Abstract
Rheumatoid arthritis (RA) is a chronic immune-mediated inflammatory disease of unknown origin. Although it mainly affects joints, it can have extra-articular manifestations, with the lung being one of the most affected organs. The estimated incidence of diffuse interstitial lung disease (ILD) is 4 cases to 4.5 cases/1000 patient-years. The most common forms are usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP; 44–46% and 33–44%, respectively), although there have been reports of cases involving all the histopathologic forms described for the disease. RA-ILD is associated with specific risk factors, such as male sex, older age, smoking, and positive rheumatoid factor (RF) and anti-citrullinated peptide antibody (ACPA) levels. The clinical course of ILD ranges from asymptomatic forms to rapidly progressive disease in a minority of cases. It has been estimated that the risk of death is up to 3-fold higher in patients with RA-ILD than in those without ILD, making RA-ILD the second most common cause of death after cardiovascular disease. Treatment of RA has improved considerably in recent years with the advent of biologics; however, the use of these agents has been restricted in patients with ILD owing to safety concerns. Many doubts continue to surround the treatment of patients with RA-ILD. Therefore, the objective of this review is to examine the current management of affected patients in terms of diagnosis, treatment, and follow-up.
Keywords
Rheumatoid arthritis, interstitial lung disease, managementIntroduction
Diffuse interstitial lung disease (ILD) comprises a group of conditions characterized by diffuse cellular and noncellular infiltration of the interstitium. ILD includes more than 200 disease entities, among which the underlying cause is found in only one-third of cases after the correct application of diagnostic techniques [1]. The diseases are all very uncommon and are defined as rare diseases. Therefore, it is paramount for the professionals who manage them to acquire sufficient experience to ensure correct diagnosis and treatment.
Within ILD, the idiopathic interstitial pneumonias (IIPs) comprise a group of 7 diseases that can be recognized by their clinical, radiological, and histological characteristics after a dynamic and multidisciplinary process [2]. The cause of the diseases is unknown, although they result from damage to the parenchyma through various patterns of inflammation and fibrosis. The most frequent IIP is idiopathic pulmonary fibrosis (IPF).
Rheumatoid arthritis (RA), as with other systemic autoimmune diseases, may be associated with ILD, whose clinical, radiological, and histological characteristics are similar to those of IIPs.
Incidence and prevalence of RA-ILD
The incidence of ILD associated with RA (RA-ILD) is estimated at between 4 cases and 4.5 cases/1,000 patient-years [3, 4]. Population-based studies in the USA suggest that the cumulative incidence is 3.5–5% at 10 years, 6.3% at 15 years, and 6.8–7.7% at 30 years of follow-up [5, 6]. The prevalence of ILD varies considerably, ranging from 10% to 30% of cases of early RA (≤ 2 years) and between 3.6% and 42% in established RA [5, 7–12]. Another prospective study, based on a population cohort (Rochester, Minnesota, USA), estimated the cumulative incidence of RA-ILD to be 3.5%, 6.3%, and 7.7% at 10, 20, and 30 years, respectively [5]. The most relevant finding of the study was that the risk of this complication in patients with RA was much greater than in the general population [hazard ratio (HR) = 8.96].
This variability is due to the great difference between the different populations included in the studies published to date and the lack of uniformity in the high-resolution computed tomography (HRCT) findings used to establish the diagnosis of ILD. All this variability means that to date not many longitudinal studies or registries have been published.
Determination of the prevalence of RA-ILD is hampered mainly by the fact that ILD is often asymptomatic until advanced stages [6]. The prevalence of subclinical ILD is variable, ranging from 19% to 57% [10, 13], and the disease can progress to the clinical form in 50% of cases [6].
Reported results are highly variable. All studies include patients with RA, although the time to diagnosis of the disease differs widely at inclusion. Markedly heterogeneous findings have also been detected in HRCT.
Risk factors for ILD
Several risk factors have been associated with ILD, including smoking [13], male sex, advanced age, late onset of RA, severe and erosive joint disease, and disease duration, with most cases of lung involvement occurring within 5 to 10 years of onset and positive anti-citrullinated peptide antibody (ACPA) titers [7, 8, 14]. The presence of rheumatoid nodules and positive rheumatoid factor (RF) have been highlighted in only a few studies [7, 8, 14].
Smoking is closely associated with the onset of ILD with an odds ratio (OR) of 3.76 [95% confidence interval (CI), 1.59–8.88] for ≥ 25 pack-years and 1.9 (95% CI, 0.68–5.24) for < 25 pack-years [13].
As for advanced age, the most common age groups are > 60 years [OR of 1.48 (95% CI, 1.01–2.18)] [15] and ≥ 65 years [relative risk (RR) of 4.58 (95% CI, 1.67–12.53)] [16].
With respect to disease activity measured by the 28-joint disease activity score (DAS28) with C-reactive protein (CRP), patients with maintained moderate-high activity are twice as likely to develop ILD as patients in remission or with low disease activity (HR = 2.22; 95% CI, 1.28–3.82). Thus, the risk of ILD according to the DAS28 score is as follows: low activity (DAS28 > 2.6–3.2), 1.41 (95% CI, 0.61–3.28); moderate activity (> 3.2–5.1), 2.08 (95% CI, 1.06–4.05), and high activity (> 5.1), 3.48 (95% CI, 1.64–7.38) [14].
Positive findings for RF and ACPA, especially at high titers, increase the risk of ILD [15, 17–21]. In one meta-analysis [7 studies, 1,685 patients; I2 (measure of heterogeneity) = 0%], the risk of ILD and pulmonary fibrosis was almost 5 times greater in the presence of ACPA [OR of 4.68 (95% CI, 2.07–10.57)] [15].
In addition to RF and ACPAs, other biomarkers potentially predictive of ILD such as antibodies directed against carbamylated proteins (anti-CarP) have been studied. It has been shown that there is a correlation between the levels of all anti-CarP specificities and the presence of ILD. Similarly, other biomarkers such as extracellular matrix metalloproteinases 7 (MMP-7) [16–18], interferon-gamma (IFNγ)-induced protein-10 (IP-10) or CXCL1049, interleukin-18 (IL-18), and 90 and 70 KDa heat shock proteins (HSP90/70) [19, 20] are currently being studied with promising results. The problem is that none of them are available in daily clinical practice and have not been shown to have greater predictive value for the development of this complication than ACPA.
Some genetic biomarkers have also been identified, such as mutations in the MUC5B gene (which encodes one of the proteins of the mucin family) and mutations in the telomerase genes that condition accelerated telomere shortening (in cases with a family history of ILD) [20–23].
Finally, the development of ILD has been related to drugs used conventionally in the treatment of this disease. This aspect will be evaluated in later sections.
Progression of lung disease and mortality
ILD continues to be the second cause of premature death in RA after cardiovascular complications, leading to 10–20% of deaths from the disease [6, 24, 25]. The clinical course and prognosis of RA-ILD are very heterogeneous. The disease remains stable or progresses slowly in a variable percentage of patients, whereas in others, pulmonary function deteriorates rapidly. This heterogeneous course makes it essential to identify prognostic factors of severe disease and mortality, which are key for follow-up and treatment of affected patients [24–26].
The prognostic factors most associated with greater mortality are advanced age at diagnosis of ILD [27–32]: male sex [33], duration of RA [32, 33], moderate-high disease activity, the usual interstitial pneumonia (UIP) radiological pattern [32–38], low baseline diffusing capacity of the lung for carbon monoxide (DLCO) and/or forced vital capacity (FVC), decrease > 10% in FVC or > 15% in DLCO during follow-up [30–33, 36], extensive lung involvement in chest HRCT [34, 37, 38], gender-age-physiology and composite physiological indices [37], and elevated serum levels of Krebs von den Lungen 6 (KL-6; Table 1) [27, 36].
Predictors of disease progression and mortality
| Conditions | Predictors |
|---|---|
| Progression | UIP pattern Extension in HRCT High ACPA values Degree of impairment in follow-up High serum IL-6 and KL-6 |
| Mortality | Advanced age at diagnosis Male sex Duration and moderate-high activity of RA UIP pattern Low baseline DLCO and/or FVC ↓DLCO > 15% or ↓FVC > 10% during follow-up Extension in HRCT > 20–30% GAP and CPI High serum KL-6 |
↓: less; GAP: gender-age-physiology; CPI: composite physiologic index
A 2014 systematic review of 10 studies investigating predictors of mortality in RA-ILD found the significant predictors of mortality to be male sex, older age, lower DLCO, UIP, and extension of fibrosis [38]. A 2019 meta-analysis of 10 studies (1,256 patients with RA-ILD) found a higher risk of mortality for the UIP pattern [OR of 1.66 (95% CI, 1.07–2.25)] [39].
The main predictive factors in progression of ILD are the UIP radiological pattern [40–44], high ACPA titers [45–47], the degree of impaired DLCO at baseline [48], a decrease of ≥ 10% (estimated theoretical percent value) in FVC during follow-up [48], extensive lung involvement in chest HRCT [40–43], and high serum levels of IL-6 and the glycoprotein KL-6 (Table 1) [27].
Interstitial pneumonia patterns associated with RA
The 2 patterns most associated with RA are UIP and nonspecific interstitial pneumonia (NSIP). UIP is the most prevalent in most series [5, 7, 8].
Patients with the UIP pattern are hospitalized more frequently owing to respiratory disorders and have a lower survival rate, although the data are not conclusive [30, 49–53].
Similar to idiopathic UIP, the clinical course of RA-UIP is variable and may be interrupted by decompensation episodes known as acute exacerbations, which are defined as acute worsening of dyspnea (i.e., within approximately 1 month) accompanied by new ground-glass opacification and bilateral consolidations, once acute pulmonary edema has been ruled out.
NSIP affects one-third of patients with RA-ILD and is generally associated with a lower risk of disease progression and a better response to treatment [6, 50, 51, 53]. The most common symptoms are dyspnea and cough, which develop over weeks or months. The clinical course of NSIP is heterogeneous: in some patients, the condition remains stable (the minority) and in others, the condition deteriorates rapidly [54].
Other less frequent patterns include cryptogenic organizing pneumonia (COP) and acute interstitial pneumonia (AIP). Diagnosis in these cases is simpler owing to the acute course of the disease and the presence of alveolar infiltration and ground-glass opacification on the chest x-ray.
The differential diagnosis is problematic and must be made with intercurrent infections or drug toxicity. In such cases, bronchoalveolar lavage (BAL), transbronchial biopsy, and even surgical lung biopsy can help to confirm the diagnosis.
Diagnosis of ILD in patients with RA
Screening for early diagnosis of ILD in patients with RA is very important since the disease is associated with significant morbidity and mortality. Subclinical ILD is highly prevalent in affected patients and may be characterized by clinical-radiological progression in approximately half of reported cases [55, 56]. Evidence from the literature suggests a higher probability of response if therapy is started early.
Recent recommendations drawn up jointly by the Spanish Society of Rheumatology and the Spanish Society of Pulmonology and Thoracic Surgery (SER-SEPAR) recommendations [57] highlight the importance of a multidisciplinary approach based on teams formed by rheumatologists, pulmonologists, radiologists, and pathologists, both at diagnosis and during follow-up. Screening criteria for ILD in patients with RA have also been developed by a SER-SEPAR expert panel following the Delphi method [58].
When taking the clinical history, physicians should aim to identify potential risk factors associated with ILD and respiratory symptoms such as dry cough and dyspnea. The approach should also involve chest auscultation and physical examination in patients with RA [57].
A 3-month history of respiratory symptoms or velcro-type dry crackles in the chest auscultation [6, 34] points to the need for systematic screening for ILD [58].
Screening should start with chest X-rays and pulmonary function tests (PFTs), including spirometry and DLCO. Depending on the result, we should evaluate the need for HRCT [58].
Chest X-rays are an inexpensive, accessible, and low-radiation technique with the added advantage of facilitating the differential diagnosis and detection of associated complications, albeit with low sensitivity and specificity. In ILD, the characteristic finding is an interstitial pattern initially affecting the lung bases.
Functional alterations in PFTs are characterized by a restrictive ventilatory defect in spirometry and a decrease in DLCO, which is more sensitive and frequently the only identifiable alteration in early stages [4, 9].
However, both respiratory symptoms and chest X-rays or PFTs are limited owing to their low sensitivity for rapid diagnosis of ILD in the earliest stages [7–9].
HRCT is the gold standard diagnostic technique in ILD [58] owing to its high sensitivity. HRCT can confirm a diagnosis in cases of clinical suspicion and normal radiographic findings and characterize the ILD pattern, which correlates well with the histologic diagnosis in most patients [59]. The HRCT pattern of ILD is also useful for determining the potential reversibility of the lesions (alveolitis/fibrosis), reaching a prognosis, and evaluating the response to treatment.
HRCT should be performed directly in patients with velcro-type crackles on auscultation [58]. In the absence of respiratory symptoms and abnormal findings on auscultation, the need for screening should be evaluated on an individual basis depending on the number of risk factors associated with ILD, irrespective of the time since diagnosis of RA.
When the overall risk factor score is 5–6, screening for ILD should be performed with chest X-rays and PFTs, and the need for HRCT depending on the results should be evaluated. If the overall score is ≥ 7, then HRCT should be performed directly.
Systematic BAL is not recommended and should only be performed in cases of doubtful diagnosis, especially when infection is suspected, in order to facilitate the differential diagnosis. Lymphocytosis in BAL seems to be more frequent in non-UIP patterns of RA-ILD, whereas neutrophilia is more common in patients with UIP and has been considered indicative of disease progression and diminished response to therapy [60].
Lung biopsy should be reserved for cases of doubt over an alternative diagnosis of ILD (e.g., hypersensitivity pneumonitis and smoking-related disease) or suspicion of lung cancer [61].
The recommendations for frequency of screening are as follows [58]:
(A) Auscultation should be performed during follow-up at least once per year, and patients should be questioned specifically about the presence of respiratory symptoms. The presence of risk factors for ILD should be evaluated.
(B) If velcro-type crackles are detected or the patient experiences respiratory symptoms (> 3-month history of cough and/or dyspnea) during follow-up, screening tests should be repeated according to the previous recommendations, independently of whether they were previously negative.
(C) In asymptomatic patients with normal findings on chest auscultation, an overall score of ≥ 5, and negative screening test results, screening should be performed once per year with spirometry and measurement of DLCO.
Treatment of RA-ILD
There is currently no consensus on the most appropriate treatment for patients with RA-ILD. Therapy should be integrated and on an individual basis, and multidisciplinary teams should have access to support from nurses, physiotherapists, and pharmacists.
The choice of therapeutic strategy in RA-ILD is complex since multiple factors must be taken into account, for example, severity of RA and ILD, presence of prognostic factors associated with both progression and mortality, comorbid conditions, and patient preferences (Figure 1) [56]. The potential risk of pneumonitis induced by some of the disease-modifying antirheumatic drugs (DMARDs) used to treat RA has been reported [61, 62].
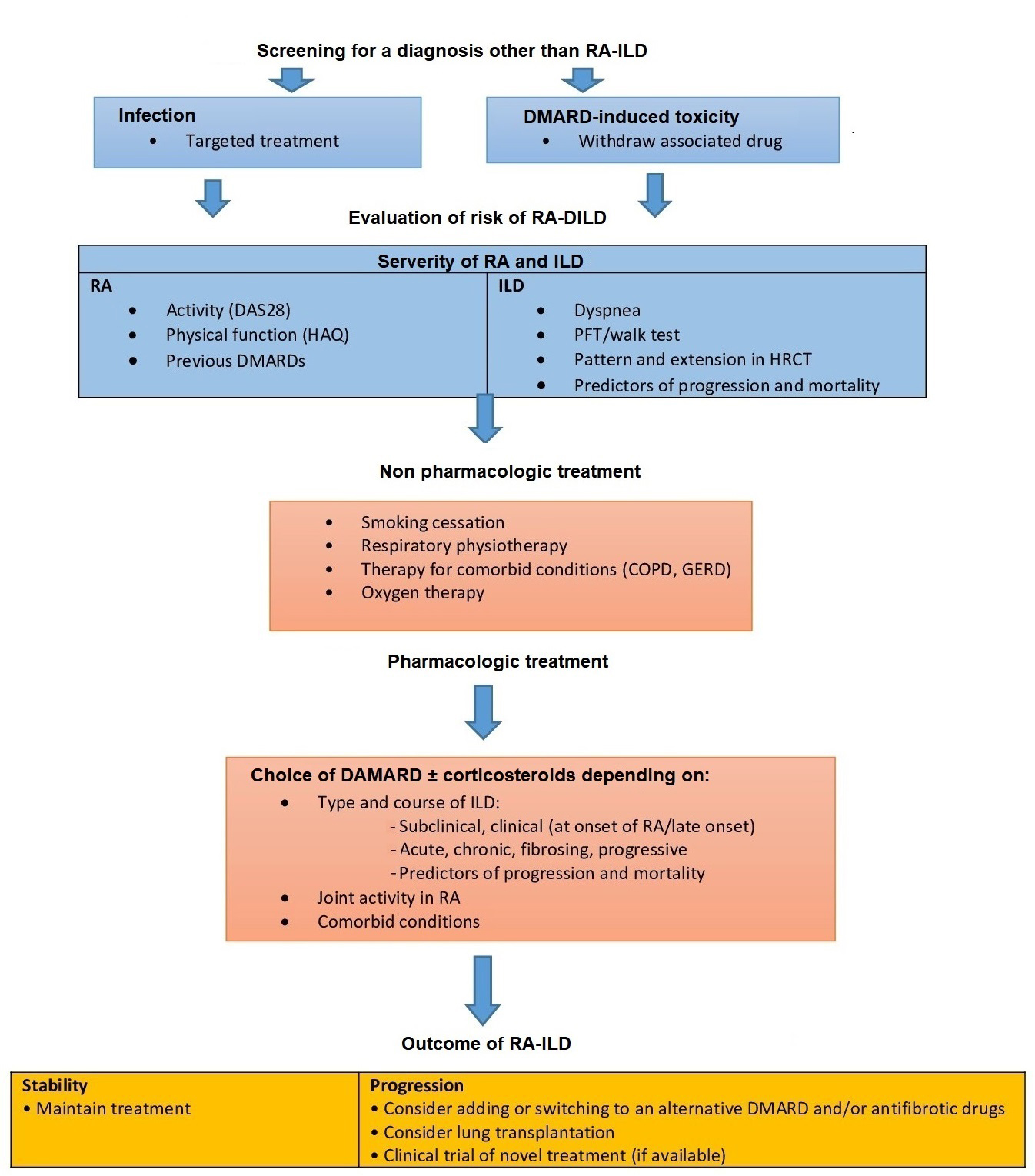
Algorithm for the management of RA-ILD. COPD: chronic obstructive pulmonary disease; GERD: gastro-oesophageal reflux disease; HAQ: health assessment questionnaire
Corticosteroids form part of the arsenal used to treat RA-ILD, especially in severe and progressive forms with a predominantly NSIP- or COP-type pattern, which respond more favorably than UIP [62, 63].
Owing to their dose- and duration-dependent adverse effects, such as infection and osteoporosis, corticosteroids are used mainly in the initial phase of RA-ILD as bridging therapy for the shortest period and at the lowest dose possible until DMARDs and/or antifibrotic drugs take effect [4, 57, 63].
Methotrexate (MTX) is a key drug in the treatment of RA, and recent evidence shows that it is not a risk factor for RA-ILD or for progression or mortality [64–66]. Therapy with MTX has been associated with later onset of RA-ILD in 2 British cohorts of early RA [63], a lower risk of hospitalization because of ILD [64], and greater survival than with other conventional synthetic DMARDs (csDMARDs) or in controls who did not receive DMARDs. However, MTX can induce pneumonitis, especially during the first year of treatment, although recent data indicate that the risk is lower than previously thought. MTX is usually avoided in clinically significant or progressive RA-ILD.
According to the recommendations of SER-SEPAR [57], in cases where ILD is diagnosed or worsens during the first year of treatment with MTX, the drug should be suspended temporarily until it is determined whether there is a causal relationship. However, MTX could be maintained if ILD is diagnosed after more than 1 year of treatment. When ILD is present at the onset of RA, MTX should be evaluated on an individual basis owing to the risk of inducing pneumonitis, given that it is better to use other csDMARDs as a risk minimization strategy.
In patients with RA-ILD who are not of Asian origin, leflunomide (LEF) is considered a safe option [57], given that the risk of LEF-induced pneumonitis has mainly been described in Asian populations [67].
The role of other csDMARDs such as mycophenolate mofetil, cyclophosphamide, azathioprine (AZA), cyclosporine A, and tacrolimus in the management of RA-ILD is even less clear. Moreover, these drugs are at a disadvantage owing to their poorer toxicity profile and modest efficacy in joint involvement in RA.
Biologic DMARDs (bDMARDs) have become increasingly important in the treatment of moderate-severe RA in recent years and have led to improvement in or stabilization of respiratory symptoms, lung function, and/or imaging findings in retrospective studies.
Findings for anti-tumor necrosis factor alpha (anti-TNFα) inhibitors are contradictory, with reports of favorable outcomes and of onset or exacerbation of RA-ILD [43, 45]. A study performed in the USA found no significant differences with respect to incidence or exacerbation of ILD for anti-TNFα agents compared with other biologics such as abatacept (ABA), rituximab (RTX), and tocilizumab (TCZ) [68, 69], although values were numerically lower for ABA. In another study [68], the authors reported a higher rate of exacerbation of ILD with anti-TNFα agents (30%) than with other biologics such as ABA and TCZ. With respect to the effect on mortality associated with RA-ILD, data from the British Society for Rheumatology Biologics Registry did not associate anti-TNFα drugs with greater mortality than csDMARDs, although the relative risk of all-cause mortality was twice as high in patients treated with anti-TNFα agents [70, 71]. Huang et al. [72] in a search in different literature databases from their inception to November 2018 selected 7 original articles and 28 case reports. All 7 cohort studies demonstrated the lack of benefit from TNF inhibitor (TNFi) treatment in patients with ILD and could be associated with pulmonary adverse events. Case reports further suggested that TNFi was harmful in 87.5% of the cases and even increased mortality [72].
In the case of ABA, observational studies show improvement or stabilization in imaging tests and in FVC and DLCO (> 85%) after a mean follow-up of up to 48 months, irrespective of the pattern of lung involvement and with no unexpected adverse events [73–76]. ABA led to significantly lower rates of exacerbation of ILD than anti-TNFα drugs [76] and was associated with a 90% reduction in the relative risk of worsening ILD after 24 months of follow-up compared with anti-TNFα drugs and csDMARDs. ABA also had a better efficacy profile than RTX and TCZ.
RTX has been associated with improvement in or stabilization of lung function in several retrospective studies with small samples, even in RA-ILD that was refractory to standard treatment [77, 78]. Data from a British registry show RTX to be associated with greater survival in RA-ILD than anti-TNFα agents, with a risk of all-cause mortality that is 48% lower than that of anti-TNFα drugs [79].
Data on TCZ are anecdotal and contradictory. The drug has been associated with improvement and stabilization in RA-ILD, although it has also been associated with fatal pulmonary adverse effects. Therefore, more studies are necessary to draw conclusions on its efficacy and safety profile [80].
As for targeted therapy, available data are scarce, although there have been reports of very low rates of ILD in registry studies and in postmarketing studies [81].
Roubille et al. [82] reviewed published cases of induced or exacerbated ILD in RA associated with non-biologic DMARDs (nbDMARDs). They conducted a systematic review of the literature from 1975 to July 2013 using Medline, Embase, Cochrane, and abstracts from the American College of Rheumatology (ACR) 2010–2012 and European League Against Rheumatism (EULAR) 2010–2013 annual meetings.
They selected a total of 88 articles [32 for MTX, 12 for LEF, 3 for gold, 1 for AZA, 4 for sulfasalazine (SSZ), 27 for TNFi, 3 for RTX, 5 for TCZ, and 1 for ABA]. No articles were found with hydroxychloroquine (HCQ) or anakinra. After analyzing the results, they noted that ILD is a rare serious adverse event that occurs mostly in the first 20 weeks after initiation of treatment, usually causing dyspnea, especially in elderly patients, and can be life-threatening. They emphasize the importance of pulmonary function monitoring in RA patients with pre-existing ILD while receiving biologic therapy or nbDMARDs [82].
Antifibrotic drugs can be used in those forms of RA-ILD that progress despite therapy. In the INBUILD study, a double-blind, randomized, controlled trial that included patients with progressive fibrosing ILD (13% RA-ILD), the authors showed that nintedanib reduced the frequency of impairment of FVC, albeit without modifying respiratory symptoms or clinical events [83]. No data are available for pirfenidone in RA-ILD, although an ongoing clinical trial in this disease TNF-related apoptosis-inducing ligand 1 (TRAIL 1) will evaluate its efficacy both in the joints and in the lungs [84]. In actual clinical practice, some clinical cases [85, 86] and a case series [87] confirm the efficacy and safety of nintedanib in the treatment of progressive fibrosing LID, administered in combination with classic synthetic DMARDs or mophetil micophenolate (MMF) and biologics (RTX, ABA, and TCZ) with a good safety profile.
Evidence on the use of biologics for the treatment of RA-ILD shows ABA and RTX to be the safest options in the Clinical Practice Guidelines for Management of Patients with RA of the Spanish Society of Rheumatology (GUIPCAR) [4], the guidelines on the safety of bDMARDs in inflammatory arthritis of the British Society for Rheumatology [88], and the recommendations of SER-SEPAR, which recommend considering targeted synthetic DMARDs or IL-6 inhibitors in the case of contraindications or insufficient response to ABA or RTX [55]. In addition, real-world data suggest that both ABA and RTX can improve or stabilize lung function.
It is necessary to remember the importance of nonpharmacologic treatment, such as recommending patients to give up smoking. Respiratory physiotherapy and evaluation of the need for home oxygen therapy have a beneficial effect on patients’ quality of life.
In the case of patients with progressive RA-ILD, should be considered the possibility of lung transplantation, since the risk of rejection and death has been reported to be similar to that of other ILDs [89].
Prognosis and follow-up
While a significant improvement has been observed in the prognosis of RA in the last 15 years, ILD is the second most frequent cause of death after cardiovascular disease. In a variable percentage of patients, ILD barely progresses and remains in a subclinical asymptomatic or pauci-symptomatic phase. In the remainder, lung function deteriorates rapidly, especially in patients with UIP, and mean survival after diagnosis in various studies has been shown to be 2 to 3 years, with a maximum of 8 years [5].
The outcome of RA-ILD and the response to treatment are monitored using PFTs, which should include total lung capacity, DLCO, the 6-minute walk test, and the evaluation of dyspnea based on clinical scales. Patients with RA-ILD should be followed up every 3–6 months, and, once the disease has stabilized, every 6–12 months. The changes considered clinically relevant include a 15% reduction in DLCO and a 10% reduction in FVC over baseline [59].
In advanced phases, echocardiography is useful for detecting secondary pulmonary hypertension [90]. The differential diagnosis of this complication is mainly with infection, drug-induced lung toxicity, and heart failure.
Conclusions
RA is a chronic immune-mediated inflammatory disease that mainly affects joints but can have extra-articular manifestations, with the lung being one of the most affected organs becoming the second cause of premature death in RA after cardiovascular complications. For this reason, screening for early diagnosis of ILD in patients with RA is very important. The treatment of RA has changed substantially in recent years since the advent of biological drugs. Therapeutic strategy in these patients is complex due to different factors.
Abbreviations
| ABA: |
abatacept |
| ACPA: |
anti-citrullinated peptide antibody |
| anti-TNFα: |
anti-tumor necrosis factor alpha |
| BAL: |
bronchoalveolar lavage |
| CI: |
confidence interval |
| csDMARDs: |
conventional synthetic disease-modifying antirheumatic drugs |
| DAS28: |
28-joint disease activity score |
| DLCO: |
diffusing capacity of the lung for carbon monoxide |
| DMARDs: |
disease-modifying antirheumatic drugs |
| FVC: |
forced vital capacity |
| HR: |
hazard ratio |
| HRCT: |
high-resolution computed tomography |
| IIPs: |
idiopathic interstitial pneumonias |
| ILD: |
interstitial lung disease |
| KL-6: |
Krebs von den Lungen 6 |
| LEF: |
leflunomide |
| MTX: |
methotrexate |
| NSIP: |
nonspecific interstitial pneumonia |
| OR: |
odds ratio |
| PFTs: |
pulmonary function tests |
| RA: |
rheumatoid arthritis |
| RF: |
rheumatoid factor |
| RTX: |
rituximab |
| SER-SEPAR: |
Spanish Society of Rheumatology and the Spanish Society of Pulmonology and Thoracic Surgery |
| TNFi: |
tumor necrosis factor inhibitor |
| UIP: |
usual interstitial pneumonia |
Declarations
Acknowledgments
The authors thank the Spanish Foundation of Rheumatology for providing medical writing/editorial assistance during the preparation of the manuscript (FERBT2023).
Author contributions
GCR and VV: Conceptualization, Writing—original draft, Writing—review & editing. Both of the authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.