should carefully be balanced versus the risk of increasing body weight and thereby worsening metabolic profile in these patients [111].
NAFLD and sex medicine
Sex and gender differences are common features of human disease and definite modifiers of the top ten causes of death and morbidity in high-income countries. For example, coronary artery disease exhibits significant differences between men and women in key areas spanning epidemiology, clinical and pathophysiological features, to management and outcome [112]. Moreover, compared to men, women are more spared from infectious as well as chronic inflammatory disease although they are generally more prone to developing autoimmune disorders [113]. Interestingly, these sex differences also regard metabolic disorders and the prevalence of pre-diabetic syndromes is also sexually biased, men exhibiting impaired fasting glucose more often than women whereas impaired glucose tolerance is more prevalent among women than in men [114]. Consistent with all the findings reported above, a robust line of research based on substantial sex differences and the effect of reproductive status in clinical and experimental NAFLD [115–118] has advocated a more widespread awareness of sex differences in NAFLD research [33]. It is widely accepted that the prevalence of disease, as well as its fibrotic progression, follow a sexually dimorphic pattern with men being more exposed to NAFLD than fertile women; however, post-menopausal women lose the protection conferred by estrogen [72, 117, 119, 120]. Owing to the lack of gender/sex consideration in clinical trial design, insufficient data are available regarding sex differences in response to drug treatment of NAFLD [33, 121]. Weight reduction and regular exercise improve NAFLD, but we do not know how sex and gender affect this [111]. Recent data suggest that engaging in physical activity is associated with a 10% higher risk reduction of NAFLD in women than in men but, compared to men, women tend to have a lower compliance with recommendations on physical activity [109]. Moderate body weight reduction may suffice to achieve the resolution of NASH in men, while a substantially greater weight loss is necessary for NASH resolution in women [122].
In conclusion, the above-summarized epidemiological background translates into potentially clinically relevant sex differences in outcome and response to treatment [111] which remain, at this moment, largely unexplored.
Endocrine NAFLD
The notion that hormones regulate body fat distribution and energetic homeostasis implies that deranged hormonal concentrations play a key role in the development of NAFLD [123]. Confirming this pioneer intuition, an innovative line of research has identified a specific role for endocrine disease and hormonal derangements in NASH [124]. Presently, excluding T2D, the most common endocrine disorders associated with NAFLD include PCOS [125], hypothyroidism [126], hypogonadism [80], and GH deficiency [80, 127].
In conclusion, more studies are eagerly awaited aimed at gaining a better understanding of NAFLD forms secondary to endocrine derangements, which have been envisaged as a naturally occurring disease model of NAFLD in humans [80]. Therefore, this path of research promises novel therapeutic approaches, which may potentially be exploited also in certain cases of primary NAFLD [80].
Moving across the diaphragm: NAFLD and the lung
Chronic pulmonary disease
Epidemiological evidence suggests that chronic lung conditions, such as chronic obstructive pulmonary disease (COPD) and sleep apnoea syndrome, may bi-directionally be associated with either the MetS or its individual components (i.e. diabesity) [128–132].
Mantovani et al. [132], by meta-analyzing six observational studies globally enrolling more than 133, 000 individuals, three quarters of whom were Asians, more than a quarter with NAFLD, observed that NAFLD was significantly associated with reductions of lung volumes at baseline and that such reductions may also be a risk factor for incident NAFLD over follow-up. Not unexpectedly, NAFLD is a risk factor for the development of long-term cardiovascular events and mortality in patients with COPD [133].
As summarized elsewhere [134], studies have illustrated the range of similarities linking COPD and NAFLD which are common non-communicable diseases affected by a genetic predisposition and associated with an unhealthy lifestyle. Moreover, the liver and the lung also have similar vascular anatomy and immune-metabolic functions, such as antigen processing and regulation of energy homeostasis [134]. First-line treatment of COPD and NAFLD includes cessation of smoking, diet and physical activity [134].
In conclusion, COPD and NAFLD are prevalent systemic diseases with a high rate of cardio-nephro-metabolic and cancer co-morbidities and outcomes which, collectively, exact a high health and economic toll. How is it possible that NAFLD and COPD live together? Which is the egg and which is the chicken? Additional studies are warranted in individuals with COPD and NAFLD to address these research questions.
NAFLD and COVID-19
Clinical vignette
A 59-year old Caucasian man was admitted to our Medical COVID Unit owing to severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) pneumonia. Fever, dysgeusia, nausea, fatigue and polyarthralgia had begun on the 16th of March, a few days following a contact with a definite case of COVID-19. This patient’s laboratory profile exhibited IL-6 240.73 pg/mL (normal range 0-10), PCR 13.9 mg/dL (0–0.7), AST 357 U/L (1–37), ALT 354 U/L (1–40), GGT 210 U/L, (18–136), lactate dehydrogenase (LDH) 1242 U/L (208–378), creatine kinase (CK) 400 U/L (10–170), total bilirubin 0.96 mg/dL (0.16–1.10), alkaline phosphatase 123 U/L (38–126), procalcitonin 0.5 ng/mL (< 0.5), prothrombin time/international normalized ratio 1.28 (0.80–1.20). Competing causes of liver disease and bacterial pneumonia were ruled out. Chest X-rays showed bilateral interstitial pneumonia (Figure 2) and hemogasanalysis disclosed partial pressure of oxygen/fraction of inspired oxygen ratio = 266.
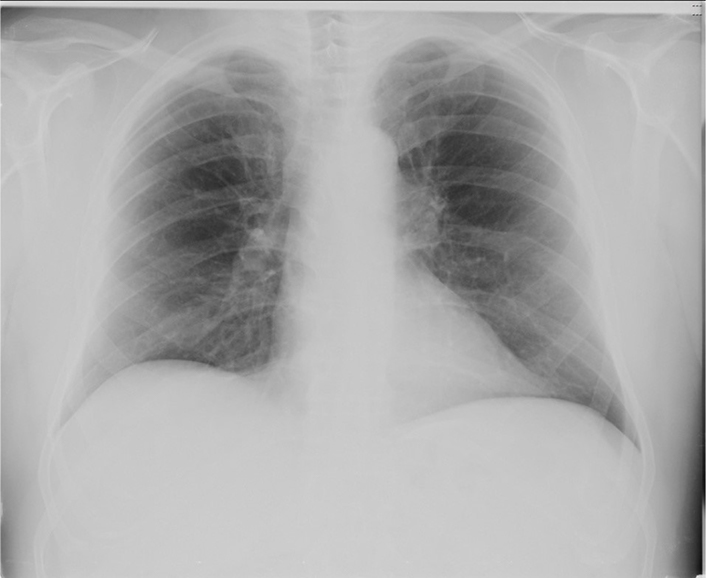
Chest X-Ray in SARS-CoV-2. Chest X-Ray displays a typical bilateral ground glass pattern, which tends to affect the posterior segments of lower lobes, with a peripheral and sub-pleural distribution
On the 30th of March, owing to worsening respiratory insufficiency, the patient received non-invasive ventilatory support and was administered oral Hydroxychloroquine and intravenous Tocilizumab at standard doses. Concurrently, laboratory data as well as dyspnea and subjective general conditions improved. The patient was discharged from hospital on the 8th of April.
COVID and the Liver
While interstitial pneumonia remains the most important, often life-threatening, organ damage occurring in the course of the SARS-Cov-2, liver injury has also been observed in severe cases in full analogy to that observed in the other two closely related highly pathogenic coronaviruses, SARS-CoV and the Middle East respiratory syndrome coronavirus [135]. Prevalence and determinants of altered liver tests are poorly characterized and given their mild and transient nature, and similar to the closely related SARS disease [136] they are expected to be easily overlooked in the setting of prominent respiratory dysfunction and overwhelming pandemic.
A large body of literature indicates that the liver is commonly involved in bacteremia, sepsis and bacterial pneumonia as expressed by altered hepatic enzymes and/or cholestasis [137–143]. However, individuals with COVID-19 pneumonia tend to have remarkably more abnormal values of AST, ALT, GGT and LDH than those with pneumonia owing to non-COVID-19 etiologies [144]. Host and viral factors modulate the risk of liver dysfunction in COVID-19. For example, men and individuals from the highly hyperendemic Wuhan area are deemed to manifest a higher risk of COVID-associated liver dysfunction [145]. Few data are available regarding the pathogenic mechanisms accounting for liver dysfunction in COVID-19 and, speculatively, this may be due to SARS-CoV-2 directly damaging bile duct epithelial cells; concurrent drug-induced liver injury; systemic inflammatory response syndrome; hypoxia-reperfusion dysfunction [145]. It is also reasonable to hypothesize that individuals with pre-existent NAFLD/NASH also display a worse laboratory profile. A recent meta-analysis by Mantovani et al. [146], however, has shown that, while the baseline prevalence of liver disease in patients with COVID-19 is relatively low, major elevations of liver enzymes occur in the course of disease paralleling alterations of coagulative and fibrinolytic pathway profile, as a result of the innate immune response against the virus.
In conclusion, the effects of the SARS-CoV-2 infection on the (fatty) liver are a topic of great clinical and biological interest. Studies will have to ascertain whether liver damage is more common among those with NAFLD/NASH and whether these are exposed to increased mortality. The worse outcome of SARS-CoV-2 infection in those with obesity and increased IL-6 levels may also be a clue to the involvement of the liver [147, 148]. An additional line of research will have to address what the metabolic complications (including development/progression of NAFLD) will be owing to the protracted physical inactivity (and, in many cases, of perturbed dietary habits) owing to the global lockdown adopted, in many countries, by Health Authorities to combat the COVID epidemic in 2020.
Conclusions
What is, in its intimate essence, NAFLD? It is an adaptive physiological response [149–151] which, in some cases, becomes maladaptive [151, 152] owing to sexual, genetic and environmental modifiers. It is also a barometer of metabolic health [153, 154]. Therefore, it is illogical to postulate that we should be looking for a pharmacological “magic bullet” which reverses the disease in all cases irrespective of the widely acknowledged [87] individual variability. The intrinsic irrationality of this approach may probably account for both the failure of some novel anti-fibrotic drugs such as Cenicriviroc and Selonsertib [155, 156] and the only partial success of others which, for example, improve liver histology, though at the cost of worsening the dysmetabolic features (i.e. increased LDL-cholesterol and HbA1c) inherent in the disease [157]. Stated otherwise, we should specifically target those cases of NAFLD with the potential to become progressive over time and diagnosing NASH is a surrogate (not the ultimate) answer to this problem. Simultaneously, the potency of lifestyle in preventing, and reversing the effects of NAFLD [158] needs to be fully appreciated and strongly re-launched thanks to adequate psychological backing and conditioning.
In conclusion, revolutionizing the previously prevailing paradigm of chronic hepatitis owing to viral etiology, over the last 20 years, NAFLD has taught us a lot regarding the pathogenic importance of an endogenous pathogenic factor, insulin resistance [45, 159, 160] but also the limitations of correcting this in the treatment of NAFLD [161], the root causes of T2D and the MetS [3, 89, 162, 163], the role of sex differences in disease [33, 116, 117, 164] and the key importance of nuclear receptors in determining NAFLD [1]. It is also possible that, thanks to its systemic nature [75] certain features of NAFLD will be exploited in the future for guiding our medical conduct in closely related conditions, such as colo-rectal adenoma and carcinoma, by modulating colonoscopic surveillance programmes based on liver status [165]. That said, the overwhelming COVID-19 pandemic, is now likely to reset the stage of our clinical interest suggesting that–for the time being– NAFLD has probably lost its primacy among contemporary public health issues.
Abbreviations
| AF: | atrial fibrillation |
| AFLD: | alcoholic fatty liver disease |
| AHEI: | alternative healthy eating index |
| ALT: | alanine aminotransferase |
| ARFI: | acoustic radiation force impulse |
| AST: | aspartate aminotransferase |
| AUROC: | area under the receiver operating characteristic curve |
| BMI: | body mass index |
| CAP: | controlled attenuation parameter |
| COPD: | chronic obstructive pulmonary disease |
| COVID-19: | Coronavirus disease 2019 |
| CT: | computed tomography |
| ELOVL2: | ELOVL fatty acid elongase 2 |
| eLP-IR: | enhanced lipoprotein insulin resistance index |
| FADS2: | fatty acid desaturase 2 |
| GCKR: | glucokinase regulator |
| GGT: | gamma-glutamyl-transferase |
| GH: | growth hormone |
| GPR120: | G-protein coupled receptor 120 |
| GRS: | genetic risk score |
| GWAS: | genome-wide association studies of nonalcoholic fatty liver disease |
| HCC: | hepatocellular carcinoma |
| HCV: | hepatitis C virus |
| HFF%: | hepatic fat fraction |
| HOMA-IR: | homeostasis model assessment of insulin resistance |
| IF: | intermittent fasting |
| IR: | insulin resistance |
| KLF6: | Kruppel like factor 6 |
| LDH: | lactate dehydrogenase |
| LPIN1: | Lipin 1 |
| MAFLD: | metabolic associated fatty liver disease |
| MBOAT7: | membrane bound O-acyltransferase domain containing 7 |
| MDS: | mediterranean-style diet score |
| MetS: | metabolic syndrome |
| MR: | magnetic resonance |
| MRS: | nuclear magnetic resonance spectroscopy |
| MTTP: | microsomal triglyceride transfer protein |
| NAFLD: | nonalcoholic fatty liver disease |
| NAS: | nonalcoholic fatty liver disease activity score |
| NASH: | nonalcoholic steatohepatitis |
| NFS: | NAFLD fibrosis score |
| PCOS: | polycystic ovary syndrome |
| PNPLA3: | patatin-like phospholipase domain-containing protein 3 |
| RCT: | randomized controlled trial |
| SAF: | Steatosis, Activity, and Fibrosis |
| SARS-CoV: | severe acute respiratory syndrome coronavirus |
| SNPs: | single nucleotide polymorphisms |
| SOD2: | superoxide dismutase 2 |
| T2D: | type 2 diabetes |
| TM6SF2: | transmembrane 6 superfamily member 2 |
| US: | ultrasound |
| US-FLI: | ultrasonographic fatty liver indicator |
| VTE: | venous thromboembolism |
Declarations
Acknowledgments
Dedicated to AL’s newborn grandson, Amedeo Juhani Lonardo
Author contributions
AL and SB together wrote the first draft of the manuscript; equally contributed to manuscript revision, read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2020.