Affiliation:
1Pediatric Orthopedic Unit, Piccole Figlie Hospital, 43125 Parma, Italy
2Department of Orthopedics, Regional Health Care and Social Agency Papa Giovanni XXIII, 24127 Bergamo, Italy
Email: depellegrin1956@gmail.com
ORCID: https://orcid.org/0000-0002-8376-7420
Affiliation:
1Pediatric Orthopedic Unit, Piccole Figlie Hospital, 43125 Parma, Italy
2Department of Orthopedics, Regional Health Care and Social Agency Papa Giovanni XXIII, 24127 Bergamo, Italy
ORCID: https://orcid.org/0009-0004-1872-3431
Affiliation:
3Orthopedic Residency Program, University of Verona, 37134 Verona, Italy
ORCID: https://orcid.org/0009-0006-3511-1749
Affiliation:
2Department of Orthopedics, Regional Health Care and Social Agency Papa Giovanni XXIII, 24127 Bergamo, Italy
ORCID: https://orcid.org/0000-0001-8237-6738
Explor Musculoskeletal Dis. 2024;2:116–129 DOI: https://doi.org/10.37349/emd.2024.00040
Received: December 09, 2023 Accepted: January 12, 2024 Published: April 10, 2024
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Baby Hip Sonography Worldwide: Experience, Results, and Recommendations
This paper provides a review of the years of experience of hip sonography since the first ultrasound (US) course in Italy in 1987. Clinical and US findings were correlated in 1,000 newborns examined consecutively in a study in 1991. Developmental dysplasia of the hip (DDH) was present even in the absence of clinical signs, including the Ortolani sign. The percentage of US diagnosis of DDH in newborns was 2.8%, while instability according to the Ortolani test was present in 0.75%. After recommendations from the American Academy of Pediatrics against universal US screening, early diagnosis decreased from 74.4% in the period 1992–2002 (43,418 hips examined) to 52.7% in 2013–2014 (5,598 hips examined). In order to answer the question of whether early treatment of DDH has better outcomes, the acetabulum maturation was studied in 93 type III hips. The statistical analysis showed a strong dependency (P < 0.001) between the alpha-angle gain and the age at which treatment was started. The first 2 weeks of life is the optimum time for early diagnosis and treatment; after 6 weeks of life, treatment is less effective and the results are less predictable. Furthermore, the role of the labrum and its morphological changes was analyzed in 86 unstable dysplastic hips (13 type D, 49 type III and 24 type IV) in patients with an average age of 53 days (range 1–134 days) at DDH diagnosis and the beginning of treatment. The labrum was never inverted and underwent a statistically significant increase in echogenicity and dimensions with a frequency of 97% and 96% respectively, suggesting the labrum’s stabilizing role. Abnormal findings such as in achondroplasia, cleidocranial dysplasia, other rare osteochondrodysplasias and in coxa vara are underlined. Uncommon findings such as incomplete acetabular bony rim and eccentric position of the femoral head nucleus are also described.
In the “pre-ultrasound era”, classifying an infant’s hip as normal or dysplastic before the stage of radiological significance, i.e., during the first three months of life, has always been entrusted to functional maneuvers causing clinical signs such as the Ortolani and Barlow tests. From the results of clinical examination, hip morphology was taken to be normal or abnormal. In other words, because insufficient radiographic significance makes it impossible to directly assess the “morphological” criterion, evaluation of the hip was carried out using an indirect “functional” criterion. The use of ultrasound (US) for infant hip evaluation was first described by Graf [1] in 1980. Graf’s technique allowed hip examination to take place in the first weeks of life and eliminates the need for symptomatic evidence of dislocation or developmental dysplasia of the hip (DDH). As a result, the “functional” indirect criterion used for early diagnosis of an infant’s hip could be replaced by a direct “morphological” criterion [2].
The first courses on hip sonography in DDH were held by Graf in Austria (in German) in 1982 and in Germany in 1985. The first US course in Italy was run by Graf himself in 1987 in German with simultaneous translation by the first author (MDP) of this paper. After a second basic course and an advanced course held by Graf in the years that followed, MDP obtained the German certificate for teaching hip US in children, and he started organizing regular basic courses in Italy. Courses were open to pediatricians, radiologists, orthopedic surgeons and obstetricians. Initially, they sparked most interest among pediatricians and neonatologists, which was followed later by interest from pediatric orthopedic surgeons. The basic US hip course followed Graf’s original format with a mixture of theoretical and practical sessions. These practical sessions were always described as fundamental by participants. Until 2023, fifty-four courses following Graf’s method have been held in Italy, mainly in Milan initially, and then later in Rome and in other cities including Trieste, Novara, Bari, Florence, and Naples. The first webinar of the Italian Orthopedic and Traumatology Society was held in 2017 with the topic of “Early clinical and US diagnosis of DDH”. It was attended by 1,052 participants, with 703 of them successfully completing the course by correctly answering the final questionnaire.
Moving on from this historical context, the aim of this review is to summarize the wealth of experience of the US technique for DDH diagnosis in Italy since it was first introduced. The most important studies relating to US screening for DDH, US examination of the hips in osteochondrodysplasias and rare conditions and abnormal and uncommon findings in infant hips are reported.
After introduction of Graf’s US technique for DDH evaluation, the first study performed and published very early in Italy, home country of the pediatrician Marino Ortolani, was a comparison between clinical and US DDH universal screening [3]. Clinical and US findings were correlated in 1,000 newborns examined consecutively. The incidence for females was 1.8% and 0.4% for males. This study found that medical history and clinical signs do not always guarantee a diagnosis of congenital hip dislocation. DDH can be present even in the absence of clinical signs, including the Ortolani sign. The percentage of DDH on US with 57/2,000 hips affected was 2.8% while instability sign according to Ortolani test was present in only 0.75% (15 hips). Although all hips with a positive Ortolani sign were found to be morphologically dysplastic or decentered on US, 39 hips with the same sonographic findings were Ortolani-negative.
Performing a selective neonatal US screening limited to the cases with risk factors (breech presentation, positive family history) has been proven to be inadequate in reducing late diagnosis and surgical treatment. According to an American study, up to 85.3% of patients with symptomatic acetabular dysplasia at skeletal maturity would not have met current recommendations for selective US screening in the USA [4]. Moreover, a significant reduction in the rate of surgery for DDH later in life was observed following introduction of universal US screening in Austria with a significant 46% decrease in the pelvic surgery rate to treat DDH [5]. The results of a universal screening program in Coventry (UK) are also described as impressive with significantly better results than from other UK centers [6]. Many authors highlight the need for a paradigm shift in DDH screening towards a universal US protocol [5, 7–9]. Universal US screening allows DDH to be identified in a number of children whose clinical examination is normal with no risk factors [9].
In one of our studies [10] data relating to clinical and US examinations in a large number of children collected during an 11-year period (1992–2002) at a DDH dedicated outpatient clinic. The data was analyzed in order to verify the importance of US hip examination and Ortolani test for early DDH diagnosis in selecting dysplastic and unstable hips. Of the 21,709 newborns (43,418 hips) examined with US and the Ortolani’s maneuver for DDH diagnosis, 431 patients (356 females, 75 males) had 574 (1.32%) unstable dysplastic hips at a median age at diagnosis of 42 days (range 36–124 days). The hips identified according to Graf’s classification were: 298 type D, 252 type IIIa, 4 type IIIb, 20 type IV. In 73.09% of the patients, no risk factors were identified, 18.56% had a positive family history of DDH, 5.57% had breech presentation and 2.78% had both risk factors. Only 10.63% had a positive Ortolani’s sign. The diagnosis was made in 21.5% of cases by the 2nd week of life, in 52.9% between the 2nd–8th week, and in 25.5% after the 8th week.
Following recommendations from the U.S. Preventive Service Task Force (USPSTF) and the American Academy of Pediatrics (AAP) against universal US screening in 2000 [11] and 2006 [12], we report an alarming decrease to 52.7% in early diagnosis between the third and eighth week of life in our outpatient clinic for DDH. This follows an established period with a high percentage of 74.4% in 1992–2002 (Table 1).
Summary of early diagnosis of DDH in six representative periods
| Category | X-rays examination* (1979) | 1st year of US examination* (1985) | General US screening** (1989) | Selective US screening*** (1990) | US outpatient clinic*** (1992–2002) | US outpatient clinic*** (2013–2014) |
|---|---|---|---|---|---|---|
| Hips | 606 | 2,370 | 2,000 | 2,664 | 43,418 | 5,598 |
| < 2 Weeks of age | 14.5% | 11.8% | 100.0% | 61.1% | 21.5% | 9.7% |
| 3–8 Weeks of age | 14.5% | 35.6% | - | 11.1% | 52.9% | 43.0% |
| > 8 Weeks of age | 70.9% | 52.6% | - | 27.7% | 25.5% | 47.3% |
* Data from Olgahospital Stuttgart, Germany; ** study performed at Orthopedic Department of Tübingen University, Germany; *** study performed at San Raffaele Hospital, Milan, Italy. -: no data
In 2019, a multidisciplinary group of experts (pediatricians, radiologists and pediatric orthopedic surgeons) was established worked together to provide recommendations for early diagnosis of DDH [13, 14]. In 2020, the following interdisciplinary consensus was published, obtained from Italian pediatric, radiology and pediatric orthopedic societies: all newborns, regardless of the presence of risk factors, must be included in a DDH screening program that involves an US examination of the hips between 4 weeks and 6 weeks of life by certified operators [15]. A significant increase in early diagnosis and treatment of DDH could be observed after publication of this statement (Table 2).
Number of hips with type distribution according to Graf, examined during 2019 and 2020
| Hip type | 2019 | 2020 | ||||
|---|---|---|---|---|---|---|
| N | % | Average age (days) | N | % | Average age (days) | |
| Ia, b | 2,488 | 88.83 | 62.2 | 1,886 | 86.28 | 67.2 |
| IIa | 258 | 9.2 | 36.5 | 239 | 10.94 | 40.3 |
| IIb | 14 | 0.5 | 140.2 | 18 | 0.82 | 127.6 |
| IIc | 13 | 0.45 | 53.3 | 16 | 0.73 | 41.3 |
| D | 13 | 0.45 | 49.5 | 14 | 0.64 | 49.7 |
| III | 13 | 0.46 | 38.2 | 11 | 0.5 | 28.2 |
| IV | 3 | 0.11 | 47.6 | 2 | 0.09 | 11 |
| Total | 2,802 | 100 | 61.0 | 2,166 | 100 | 52.2 |
Average age at first US examination and at diagnosis. In 2020 lockdown for COVID pandemic and restrictions caused a decrease of outpatients number (n). COVID: coronavirus disease
In the literature, “overtreatment” was associated with US examination and universal screening [16] and the high rate of reported spontaneous resolution was given as a counter argument to this strategy [12]. However, these authors considered treatment of all hips with abnormal US findings. The majority of abnormal US findings in a universal neonatal screening program are classified as immature hips while true DDH is present in only 1–4% [17]. As shown in Table 2, 9.2% and 10.94% at the age of 36.5 days and 40.3 days in 2019 and 2020, respectively, are classified as immature hips (type IIa) which do not need treatment in the majority of the cases. In fact, since the introduction of the universal US screening program, a decrease of treatment rate is reported [5].
An interdisciplinary approach for DDH detection is important for successful outcomes [14, 18]. Early diagnosis is correlated with early treatment which represents the most effective tool for achieving excellent results in DDH. Acetabular development in severe dysplasia is evidently age-related with treatment starting before the 6th week of life as the gold standard [19–21]. Moreover, effective communication between obstetricians, neonatologists, pediatricians and radiologists is essential for early referral to a pediatric orthopedic surgeon for DDH treatment. Potential risk factors such as a positive family history, breech presentation and female sex must be taken into account by the obstetricians. Swaddling of the hips should be avoided and this recommendation should be included in prenatal information which must be reiterated by neonatologists after delivery [17].
In a retrospective observational study, data was analyzed relating to DDH US screening performed at two pediatric orthopedic centers: an academic teaching hospital and pediatric trauma center (T) and a university hospital and DDH referral center (H). Both these centers were located in Northern Italy, one of the most critically affected areas in Europe during the COVID-19 lockdown [22].
Graf’s method was applied in both centers. Patients already being followed up for DDH or evaluated for a second check were excluded. In total, during the lockdown, 95 patients (190 hips) and 199 patients (398 hips) were screened at centers T and H respectively. In addition, data was evaluated from all patients (n = 1,083) who underwent US at center H throughout 2020.
At center T, the average age at screening was 3.85 months (range 0.1–7.4 months). In total, 10/95 patients had pathological findings or needed a second check (9/190 type IIa, 5/190 type D and 1/190 type IV). Out of 95 patients, 71 (74%) were older than 3 months of age at screening.
At center H, from the start of the first lockdown (10.03.2020) until the end of the year, the following hip types of DDH with a late diagnosis were collected in 28 patients with an average age of 114 days (range 96–146 days): 8 IIb, 5 IIc, 8 D, 11 III. The number and the age distributions of patients who underwent US for the whole of 2020 were compared with the data from 2019. In 2020, US hip examinations decreased by 22% compared to 2019, with 1,083 and 1,401 patients examined, respectively. The difference in age at US calculated between 2019 and 2020 was statistically significant (P < 0.0001). In the same period in 2019, only 8 patients with 11 hips (8 IIb, 1 D, 1 III, 1 IV) and with an average age of 142 days (range 92–305 days) had a late diagnosis. Due to a 22% reduction in examinations in 2020, there is expected to be an increased rate of late DDH diagnosis in the future (Figure 1) [23–27].
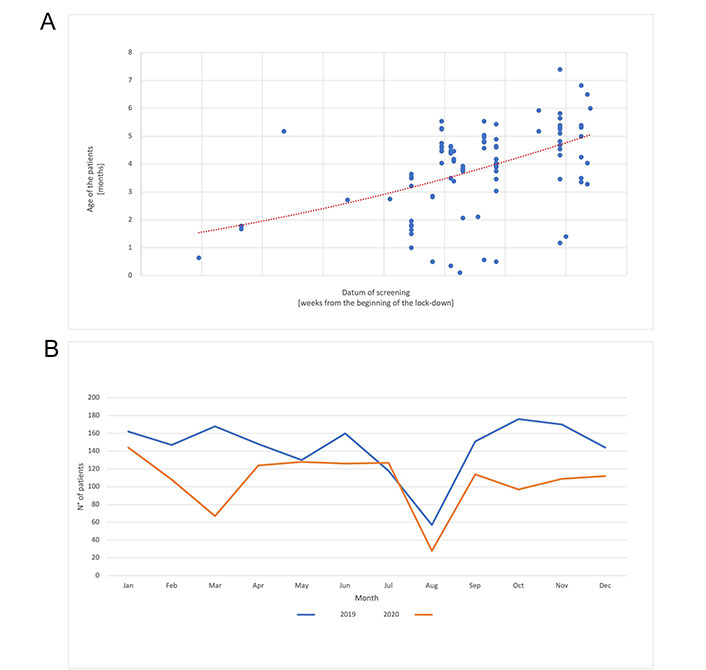
DDH screening timing in 2020 (COVID lockdown) compared to 2019 data. A. The age of each patient (y-axis) at screening is plotted in relationship to the appointment date, displayed as weeks from the beginning of the lock-down (x-axis). The polynomial regression (dotted red line) shows an increase of the patient mean age in relationship to the duration of the lock-down; B. number of US examinations (y-axis) performed every month (x-axis) in 2019 (blue line) and in 2020 (orange line). Monthly decrease of the number of patients screened during pandemia in 2020
The labrum has always been an interesting topic in the discussion of DDH, especially because of its relationship with the femoral head. In the past, the term “labrum” has often been, incorrectly, confused or substituted with “limbus” and “neolimbus”. The neolimbus, described in detail by Ortolani and visible in his anatomical collection, is a “new” structure which appears as a consequence of the forces applied by the dislocated femoral head (Figure 2). Since the 1980s, with the introduction of US for newborn hip evaluation, this anatomical region has been more accurately defined. The US examination distinguishes very clearly the echogenic fibrocartilaginous acetabular labrum from the cartilaginous rim, which is not echogenic since it consists of hyaline cartilage, and from the bony acetabular rim, which is very strongly echogenic. With US examination, the labrum has acquired its own unique identity [28].
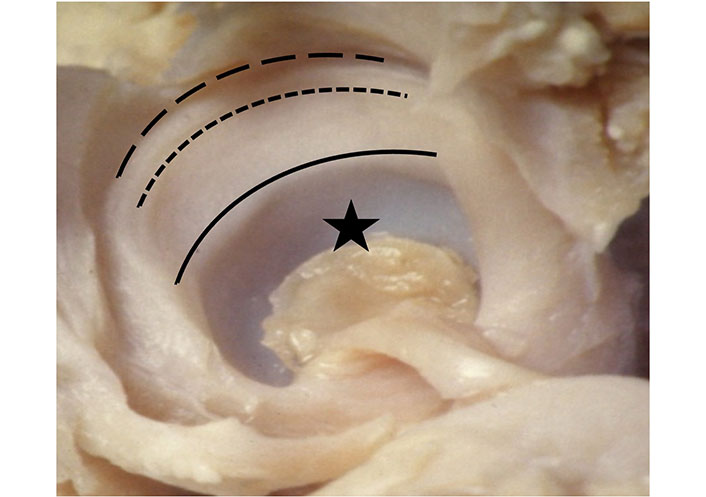
Ortolani specimen. Acetabular socket (star); bony rim (black line); neolimbus cartilage between bony rim and acetabular labrum attachment (dashed line); lateral border of acetabular labrum (dotted line)
Previously, many authors considered the labrum to be an obstacle [29–31] to femoral head reduction. According to other authors, however, more recent clinical experience has shown that the labrum does not represent an obstacle to reduction of the dislocated femoral head [10, 32, 33].
In a study of 20 dislocated hips evaluated with US, we reported that an inverted labrum was never present (it is important to highlight that the average age of the patients was 42 days) [13]. Similarly, in Eberhardt et al.’s [33] arthroscopic study in 2015, the labrum is described as a fibrocartilaginous margin, which does not protrude and never represents an obstacle to reduction.
The role of the labrum was analyzed in a recent study, with particular attention to its morphological changes in unstable dysplastic hips during treatment [32]. In total, 86 unstable, dysplastic hips were classified as type D (n = 13), type III (n = 49) and type IV (n = 24). The labrum was evaluated with US examination for echogenicity and dimensions with inter-/intra-observer tests comparing the US images at diagnosis and at the end of treatment. At the end of treatment of unstable, dysplastic hips, the labrum appeared more echogenic with a frequency of 97% and was larger with a frequency of 96%. The labrum undergoes a statistically significant increase in echogenicity and dimensions after treatment (Figure 3) [34].
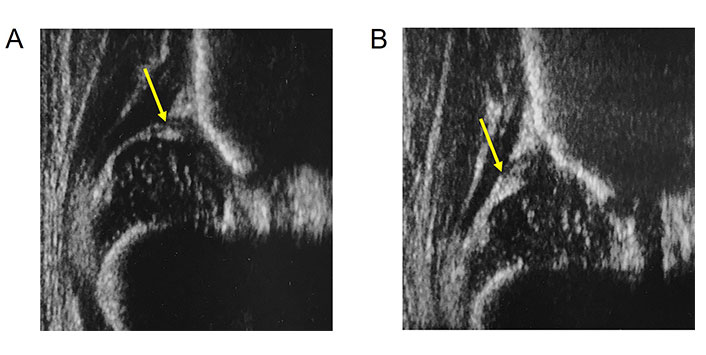
Four days old baby with type IIIa hip. A. Labrum (arrow) above the decentred femoral head; B. same hip 4 weeks later during brace treatment in human hip position. Increased echogenicity and dimension of the labrum (arrow)
The increase in echogenicity of the labrum, which corresponds to an increase in its fibrous component, suggests a stabilizing role, as the centered femoral head is kept in place by a histologically more robust structure during DDH treatment. The new hypothesis is that the labrum plays an active stabilizing role and is not an obstacle to reduction in early treatment as previously reported.
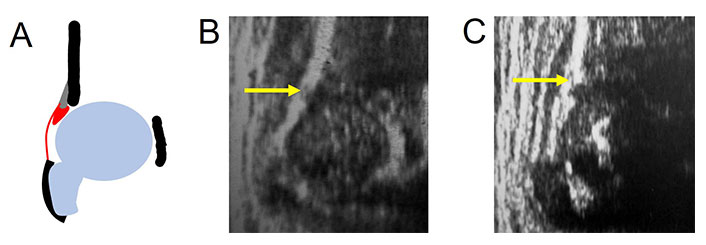
In uncommon anatomical situations, an incomplete acetabular bony rim may be observed on occasion while performing US examination in newborns hips and in DDH treated hips. If present, the shape of the bony rim has a defect in its profile, displaying a “step”, which is clearly distinct from DDH pathoanatomy at onset. This defect is filled with hyaline cartilage appearing anechoic/hypoechoic on the US (Figure 4). According to the anatomy, a correct angle measurement is needed [35].
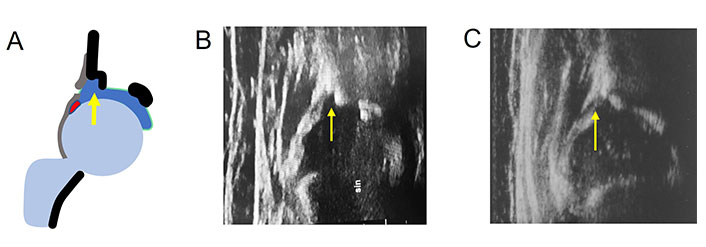
Incomplete acetabular bony rim (arrow). A. Diagram; B. occasionally in a 3 months old child with mature hip; C. after DDH treatment. sin: left hip
Ossification of the proximal femoral epiphysis appears radiographically between the third and twelfth months of life; in rare cases, however, it becomes visible earlier at the sixth week. We reported the presence of the ossific nucleus bilaterally in the first week of life in 3 of the 1,000 newborns studied in a universal DDH screening [3]. Asymmetry in appearance and its persistence during development of the nuclei for 6 months to 12 months is well recognized; moreover, delayed appearance in DDH is also well known [36].
The eccentric development of the nucleus is rarely mentioned in the literature. In as early as 1986, Imhäuser [37] reported the possibility of an eccentric ossification of the cartilaginous femoral epiphysis in hip dislocation, while in babies with normal hips, the osseous nucleus develops in the center of the epiphysis. In hip dislocation, ossification begins in a more lateral and cranial position and sometimes a second nucleus forms and grows together with the first nucleus, or the eccentric nucleus extends in a caudal direction. Both alternatives represent physiological processes and should not be confused with necrosis of the epiphysis. Ignoring the phenomenon of eccentric ossification may lead to false assessment of the femoral head position. The relationship of ossific nucleus eccentricity of the femoral head after congenital hip dislocation with the shape of the cartilaginous femoral head and the prognosis of these findings has been studied by other authors. No statistically significant correlation of eccentric nucleus ossification and cartilaginous femoral head shape has been found [38]. However, an eccentric nucleus in normal hips has never been studied in the literature to date. In our US DDH outpatient clinic activity since 1989 with screening of more than 100,000 babies, we have found 3 cases of eccentric nucleus position in the cartilaginous femoral head in normal hips (Figure 5).
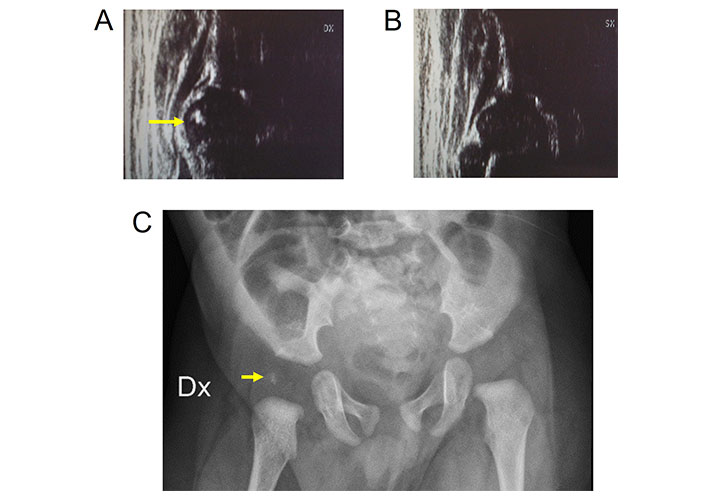
Eccentric ossific nucleus in a 4 months old baby. A. Right hip with eccentric echoic ossific nucleus (arrow) within the anechoic femoral head; B. left hip; C. pelvis X-ray. Radiopaque structure (arrow) with uncertain position in relation to the radiolucent cartilaginous femoral head. Dx: right; SX: left
We reported the results of an US evaluation performed using Graf’s method in 22 children (44 hips) aged between 7 days and 29 months with achondroplasia. All hips had a sharp acetabular bony rim, a horizontal acetabular roof, thickened acetabular cartilage, normal echogenicity and no coxa vara (Figure 6). Due to the absence of the lower limb of the ilium, measurement of the alpha angle was impossible. However, the femoral head was well centered and deeply contained in the acetabular fossa with a mean coverage of 87% (range 78–90%) according to Morin’s method. The mean value of the beta angle was 20° (range 8°–38°). The value of the beta angle tended to decrease as age increased. All hips were stable. An ossific nucleus was present in 5 children. The characteristic findings in hip ultrasonography in children with achondroplasia can aid in its early diagnosis [39, 40].
Achondroplasia. A. Diagram; B. nine days old baby. Hip US showing the sharp bony rim (arrow), the absence of the lower limb of the ilium and the abnormal small beta-angle; C. twenty-one months years old child. Sharp bony rim (arrow), ossific nucleus, femoral head deeply contained in the acetabular fossa
Cleidocranial dysplasia (CCD) is a rare skeletal disorder primarily affecting bones formed by intramembranous ossification. Among the patients who were referred to our DDH dedicated outpatient clinic, we performed US using Graf’s technique on 8 hips (4 patients) with CCD. The patients’ age at examination ranged from 2 weeks to 4.9 months. In all hips, the characteristic finding was a short bony roof due to the thickened cartilage component with absence of the lower limb of the ilium and a rounded bony rim. This finding was substantiated by the pelvis X-ray and diagnosis was later confirmed clinically and genetically.
Early US hip examination can detect a pathognomonic finding of CCD allowing a differential diagnosis between osteochondrodysplasias before clinical and genetic determination (Figure 7) [41].
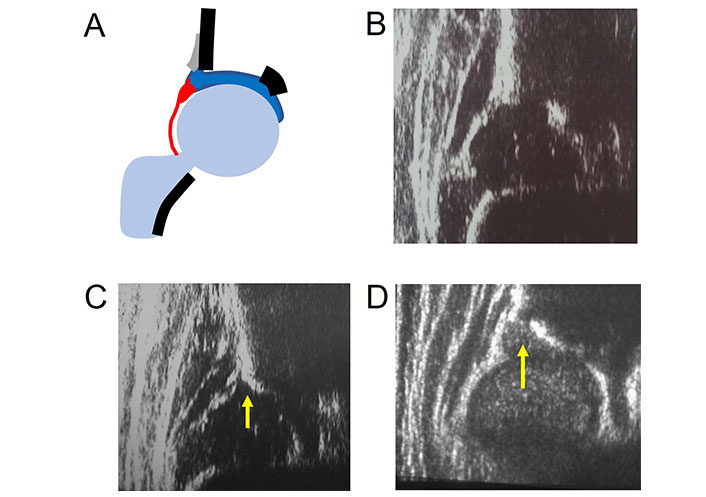
Abnormal bony rim shape in CCD and metatropic dysplasia. A. Normal hip diagram; B. normal hip image; C. CCD with rounded bony rim (arrow) and absence of the lower limb in the fossa acetabuli; D. lateral bony roof deficiency (arrow), notched bony rim with thickened cartilaginous roof in metatropic dysplasia
We described the ultrasonographic findings of the hips of twenty-four children (14 males and 10 females) aged between 9 days and 3 years with the following osteochondrodysplasias: spondyloepiphyseal dysplasia congenital, spondyloepiphyseal dysplasia corner fracture type, spondylo-meta-epiphyseal dysplasia, Kniest dysplasia, metatropic dysplasia, campomelic dysplasia, osteogenesis imperfecta, diastrophic dysplasia, CCD and achondroplasia (Figure 7). Lateral bony roof deficiency with notched or irregular bony rim and thickened cartilaginous roof are often present except in osteogenesis imperfecta. Coxa vara was present in 36% of the hips. A small beta angle (42° on average) is a common finding. Nucleus ossification is delayed with only two cases with the presence of the ossific nucleus described. Due to the pathognomonic findings, CCD and achondroplasia have been described separately above [39].
Coxa vara deformity is a common finding in skeletal dysplasia and frequently occurs also in congenital lower limb malformations such as proximal femoral deficiency (PFD). US is very useful for the detection of coxa vara. The greater trochanter is lateral to the femoral head with soft tissues and muscular septa less vertical than in normal hips. Anechoic greater trochanter appearing like a rounded structure can be confused with the femoral head. This finding can be misinterpreted as femoral head dislocation or hip instability. Struwe et al. [42] described this phenomenon as “the apparent (double) femoral head” in 13 cases (Figure 8). However, US dynamic evaluation can distinguish instability from abnormal hip anatomy and an unnecessary orthopedic treatment can be avoided. We analyzed 9 children with unilateral PFD and 17 children with different osteochondrodysplasias using US. For quantitative US measurement of the deformity in the first year of life, a new measurement technique was introduced (Figure 9). Twenty-five hips with coxa vara were measured showing a delta angle (δ) of 110° (range 96°–122°) and the apex of the greater trochanter stably above the center of the femoral head while normal hips had a delta angle (δ) of 82° (range 82°–88°) and the apex of the greater trochanter always below the center of the femoral head [43].
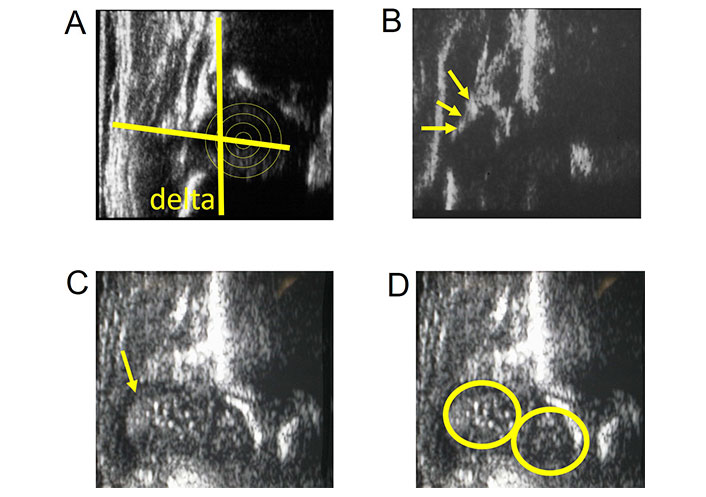
Coxa vara deformity. A. Coxa vara deformity US image with delta angle (δ) of 102° and femoral head center below the greater trochanter apex; B. US of coxa vara with the anechoic greater trochanter lateral to the femoral head (arrows); C. rounded echoic signal of the greater trochanter misintepreted as femoral head (arrow); D. phenomenon of “the apparent (double) femoral head”
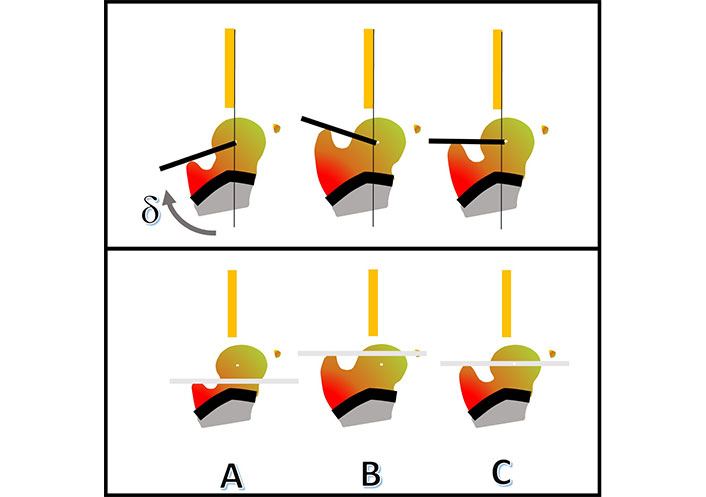
Coxa vara. US measurement with delta angle (δ) and ABC techiques. The δ subtended between the line from the trochanter apex to the center of the femoral head and the base line. The ABC-method for visualizing the position of the femoral head in relationship to the apex trochanter line. A: above; B: below; C: central
In conclusion, after decades of experience on hip sonography, following key points can be underline:
Interdisciplinary approach for DDH is the most important tool for early diagnosis and successful outcomes.
Universal US screening can detect DDH in children without risk factors.
Acetabular development in severe dysplasia is certainly age-related with treatment start before the 6th week of life as gold standard.
Achondroplasia and CCD have pathognomonic findings on hip US.
CCD: cleidocranial dysplasia
COVID: coronavirus disease
DDH: developmental dysplasia of the hip
US: ultrasound
MDP: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. DF, LM, and NG: Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Institute of San Raffaele Hospital, Milan. San Raffaele Hospital is a Scientific Institute and the Center of “Vita-Salute San Raffaele University”.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Hakan Ömeroğlu ... Feza Korkusuz
Beat Dubs
Beat Dubs
Giovanna Galvão Braga Motta ... Alexandre Francisco de Lourenço
Nicholas Birkett ... Claudia Maizen
Tanja Kraus, Catharina Chiari
Konstantinos Chlapoutakis ... Maria Raissaki
Jingnan He ... Xuemin Lyu
