Affiliation:
1Osakidetza, OSI EE-Cruces, Rheumatology Division, Cruces University Hospital, 48903 Barakaldo, Spain
2BIOBizkaia Health Research Institute, 48903 Barakaldo, Spain
†These authors contributed equally to this work.
Affiliation:
3Department of Medicine, Medicine and Nursery School, University of the Basque Country UPV/EHU, 48903 Barakaldo, Spain
ORCID: https://orcid.org/0009-0000-4140-8262
Affiliation:
1Osakidetza, OSI EE-Cruces, Rheumatology Division, Cruces University Hospital, 48903 Barakaldo, Spain
2BIOBizkaia Health Research Institute, 48903 Barakaldo, Spain
Affiliation:
1Osakidetza, OSI EE-Cruces, Rheumatology Division, Cruces University Hospital, 48903 Barakaldo, Spain
2BIOBizkaia Health Research Institute, 48903 Barakaldo, Spain
ORCID: https://orcid.org/0000-0002-4983-5506
Affiliation:
1Osakidetza, OSI EE-Cruces, Rheumatology Division, Cruces University Hospital, 48903 Barakaldo, Spain
2BIOBizkaia Health Research Institute, 48903 Barakaldo, Spain
3Department of Medicine, Medicine and Nursery School, University of the Basque Country UPV/EHU, 48903 Barakaldo, Spain
†These authors contributed equally to this work.
Email: fernando.perezruiz@osakidetza.eus
ORCID: https://orcid.org/0000-0002-5268-1894
Explor Musculoskeletal Dis. 2023;1:186–193 DOI: https://doi.org/10.37349/emd.2023.00021
Received: May 22, 2023 Accepted: September 22, 2023 Published: October 31, 2023
Academic Editor: Peter Mandl, Medical University of Vienna (MUW), Austria
Aims: To study factors associated with the development of calcium pyrophosphate (CPP) arthritis and the severity phenotype.
Methods: Transversal case-control study. Cases had to be confirmed by both X-ray chondrocalcinosis and CPP crystals in synovial fluid. Controls had neither chondrocalcinosis nor CPP crystals in synovial fluid. Patients and controls with hemochromatosis or primary hyperparathyroidism were not included. Mutations of hemochromatosis genes (HFE), magnesium (Mg), calcium (Ca), phosphate, iron (Fe), transferrin saturation, ferritin, parathyroid hormone (PTH), and calcifediol levels were studied.
Results: Three hundred patients and 300 sex and age matched controls were compared. Lower serum Mg (sMg) and higher ferritin levels were found among patients. Hypomagnesemia (HypoMg) and HFE mutations were more frequent among patients. Involvement of over one joint was observed in 199 (66.4%) patients whereas persistent joint inflammation was retrieved in 154 (51.4%) of the patients. Initial analysis showed that the frequency of polyarticular and inflammatory phenotypes seemed to be progressively overrepresented in patients with HFE mutations. Further bivariate and multivariate analysis adjusted for the time from onset disclosed that the presence of genotypes with C282Y mutations was associated with polyarticular disease (hazard risk 3.501, 95% confidence interval 1.862–6.581, P < 0.001). Although C282Y mutations also seemed to be associated with inflammatory patterns, the association did not reach statistical significance (P = 0.173).
Conclusions: Low sMg and high ferritin levels are associated with CPP arthritis (CPPA). In patients without hemochromatosis, HFE mutations, and specifically C282Y mutations seem to associate with the polyarticular disease phenotype, and plausibly with the chronic inflammatory phenotype.
Chondrocalcinosis, the presence of calcium pyrophosphate crystals (CPPC) within hyaline or fibrous cartilage, is very prevalent in the elderly population, even as much as 10% in subjects over 60 years [1]. Calcium pyrophosphate arthritis (CPPA), either as acute CPPA or chronic inflammatory CPPA [2], derives from the shedding of CPPCs from cartilage to synovial joints, inducing either acute flares, previously named as pseudogout, or a chronic inflammatory response deriving in progressive destruction of joint structures. The diagnosis of certain CPPA relies on the observation of CPPC in synovial fluid samples [2].
Factors associated with the presence of chondrocalcinosis, and therefore the risk of developing CPPA, include constitutional factors, such as aging [1], diseases inducing disturbances in calcium (Ca), iron (Fe), and magnesium (Mg) [3], and genetic such as polymorphisms of genes, ankylosis protein homolog human gene (ANKH) and hemochromatosis genes (HFE) [4]. Overexpression of the HFE allele C282Y has been associated with chondrocalcinosis [5] and hand osteoarthritis [6] and homozygosis for HFE allele H63D with chondrocalcinosis and osteoarthritis [7].
Most studies relate on the association of these factors to the presence of X-ray chondrocalcinosis or they limit acute pyrophosphate arthritis, retrieved from databases such as the Veteran Administration (VA) data warehouse [3] and The Health Improvement Network (THIN) [8] database. Such databases lack data on disease phenotype regarding the severity of the disease, genetic studies, or complete on associated factors such as Mg, Ca, Fe levels, or a dose of diuretics.
The objective of this study is to evaluate factors associated with the development and severity of CPPA in patients with definite, crystal-proved CPPA.
Transversal, case-control unicentric study in a 3rd level university hospital with a referral population of 400,000, from January 2009 to December 2018. The study was included within a prospective inception gout cohort study approved by the Cruces University Hospital Ethics and Clinical Investigation Committee (CEIC Cruces CEIC07/15). Written, informed consent was obtained and signed by all patients.
Cases were defined by the presence of CPPC within leukocytes in synovial fluid samples in addition to the presence of chondrocalcinosis in conventional X-ray of joints not necessarily restricted to those clinically involved. Controls were defined as the absence of CPPC in synovial fluid and no chondrocalcinosis in conventional X-ray of the affected joint.
Clinical phenotypes were defined according to the European League Against Rheumatism (EULAR) classification [1] into acute pyrophosphate arthritis (unique or intermittent flares without chronic inflammation or joint structural damage), and chronic pyrophosphate inflammatory arthritis (presence of persistent inflammation or stablished joint structural damage not attributable to primary osteoarthritis). Further characterization of phenotype included joint distribution as monoarticular (1 joint ever involved), oligoarticular (2–4 joints ever involved), and polyarticular (> 4 joints ever involved).
Registers with a previous diagnosis of genetic hemochromatosis (C282Y/YY genotype), a family history of hemochromatosis, known Fe overload due to any cause, primary or tertiary hyperparathyroidism were excluded.
Metabolic factors studied included serum levels of Mg, Ca, phosphate, Fe, Fe saturation (SatFe%), ferritin, parathyroid hormone (PTH), 25-hydroxyvitamin D [25(OH)D], and high-sensitivity C-reactive protein. HFE testing included C282Y, H63D and S65C alleles. Hypomagnesemia (HypoMg) was defined as per the reference range to be < 1.70 mg/dL.
The registers of 300 cases and 300 controls were analyzed and compared. Genetic studies were available for 284 cases and 281 controls. Three cases showed a double mutation of HFE allele C282Y, they were considered as genetic type 1 hemochromatosis cases and were not included in genetic analysis. Controls included different diseases, such as gout (n = 128), osteoarthritis (n = 102), and rheumatoid arthritis (n = 70).
The mean time from onset of the first symptom to recruitment was 4.5 years [interquartile range (IQR) 1‒6] and 108 (36%) of the cases were recruited during their first year of clinical manifestations.
Monoarticular involvement was present in 101 (33.6%) patients, whereas oligoarticular and polyarticular involvement was present in 154 (51.4%) and 45 (15.0%) respectively. An only-acute clinical pattern was observed in 146 (48.6%) patients, whereas persistent joint inflammation was retrieved in 154 (51.4%) of the patients.
In cases recruited during the first year of symptoms, monoarticular (76/101, 75%) and only-acute presentation (99/146, 67.8%) were predominant, whereas multiple joint involvement and chronic inflammatory pattern were observed with increasing time from onset (Figure 1).
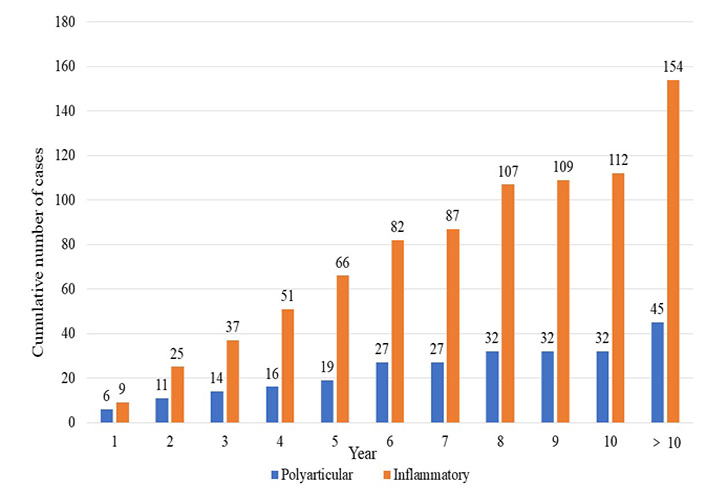
Cumulative number of cases (displayed in the y axis out of 300) developing polyarticular and inflammatory phenotypes from the time of onset of symptoms (displayed in the x axis in years)
Comparisons between cases and controls showed no differences in Ca, phosphate, Fe, SatFe%, PTH, or 25(OH)D. Lower serum Mg (sMg) levels and higher plasma ferritin levels were found in patients compared to controls. HypoMg, any HFE mutation, was also more common in cases than in controls (Table 1).
Comparisons between patients and controls
| Variable | Cases (%) | Controls (%) | P |
|---|---|---|---|
| C-reactive protein (mg/L) | 16 ± 8 | 4 ± 4 | < 0.001 |
| sMg (mg/dL) | 1.99 ± 0.28 | 2.07 ± 0.23 | < 0.001 |
| HypoMg (< 1.70 mg/dL) | 36/294 (12.2) | 19/294 (6.5) | 0.016 |
| Ferritin (mg/dL) | 257 ± 241 | 206 ± 173 | 0.004 |
| Any HFE mutation | 156/281 (55.5) | 103/281 (35.6) | < 0.001 |
| Any C282Y mutation | 35/281 (12.5) | 12/281(4.3) | 0.001 |
| Any H63D mutation | 122/284 (42.9) | 87/281 (30.9) | 0.007 |
| Any S65C mutation | 6/284 (2.1) | 9/281 (3.2) | 0.658 |
The data is represented as means ± standard deviation for continuous variables, and number/total (percentage for discrete variables)
Only mutations in C282Y and H63D alleles were found more frequently in cases than in controls, but no difference in the S65C alleles was observed. The frequency of the different genotypes in cases and controls are displayed in Table 2.
Genotypes for statistically associated HFE alleles C282Y and H63D (%)
| Population | CCHH | CCHD | CCDD | CYHH | CYHD | N |
|---|---|---|---|---|---|---|
| Controls | 189 (67) | 74 (26) | 6 (2) | 5 (2) | 7 (3) | 281 |
| Cases | 135 (47) | 93 (33) | 24 (8) | 18 (6) | 11 (4) | 281 |
| N | 324 | 167 | 30 | 23 | 18 | 562 |
The data is represented as “n (%)” or “n”. Chi-square (χ2) test for distribution with Bonferroni adjustment P < 0.001. CCHH: non mutated; CCHD: heterozygote mutation for H63D; CCDD: homorozygote mutation for H63D; CYHH: heterozygote for C282Y; CYHD: combined heterozygote mutation for C282Y and for H63D
The frequency of polyarticular and inflammatory phenotypes seemed to be progressively overrepresented in cases with HFE mutations, as may be observed in Figure 2.
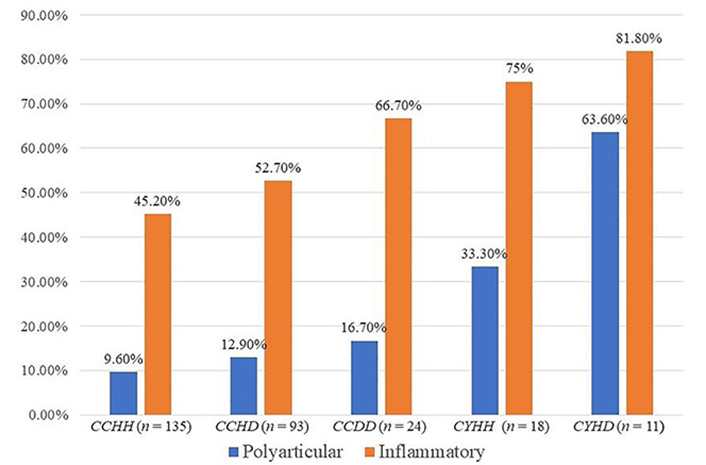
Frequency of polyarticular or chronic inflammatory phenotypes in cases. The “n” in the figure represents the number of cases
As the phenotype in a transversal study for a disease that can progress over time may be influenced by the course of the disease, further analysis was performed. In fact, as mentioned before, clinical phenotypes for polyarticular joint distribution and chronic inflammatory arthritis were more frequent with increasing time from the onset of the disease.
Therefore Kaplan-Meyer analysis was performed, with time from onset as exposure and polyarticular distribution and inflammatory pattern as outcomes. HypoMg, any HFE mutation, or H63D mutations were not associated anymore with either polyarticular or inflammatory phenotypes but C282Y and combined C282Y/H63D mutations were associated with polyarticular joint distribution.
Multivariate Cox proportional hazard analysis showed that the presence of genotypes with C282Y mutations was the only variable associated with polyarticular disease (hazard risk 3.501, 95% confidence interval 1.862–6.581, P < 0.001). Although C282Y mutations also seemed to be associated with the inflammatory pattern, the association did not reach statistical significance (P = 0.173).
CPPA is one of the most common inflammatory arthritis, especially in the elderly, and is caused by the formation of Ca pyrophosphate (CPP) crystals in cartilage structures that may induce both acute (also known as pseudogout) and chronic inflammatory arthritis when shed into synovial lined joints [2]. Disturbances in divalent cations, namely Ca, Mg, and Fe have been associated with chondrocalcinosis, as they influence the activity of pyrophosphatases [9, 10]. Therefore, along with aging, primary hyperparathyroidism, HypoMg, and hemochromatosis are clinically associated with CPPA [2].
Studies of factors associated with CPPA using a database and International Classification of Diseases-9th (ICD-9) coding have found associations between CPPA with HypoMg, hemochromatosis, and primary hyperparathyroidism [3], but also found association with diseases that may show similar clinical features, as osteoarthritis (OA), gout, and rheumatoid arthritis rheumatoid arthritis (RA) [3]. Therefore, diagnosis based on CPPC crystal identification is synovial fluid samples would be gold-standard [2] to avoid misclassification of cases. Other studies restricted to acute CPPA (formerly pseudogout) using the THIN databases only found associations with hyperparathyroidism and the use of loop diuretics, but not with RA, gout, OA, or thiazide diuretics [8]. This is a curious discrepancy, as in a prospective study of 9,820 patients, thiazide diuretics induced lower sMg levels and more frequently HypoMg than loop diuretics [11]. In addition to the risk of misdiagnosis, databases not designed specifically do not retrieve data on clinical phenotype.
To avoid these limitations, this study intended to evaluate factors associated with gold-standard definition of CPPA cases (CPP crystals in synovial fluid plus radiographic chondrocalcinosis) compared to controls with gold-standard controls (absence of both crystals in synovial fluid and chondrocalcinosis). That also allowed to obtain HFE testing, along with serum levels of divalent cations Ca, Mg, Fe, along with SatFe% and ferritin levels. More importantly, and to our knowledge first in literature, to determine clinical phenotype as only-acute CPPA and chronic inflammatory CPPA.
Common shared pathogenic pathways, not related directly to cartilage damage, but more to altered mechanisms in repair of the cartilage damage, have been considered to link hemochromatosis arthritis and CPPA [12].
Mutations of the HFE gene Fe overload and a common cause of hereditary (type 1) hemochromatosis. HFE gene expression induces the production of a protein that regulates the production of hepcidin, a protein that regulates the absorption of Fe by the intestinal cells, and its C282Y and H63D mutations may induce Fe overload [13].
We have found a higher prevalence of any C282Y and H63D HFE mutations in CPPA patients (55%) than in controls (35%). The high prevalence in controls is consistent with that published for newborns in Northern Spain [14] and adults in the Spanish Basque Country, where it reached 29% for mutations of the allele H63D and 13% allele C282Y [15].
The results from this case-controlled study support that both HypoMg and Fe overload, related to HFE gene mutations, are more frequent in patients with CPPD than in controls, supporting that inhibition of pyrophosphatases may be a common mechanism.
We have also observed, and reported for the first time, that in patients with CPPD, there is an apparent relationship between clinical phenotypes of polyarticular distribution and chronic inflammatory arthritis with HFE genotypes.
To avoid the bias of transversal design, where one-third of the patients had early (< 1 year) disease we used survival analysis with clinical phenotypes as outcomes with time from onset as time variable. It showed that only C282Y, but not H63D or combined heterozygous mutations, were associated with increased hazard ratio to develop polyarticular distribution as a surrogate of increased burden of CPP deposits. It may indicate that altered pyrophosphatase activity due to Fe overload may influence the extension of CPP crystal deposition.
Nevertheless, although close to significance, we could not demonstrate that HFE mutations were associated with the chronic inflammatory pattern. To that point, and considering that CPP crystal inflammation is derived from the nucleotide-binding oligomerization domain leucine-rich repeat-containing protein 3 (NRLP3)-caspase-interleukin-1 pathway [16], other factors may be at work that could be elucidated in the future.
There are limitations to the design of the study. Prospective recruitment from first clinical presentation and longitudinal follow-up would have been better, but would take decades. Also, hospital-based recruitment may induce a selection bias to the most severe phenotype.
In conclusion, HFE mutations, and especially C282Y, may increase the risk of CPPA and result in a more severe polyarticular disease. From the clinical point of view, study of associated factors such as Ca and phosphate disorders, HypoMg, and Fe overload should be performed, but HFE testing is of no practical use in the absence of Fe overload.
Ca: calcium
CPP: calcium pyrophosphate
CPPA: calcium pyrophosphate arthritis
CPPC: calcium pyrophosphate crystals
Fe: iron
HFE: hemochromatosis genes
HypoMg: hypomagnesemia
Mg: magnesium
SatFe%: iron saturation
sMg: serum magnesium
JASdB and FPR contributed eaqually to: Conceptualization, Data curation, Writing—review & editing. Nuria PH, Nerea PH, CVP, and MdCMC: Conceptualization, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was included within a prospective inception gout cohort study approved by Cruces University Hospital Ethics and Clinical Investigation Committee (CEIC Cruces CEIC07/15), and it complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Data can be obtained on request by contacting Fernando Perez-Ruiz (fernando.perezruiz@osakidetza.eus).
FPR was funded by a grant from
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
