Affiliation:
1Endocrinology and Diabetes, University Hospitals Coventry & Warwickshire, Coventry CV2 2DX, UK
2Warwick Medical School, University of Warwick, Coventry CV4 7AL, UK
ORCID: https://orcid.org/0000-0002-8410-0433
Affiliation:
2Warwick Medical School, University of Warwick, Coventry CV4 7AL, UK
3Department of Rheumatology, University Hospitals Coventry & Warwickshire, Coventry CV2 2DX, UK
Email: Nicola.gullick@uhcw.nhs.uk
ORCID: https://orcid.org/0000-0001-8970-4116
Explor Musculoskeletal Dis. 2024;2:216–234 DOI: https://doi.org/10.37349/emd.2024.00050
Received: November 11, 2023 Accepted: March 28, 2024 Published: June 12, 2024
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Comorbidities in rheumatoid arthritis
Background: We performed a service evaluation of local patients with rheumatoid arthritis (RA) treated with biologic or targeted systemic disease-modifying anti-rheumatic drug (b/ts)DMARDs to see if patients who were obese had different outcomes, and whether referral to specialist obesity services was considered. In addition, we undertook a systematic review of the impact of obesity on treatment outcomes in patients with RA receiving biologics.
Methods: A retrospective case note review was performed for 220 patients with RA attending clinic on treatment with a (b/ts)DMARD. BMI, DAS28, DAS components and demographics were recorded. Referrals to weight management services were evaluated. A systematic review was performed according to PRISMA guidelines (PROSPERO CRD42023433669). Electronic databases were searched for papers reporting RA patients receiving biologics with clinical responses in patients with and without obesity.
Results: Within our service, 24% of patients were obese; 12% were morbidly obese. Patients with obesity had higher disease activity scores. Only 25% of eligible patients were referred to weight management services. 238 records were identified through database searches. 69 full-text records were assessed for eligibility and data extracted from 39 records including 40,445 patients receiving a variety of biologic agents. Reduced responses, remission rates, and drug retention were seen in patients with obesity receiving TNF inhibitors (TNFi), but this was not seen for abatacept, rituximab, or tocilizumab.
Discussion: Obesity is common in patients with RA and can be associated with higher disease activity. Patients who are obese are less likely to reach remission with TNFi. The use of non-TNFi biologics should be considered earlier in the treatment pathway alongside holistic approaches to aid lifestyle change for this patient group.
Obesity is common in patients with rheumatoid arthritis (RA) and can be associated with more severe symptoms and a greater rate of disability [1, 2]. Obesity can lead to an inflammatory state; excess central obesity is associated with elevated systemic cytokines and inflammatory markers [3, 4]. Therefore, it is thought that obesity may exacerbate the development of systemic inflammatory conditions such as inflammatory arthritis. Strong evidence regarding obesity and the severity of psoriatic arthritis is available however the relationship between obesity and RA is complex and poorly understood [5, 6].
In recent years, a plethora of studies have analysed treatment response in obese RA patients based on patient-reported outcomes, disease activity scores [e.g., DAS28 (disease activity score 28 joints)], inflammatory markers [e.g., CRP (C-reactive protein) and ESR (erythrocyte sedimentation rate)], and radiographic progression. Patients with obesity report greater pain, poorer quality of life, higher patient global scores, and higher multi-dimensional health assessment questionnaire scores [7, 8]; possibly due to obesity and its associated central pain sensitisation [9]. Obesity can be a significant confounder in the assessment of clinical activity based on subjective patient-reported outcomes. Patients who are underweight have also been reported to have poorer outcomes, with an increased risk of worsening disability [7]. However, available data is limited, and patients included in these studies also had greater comorbidities and frailty.
Assessment of clinical disease activity using measures such as DAS28 which include measurement of tender and swollen joint count and levels of inflammatory biomarkers can also be overestimated as obese RA patients show more tender and swollen joints and raised inflammatory markers despite lower magnetic resonance imaging scores for synovitis [10–14]. Overall, obesity in RA is associated with higher inflammatory activity, reduced functional capacity and quality of life, but not increased joint damage.
In addition to discrepancies in clinical disease activity measurement, differences in response to treatment also occur. Patients with RA and obesity may exhibit higher disease activity scores irrespective of appropriate treatment escalation. Most medications for RA are not dosed by weight which could potentially result in under-dosing [15]. However, reduced response to treatment is also seen with intravenous infliximab which is dosed by weight [16]. Some studies suggest differences in the pharmacokinetics of disease-modifying antirheumatic drugs (DMARDs) in obese RA patients [17]. Co-existence of other conditions with obesity such as metabolic dysfunction associated with steatotic liver disease, diabetes, hypertension, and cardiovascular disease can also affect treatment decisions including early discontinuation of medications due to increased risks of serious infections and other adverse effects [18–21].
The relationship of obesity in RA and response to treatment with biologics has gained significant attention in the recent years. Poorer responses have been reported following treatment with biologics in patients who are obese but it is unclear if this is true reduced efficacy, or an artefact due to difficulties in disease assessment [22–24]. Therefore, understanding the effect of obesity on the assessment of disease and treatment response is important to aid the selection of appropriate therapies for this patient group to achieve optimal outcomes.
We performed a service evaluation of local patients treated with biologics or targeted synthetic (b/ts)DMARDs to see if patients who were obese had different outcomes, and if patients with more severe obesity were referred to specialist obesity services. In addition, we undertook a systematic review of the impact of obesity on treatment outcomes in patients with RA receiving biologic therapies.
A single centre retrospective analysis of response to treatment in obese RA patients, defined as body mass index (BMI) > 30, who were on (b/ts)DMARDs using DAS28 scores was performed. The project was registered with the Research & Development Department at University Hospitals Coventry & Warwickshire (Ref: SE0353). Ethical approval was not required.
Data were collected retrospectively from 220 RA patients who attended the clinic between May 2021 and August 2021. We included patients > 18 years of age with a confirmed diagnosis of RA who were prescribed a (b/ts)DMARD at the time of assessment. At the time of the evaluation, the following modes of action were available: tumour necrosis factor alpha inhibitors (TNFi), B cell depletion (Rituximab), interleukin-6 inhibitors (tocilizumab and sarilumab), T cell co-stimulation modulator (abatacept), and Janus kinase inhibitors (JAKi). We excluded patients with missing BMI data. We recorded BMI, gender, age, DAS28, patient global, anti-cyclic citrullinated peptide antibody and rheumatoid factor status, smoking and alcohol use, and presence of comorbidities. BMI was calculated as weight in kilograms divided by height in square metres. According to the World Health Organization (WHO) criteria, non-obese BMI was defined as < 30 kg/m2, class 1 obesity 30–35 kg/m2, class 2 obesity 35–40 kg/m2, and class 3 obesity > 40 kg/m2 [25]. Clinical response was assessed using DAS28, ESR, CRP, and visual analogue scale (VAS) scores. At the time of the case note review patients were required to have high disease activity (DAS28 > 5.1) to qualify for advanced treatment. DAS28 < 4 was used as a cut-off for clinical response as per European Alliance of Associations for Rheumatology (EULAR) guidance for patients on (b/ts)DMARDs [26, 27].
Data were expressed as percentages. Statistical analyses were performed using Pearson Chi-squared tests for categorical data and one-way analysis of variance for continuous data to compare groupings by BMI categories using IBM SPSS Statistics version 29. Analyses were considered statistically significant at P < 0.05.
Referrals to weight management services were also evaluated in this population as per local guidelines [28]. These guidelines advise that patients who have a BMI > 40 or those with a BMI between 35 and 40 with related comorbidities such as diabetes, hypertension, or obstructive sleep apnoea should be considered for referral to Tier 3 weight management specialist clinics run by dieticians and endocrinologists.
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. The protocol was published on the International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD42023433669).
The electronic databases MedLine, Embase, CINAHL, ProQuest, and Google Scholar were searched between 17th July 2023 and 21st July 2023, restricted to humans, adults, and publications in the English language. The reference lists of included studies were screened for further potentially eligible studies.
Study selection criteria were defined as follows: adult patients of any ethnicity who had been diagnosed with RA and were receiving biologic therapies or placebo/usual care in the context of either observational studies or randomised controlled trials. Selected outcomes of interest (response to treatment by validated measures, attainment of remission or low disease activity by validated criteria and treatment persistence) were compared between patients with normal BMI and patients who were obese.
Search results were uploaded into Rayyan (Qatar Computing Research Institute, Doha, Qatar) [30] and duplicates were removed. The screening was carried out by two authors independently (ZI and NJG), initially by screening titles and abstracts and secondly by full-text review for final selection. Discrepancies were resolved by consensus.
Data extraction was conducted by two authors using a data extraction form based on a Cochrane template [31]. Data extracted included study characteristics, participant demographics, details of groupings by BMI category, and outcome measures of interest.
Critical appraisal was conducted using the Cochrane risk of bias tool for randomised controlled trials [32] and the risk of bias in non-randomised studies tool for observational studies [33].
A narrative synthesis was performed. Where available, synthesis was sub-grouped by drug class for each outcome of interest. Due to differences in populations, medications, and outcomes used, meta-analysis was not performed.
Records for a total of 220 RA patients receiving (b/ts)DMARDs were reviewed retrospectively. Seventy patients were excluded as no BMI was documented. Patient demographics are shown in Table 1. In accordance with WHO cut-offs in Caucasian populations [25], the number of patients in normal/overweight, and three subclasses of obesity were 97, 23, 15, and 15 respectively. A total of 76% of our RA population were females. Patients in the BMI sub-groups did not differ in smoking status, alcohol consumption, or presence of anti-CCP (cyclic citrullinated protein) antibodies, although there were numerical differences in the presence of rheumatoid factor between groups (Table 1). There were few differences in the treatments used across groups, whether patients received additional conventional DMARDs or rates of comorbidities such as diabetes, hypertension, fibromyalgia, or osteoarthritis. Despite evidence of increased disease activity assessed by DAS28, only 11 patients in the sample of 150 were on long-term corticosteroid treatment.
Patient demographics, treatment, and disease assessment. Categorical variables were analysed using Pearson Chi-squared tests; continuous variables were analysed using one-way analysis of variance. Statistical significance was considered at P < 0.05
| Variable | Normal/overweight BMI < 30 kg/m2(n = 97) | Obese class 1BMI 30–35 kg/m2(n = 23) | Obese class 2BMI 35-40 kg/m2(n = 15) | Obese class 3BMI > 40 kg/m2(n = 15) | P |
|---|---|---|---|---|---|
| Mean age (SD) | 61 (13) | 61 (12) | 59 (10) | 58 (7) | NS |
| Female, n (%) | 74 (76) | 21 (91) | 12 (80) | 13 (87) | |
| Smoking status, n (%) | |||||
| Never | 38 (39) | 16 (70) | 8 (53) | 5 (33) | NS |
| Current | 22 (23) | 1 (4) | 1 (7) | 3 (20) | |
| Former | 23 (24) | 3 (13) | 5 (33) | 4 (27) | |
| Unknown | 14 (14) | 3 (13) | 1 (7) | 3 (20) | |
| Alcohol use, n (%) | |||||
| Never | 27 (28) | 6 (26) | 7 (47) | 5 (33.3) | NS |
| Current | 48 (49) | 13 (57) | 7 (47) | 5 (33.3) | |
| Unknown | 22 (23) | 4 (17) | 1 (7) | 5 (33.3) | |
| RF, n (%) | |||||
| Positive | 66 (68) | 11 (48) | 11 (73) | 8 (53) | < 0.001 |
| Negative | 19 (20) | 11 (48) | 4 (27) | 7 (47) | |
| Unknown | 12 (12) | 1 (4) | 0 | 0 | |
| Anti-CCP, n (%) | |||||
| Positive | 53 (55) | 12 (52) | 7 (47) | 10 (67) | NS |
| Negative | 25 (26) | 9 (39) | 7 (47) | 3 (20) | |
| Unknown | 19 (20) | 2 (9) | 1 (7) | 2 (13) | |
| DAS28, Mean (SD) | 3.5 (1.6) | 2.7 (1.1) | 4.5 (1.4) | 4.9 (1.4) | < 0.001 |
| DAS28 remission, n (%) | 33/94 (35) | 7/15 (47) | 1/13 (8) | 0/12 (0) | 0.011 |
| ESR, mean (SD) | 17 (16) | 10 (6) | 30 (24) | 30 (29) | < 0.001 |
| CRP, mean (SD) | 11 (11) | 5 (4) | 13 (13) | 11 (7) | 0.008 |
| Global VAS, mean (SD) | 46 (24) | 42 (24) | 58 (15) | 61 (17) | NS |
| (b/ts)DMARD n (%) | |||||
| JAKi | 28 (29) | 3 (13) | 8 (53) | 4 (27) | NS |
| TNFi | 33 (34) | 9 (39) | 1 (7) | 6 (40) | |
| RTX | 20 (21) | 6 (26) | 3 (20) | 4 (27) | |
| IL6i | 5 (5) | 3 (13) | 1 (7) | 0 | |
| ABA | 5 (5) | 2 (9) | 2 (20) | 0 | |
| Treatment pause | 6 (6) | 0 | 0 | 1 (7) | |
| Plus csDMARD n (%) | |||||
| Yes | 31 (32) | 18 (78) | 9 (60) | 12 (80) | NS |
| Previous (b/ts)DMARD n (%) | |||||
| 0 | 57 (59) | 8 (35) | 9 (60) | 7 (47) | NS |
| 1 | 22 (23) | 8 (35) | 5 (33) | 5 (33) | |
| ≥ 2 | 18 (19) | 7 (30) | 1 (7) | 3 (20) | |
| Comorbidities, n (%) | |||||
| Type 2 diabetes | 9 (9) | 4 (17) | 5 (33) | 3 (20) | NS |
| Fibromyalgia | 6 (6) | 0 | 1 (7) | 1 (7) | |
| Hypertension | 11 (11) | 2 (9) | 4 (27) | 4 (27) | |
| Osteoarthritis | 21 (22) | 2 (9) | 3 (20) | 3 (20) | |
ABA: abatacept; BMI: body mass index; (b/ts)DMARD: biologic/targeted systemic disease-modifying anti-rheumatic drug; csDMARD: conventional synthetic disease-modifying anti-rheumatic drug; CCP: cyclic citrullinated peptide; IL-6i: interleukin-6 inhibitor; JAKi Janus kinase inhibitors; MOA: mechanism of action; NS: non-significant; RF: rheumatoid factor; RTX: rituximab; SD: standard deviation; TNFi: tumour necrosis factor inhibitor; DAS28: disease activity score 28 joints; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; VAS: visual analogue scale
Mean DAS28 was lower in patients with lower BMI (non-obese and class 1 obesity) with higher DAS28 observed in patients with BMI > 35. In addition, patient global scores, ESR, and CRP were significantly higher in patients with BMI > 35. Data are shown in Table 1 and Figure 1. We also observed very low rates of DAS28 remission in patients with a BMI of 35–40 (1 patient, 8%) with no patients in DAS28 remission with a BMI > 40. Conversely, in patients who were not obese, we found DAS28 remission in 35% of patients, with 47% of patients with class 1 obesity in DAS28 remission.
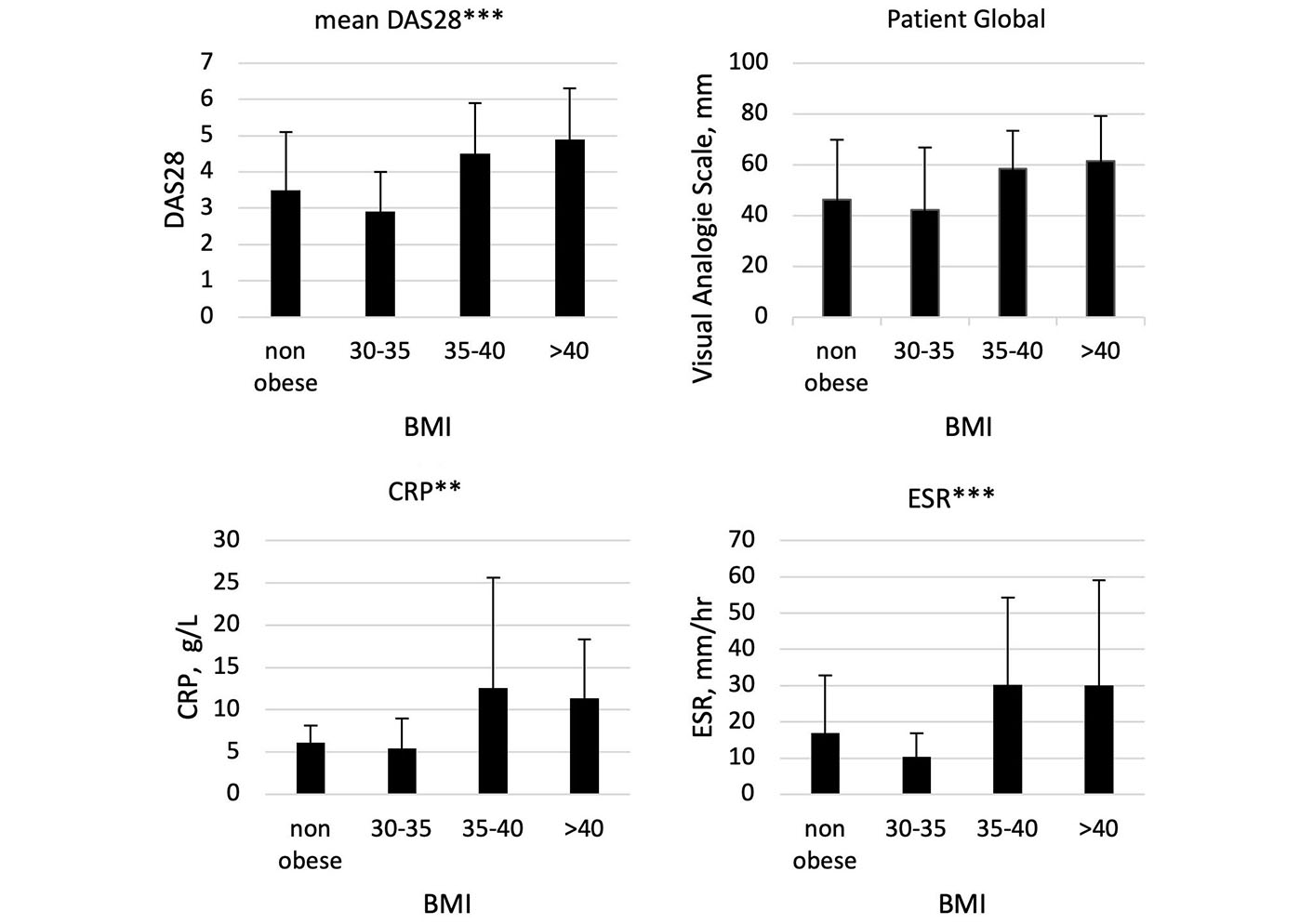
Comparison of DAS28, patient global VAS, ESR, and CRP across BMI groupings: non-obese, class 1 obesity (BMI 30–35), class 2 obesity (35–40) and class 3 obesity (BMI > 40) in patients receiving biologic or targeted systemic DMARDs. Graphs demonstrate mean values with standard deviation. *** significant at < 0.001; ** significant at < 0.01. BMI: body mass index; CRP: C-reactive protein; ESR erythrocyte sedimentation rate; VAS: visual analogue score; DAS28: disease activity score 28 joints; DMARDs: disease-modifying anti-rheumatic drugs
We also evaluated rates of comorbidity in each obesity subgroup. A wide range of comorbidities were reported (Table 1). The most common comorbidities were osteoarthritis, type 2 diabetes, and hypertension. Although there were proportionally higher numbers of patients with type 2 diabetes in all 3 obesity categories, this was not statistically significant. Fibromyalgia was reported only in a small proportion of patients (up to 7% per group).
We found that only 25% of patients who reached the criteria for referral to specialist weight management clinics and/or dieticians were referred. Following the Covid pandemic, waiting times for this service have increased considerably. We liaised with our specialist weight management team and produced an information leaflet to signpost patients from rheumatology clinics to weight management strategies available in the local community while waiting for specialist support.
235 records were identified through database searches, and one duplicate was excluded. After title and abstract screening, 69 full-text records were assessed for eligibility and 39 records were selected for data extraction. References from selected papers identified three additional reports for review and extraction. The PRISMA flow diagram is shown in Figure 2.
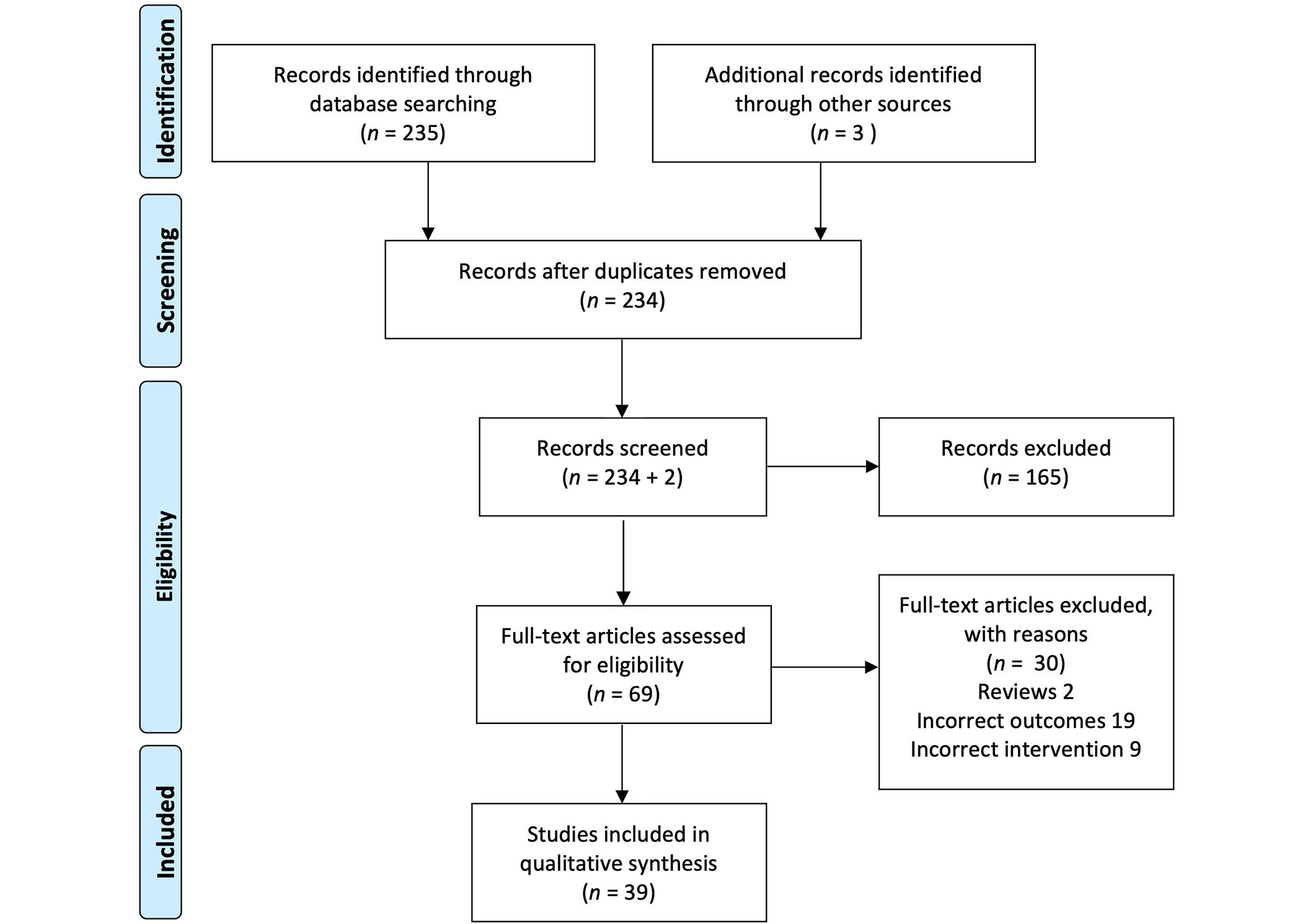
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram
Most studies were reports from observational studies and registries, with some reports describing post hoc analyses of randomised controlled trials. Additionally, there were seven studies reporting results from retrospective cohorts. Data were available for TNFi, as well as other modes of action including co-stimulation blockade (abatacept), interleukin-6 inhibitors (IL-6i, tocilizumab), and B cell depletion (rituximab). In total, 40,445 patients were included. Most studies recruited patients with poor response to DMARDs starting their first biologic (usually TNFi) with high rates of seropositivity for either rheumatoid factor or anti-CCP and high disease activity at treatment initiation. A small number of patients were DMARD naive. Data from Mariette et al. [34] was excluded to avoid double counting as this was a single-country sub-analysis of the larger AbataCepT In rOutiNe clinical practice observational cohort [35].
For most studies, obesity was defined using standard WHO cut-offs [25], however, some reports in non-Caucasian populations used lower BMI cut-offs, for example, Kim et al. [36], Hirai et al. [37], and Inanc et al. [38] defined obesity as BMI greater than 25. Huang et al. [39] allocated anyone with a BMI greater than 23 in a combined overweight and obese category. Overall, the risk of bias was low in most studies with attempts made to correct for confounding factors. However, some studies only recruited small numbers of patients from a single centre and results may be less precise.
Response to biologic treatment was assessed using initial response using validated response measures e.g., EULAR good/moderate response [26, 27], and achievement of validated measure of remission or low disease activity e.g., DAS28 remission [40], clinical disease activity index (CDAI) remission [41], or simplified disease activity index (SDAI) remission [42]. Results are shown in Table 2. Twelve reports examined response to TNFi, five to abatacept, one to rituximab, seven to tocilizumab, and the remainder investigated response to multiple bDMARDs.
Influence of obesity on response to biologic treatment
| Author, year | Study design, duration | Biologic | Total (n) | Obese (n) | Bias & risk of imprecision | Response to treatment/Achievement of remission |
|---|---|---|---|---|---|---|
| TNFi | ||||||
| Baganz et al. [43], 2019 | Observational, 3 years | TNFi not specified | 388 | 92 | Low | No influence on achieving remission within 6 months of starting first TNFi OR 0.67 (95% CI 0.45–1.70). |
| Baker et al. [44], 2011 | RCT, 52 weeks | GLM | 499 | 127 | Low | Higher BMI is independently associated with less joint damage progression. |
| Bykerk et al. [45], 2021 | Pooled RCT data, variable duration | CTZ | 8,747 | 1,180 | Low | Increased risk of serious infections and major cardiovascular events, if BMI > 35. |
| George et al. [13], 2017 | Pooled data from 2 RCT, 6 months | GLM | 470 | 103 | Low | Lower rates of DAS28 remission in obese patients 17% vs. normal weight 28%. OR remission 0.47 (95% CI 0.24–0.92). |
| Gremese et al. [46], 2013 | Registry, 12 months | ETN, IFX, ADA | 575 | 66 | Low | Lower rates of DAS28 remission in obese patients 15.2% vs. non-obese 32%, OR not remission 2.63 (95% CI 1.31–5.26). |
| Hamann et al. [47], 2019 | Observational | CTZ, ETN, IFX, ADA | 14,436 | NR | Low | Increasing BMI associated with reduced likelihood of sustained remission, OR 0.98 (95% CI 0.97, 0.99) per kg/m2 increase. |
| Klaasen et al. [15], 2011 | Prospective cohort, 16 weeks | IFX | 89 | 15 | Low, risk of imprecision | BMI higher in EULAR non-responders vs. responders. |
| Law-Wan et al. [48], 2021 | Pooled analysis of 29 RCTs | ADA, ETN, CTZ, GLM, IFX | 14,838 | NR | Low | Higher rates of EULAR non-response rate were observed in obese patients, OR 0.52 (95% CI 0.43, 0.63) vs. 0.36 (95% CI 0.30, 0.45) for non-obese. |
| Levitsky et al. [24], 2017 | Sub-analysis of RCT, 24 months, treatment-naive | DMARDs or TNFi | 403 | 43 | Low | Non-obese patients were more likely to reach remission. Non obese OR 4.6 (95% CI 2.0–10.5) vs. obese OR 3.3 (95% CI 1.4, 8.2). Obesity independent predictor of non-remission at 24 months (adjusted OR 5.2; 95% CI 1.8 to 15.2). |
| Ottaviani et al. [16], 2015 | Retrospective cohort, 6 months | IFX | 76 | 22 | Low, risk of imprecision | BMI was significantly lower in patients with EULAR good response, adjusted multivariable analysis OR 0.87 (95% CI 0.76, 0.99), no significant difference for remission. |
| Reams et al. [49], 2020 | Retrospective cohort | 2nd TNFi | 322 | 133 | Low | Similar response rates to 2nd TNFi across BMI categories. |
| Sapundzhieva et al. [50], 2019 | Observational, 6 months | IFX | 30 | 19 | Low, risk of imprecision | Higher DAS28 at 6 months in obese vs. normal BMI 3.89 ± 1.18 vs. 2.50 ± 0.62. Higher rates of DAS28 remission in normal weight vs. overweight or obese 60% vs. 33% vs. 0%. |
| Other modes of action | ||||||
| D’Agostino et al. [51], 2017 | Post hoc analysis of RCT, 6 months | ABA | 1,457 | 433 | Low | No impact of obesity on DAS28, SDAI, or CDAI remission. Lower fall in CRP in the obese group. No difference in remission rates between IV or SC routes. |
| Di Carlo et al. [52], 2019 | Post hoc analysis of prospective cohort, 6 months | ABA | 130 | NR | Low, small numbers, risk of imprecision | No difference in mean BMI between responders and non-responders (DAS28-ESR remission and/or Boolean remission). |
| Gardette et al. [53], 2016 | Retrospective6 months | ABA | 141 | 39 | Low | No difference in mean BMI between EULAR responders or those achieving remission. |
| Iannone et al. [54], 2017 | Pooled analysis of European registries, variable duration | ABA | 2,015 | 380 | Low | Moderate or good EULAR response rates at 6 months are similar across. BMI categories 39.8% normal BMI, and 40.0% obese. |
| Mariette et al. [35], 2017 | Observational, 6 months analysis | ABA | 672 | 155 | Low | No significant difference in EULAR response at 6 months with the obese BMI subgroup. |
| Ottaviani et al. [55], 2015 | Retrospective cohort, 6 months | RTX | 114 | 35 | Low | No association between BMI and response to RTX in adjusted multivariable analysis. |
| Abuhelwa et al. [56], 2020 | Pooled data of several RCT | TCZ | 5,502 | 1,654 | Low | Obesity associated with less frequent remission by SDAI HR 0.80 (95% CI 0.70–0.92) and CDAI HR 0.77 (95% CI 0.68–0.87). |
| Arad and Elkayam [57], 2019 | Open-label, 24 weeks | TCZ | 100 | 30 | Low, risk of imprecision | Inverse association between change in CDAI and BMI between weeks 1 and 12. No association between BMI and achieving remission or LDA at 24 weeks. |
| Gardette et al. [58], 2016 | Retrospective, 6 months | TCZ | 115 | 25 | Low, risk of imprecision | No influence of BMI on EULAR moderate or good response or remission. |
| Huang et al. [39], 2019 | Prospective cohort | TCZ | 52 | 6 | Low, high risk of imprecision | No difference in mean BMI between DAS28 responders and non-responders or CDAI LDA or remission or DAS28 LDA or remission. |
| Inanc et al. [38], 2023 | Retrospective cohort | TCZ | 124 | 38 | Low | No difference in response between obese and non-obese. |
| Pappas et al. [59], 2020 | Registry, 6 months | TCZ | 805 | 356 | Low | No difference in mean change in CDAI between obese/non-obese. |
| Pers et al. [60], 2015 | Retrospective cohort, 6 months | TCZ | 222 | 32 | Low | No effect of BMI on EULAR response or remission. |
| Mixed bDMARDs | ||||||
| Baker et al. [61], 2022 | Register, response analysed after 3 months | TNFi, non-TNF biologic | 5,901 | 1,299 | Low | Reduced MCID response in obese patients. OR 0.88 (95% CI 0.72, 1.08) TNFi. OR 0.82 (95% CI 0.67, 1.01) non TNFi bDMARD. Less likely to achieve CDAI LDA if obese OR 0.85 (95% 0.74, 0.99). |
| Hirai et al. [37], 2020 | Retrospective notes review | IFX, TCZ, and ABA | 324 | 33 | Low | BMI ≥ 25 is associated with a lack of efficacy. OR 4.22 (95% CI 1.69–10.5). |
| Iannone et al. [62], 2015 | Retrospective review, up to 11 years | CTZ, ETN, IFX, GLM, ABA, RTX | 292 | 66 | Low | 1st TNFi:Lower rates of good EULAR response in obese vs. normal 42% vs. 68%; Lower rates of DAS28 remission in obese vs. normal 17% vs. 38%.2nd TNFi:Lower rates of good EULAR response in obese vs. normal 33% vs. 57%; Lower rates of DAS28 remission in obese vs. normal 12.5% vs. 46%.RTX:Lower rates of good EULAR response in obese vs. normal 27% vs. 67%; Lower rates of DAS28 remission in obese vs. normal 7% vs. 33%. |
| Kearsley-Fleet et al. [63], 2018 | Registry, 20 years | TNFi, non TNFi bDMARD | 13,502 | NR | Low | bDMARD refractory disease independently associated with obesity, HR 1.2 (95% CI 1.0–1.4). |
| Kim et al. [36], 2016 | Observational, 24 weeks | ABA, TCZ, ETN, ADA | 68 | 13 | Low, risk of imprecision | No relationship between BMI with EULAR response or DAS28 remission. |
| Novella-Navarro et al. [64], 2022 | Prospective cohort, 6 months | TCZ, TNFi | 105 | NR | Low, risk of imprecision | The higher mean BMI in patients who did not attain CDAI LDA or remission was 28.7 ± 5.1 vs. 24.5 ± 4.6 with TNFi. No difference in mean BMI between responders and non-responders to TCZ. |
| Schafer et al. [65], 2020 | Observational, 10.3 years | ADA, TCZ, ETN, ABA, CTZ, GLM, RTX | 10,593 | 2,910 | Low | Less likely to attain EULAR response or remission with TNFi in females only. Good response 0.83 (95% CI 0.72, 0.95). Remission RR 0.73 (95% CI 0.61, 0.88). No relationship seen in men, or for ABA, RTX, and TCZ. |
| Vallejo-Yagüe et al. [66], 2021 | Registry | TNFi, non TNF bDMARD, tsDMARD | 3,217 | 546 | Low | Higher DAS28 in obese patients. |
ABA: abatacept; ADA: adalimumab; bDMARD: biologic disease-modifying antirheumatic drug; BMI: body mass index; CDAI: clinical disease activity index; CI: confidence interval; CTZ: certolizumab; DAS28: disease activity score 28 joints; ETN: etanercept; GLM: golimumab; HR: hazard ratio; IFX: infliximab; LDA: low disease activity; NR: not reported; OR: odds ratio; RR: risk ratio; RTX: rituximab; SDAI: simplified disease activity index; TCZ: tocilizumab; TNFi: tumour necrosis factor alpha inhibitor; tsDMARD: targeted systemic disease-modifying antirheumatic drug; RCT: randomised controlled trial; EULAR: European Alliance of Associations for Rheumatology; IV: intravenous; SC: subcutaneous; ESR: erythrocyte sedimentation rate; MCID: minimal clinically important difference
The association between response to treatment and increased BMI or obesity varied across the studies. Some studies did not report separate data for BMI groups but noted an association with poorer response due to higher BMI or obesity on multivariable regression analysis [43, 47, 63]. For TNFi, most studies demonstrated poorer responses in patients with obesity either for EULAR response or achievement of remission using one of the validated measures. Four studies demonstrated reduced rates of remission in patients who were obese [13, 24, 46, 50] (two observational studies, one registry, and one study pooling data from two randomised controlled trials of certolizumab) with rates of remission (predominantly DAS28 remission) in obese patients as low as 0% in one study [50]. Hamann et al. [47] noted a small reduction in the likelihood of sustained remission [odds ratio (OR) 0.98, 95% confidence interval (CI) 0.97 to 0.99] for each increase in BMI of 1.0 kg/m2 but did not report rates of remission in obese vs. non-obese patients. Baganz et al. [43] reported an OR of 0.67 for achieving remission within 6 months of starting the first TNFi, but confidence intervals were wide (95% CI 0.45–1.70) and the result was non-significant. A further small Italian study did not find a significant difference in BMI in patients who achieved remission compared to those who did not achieve remission [16]. Reams et al. [49] reported similar response rates to a second TNFi across BMI categories.
Similar relationships were seen for the achievement of EULAR response: Klaasen et al. [15] noted a higher BMI in patients who did not achieve an EULAR response after 16 weeks of infliximab therapy, and Ottaviani et al. [16] noted a lower mean BMI in patients achieving a EULAR good response. Law-Wan et al. [48] found higher rates of EULAR nonresponse in patients with obesity. Two studies did not report either remission or reduction in disease activity but noted other important outcomes. Baker et al. [44] reported an independent association between higher BMI and lower radiographic progression, while Bykerk et al. [45] reported an increased risk of serious infections or major cardiovascular events in patients with BMI > 35.
For other modes of action bDMARDs, only one study reported data for rituximab, which did not show any association between BMI and response at 6 months in an adjusted multivariable analysis [55].
Five studies investigated the impact of obesity on response to abatacept treatment. Di Carlo et al. [52] did not find a significant difference in BMI between patients achieving DAS28-ESR remission and/or Boolean remission, and there was no difference in mean BMI between EULAR responders or those achieving remission in a retrospective cohort [53]. A post hoc analysis of randomised controlled trial data did not demonstrate any impact of obesity on DAS28, SDAI, or CDAI-defined remission. However, a lower fall in CRP was reported in the obese patient group [51]. This study reported data from patients treated with either subcutaneous (not weight-based dosing) or intravenous abatacept (weight-adjusted dosing) and did not find a difference in remission rates between routes of administration.
Seven studies investigated responses to tocilizumab in patients with RA. One study analysed pooled data from 5,502 patients enrolled in several randomised controlled trials [56]. Obesity was associated with less frequent remission defined by SDAI and CDAI (hazard ratios, HR of 0.80 and 0.77 respectively). The remaining six studies did not show an influence of BMI on EULAR responses, mean change in CDAI, or achievement of remission [38, 39, 57–60]. One study noted lower tocilizumab drug levels in patients with obesity, but this did not result in a poorer clinical response than in non-obese patients [57].
Eight studies studied clinical responses to more than one mode of biological action. Baker et al. [61] included 5,901 patients in a national register; there was a non-significant reduction in the achievement of the minimal clinically important difference in patients with obesity, although obese patients were less likely to achieve low disease activity by the CDAI. A second study noted a BMI of over 25 was associated with a lack of efficacy (OR 4.2; 95% CI 1.69–10.5) [36].
A retrospective review of 292 patients with up to 11 years of follow-up found lower rates of good EULAR responses and DAS28 remission in obese patients after treatment with the 1st TNFi, 2nd TNFi, or rituximab compared to patients without obesity [62].
Analysis from a second national register involving 13,502 patients found an independent association between obesity and biologic refractory disease (HR 1.2), although confidence intervals included 1.0 and this result is imprecise [63].
Kim et al. [36] reported no relationship between BMI and EULAR response or achievement of remission in patients treated with adalimumab, etanercept, abatacept, or tocilizumab at 24 weeks.
A further small prospective cohort, with follow-up to six months found a higher BMI in patients who did not achieve CDAI low disease activity or remission only in TNFi-treated patients, with no relationship seen in patients treated with tocilizumab [64].
The largest study, involving 10,593 patients reported that female patients with obesity were less likely to achieve a EULAR good response or remission when treated with TNFi [65]. This relationship was not seen in men, or in patients treated with tocilizumab, rituximab or abatacept. Finally, data from the Swiss registry noted a higher mean DAS28 in patients with obesity [66].
Although short-term effectiveness data can be helpful in predicting response to treatment, successful therapy in a chronic condition such as RA needs to be effective in the longer term. Drug retention was reported by 8 studies, summarised in Table 3. Three studies reported data for TNFi alone and had conflicting results. A large international register with follow-up centred at 5,000 days showed that both underweight patients and patients with a BMI over 35 were more likely to discontinue treatment with TNFi [67]. A smaller registry study involving 521 patients found that BMI was inversely associated with drug survival and those with a BMI more than 40 were over twice as likely to discontinue treatment compared with patients who have normal weight [68]. Iannone et al. [62] reported data from patients receiving bDMARDs with more than one mode of action and found lower drug survival only in obese patients receiving their 2nd TNFi. Conversely, McCulley et al. [18] found the opposite, with a small increase in the likelihood of treatment discontinuation in patients with a normal BMI compared to those who were overweight. Rashid et al. [69] also reported outcomes for patients receiving any bDMARD and found that obesity was a predictor of the need to switch treatment (OR 1.51).
Impact of obesity on drug retention
| Author, year | Study design, duration | Biologic | Total (n) | Obese (n) | Retention rates |
|---|---|---|---|---|---|
| TNFi | |||||
| Bergstra et al. [67], 2020 | International register, follow up censored at 5000 days | Any TNFi | 5,232 (4,116 BMI data) | 734 | Underweight and overweight patients discontinued TNFi treatment earlier than normal-weight patients. Adjusted(Adj) HR underweight: 1.3 (95% CI 1.07, 1.58); Adj HR BMI 35–39.9: 1.28 (95% CI 1.06, 1.54); Adj HR BMI > 40: 1.67 (95% CI 1.29, 2.18). |
| Elalouf et al. [68], 2021 | Registry, maximum follow-up 5 years | ETN, IFX, ADA, GLM | 521 | 223 | BMI is inversely associated with drug survival. BMI > 40 is more likely to discontinue treatment compared with normal weight patients HR 2.28 (95% CI 1.67–3.10). |
| McCulley et al. [18] 2019 | Retrospective cohort | TNFi | 46,970 | 19,216 | Patients with normal BMI are more likely to discontinue treatment in fully adjusted model HR 1.14 (95% CI 1.07, 1.22) vs. overweight. |
| Other modes of action | |||||
| Alten et al. [70], 2017 | Observational bio-naive biologic failure, 12-month interim analysis | ABA | 674 (biologic naive); 1676 (biologic failure) | 155 (biologic naive); 405 (biologic failure) | No significant impact of BMI regardless of antibody status, or biological naive/exposed. |
| Iannone et al. [54], 2017 | Pooled analysis of European registries, variable duration | ABA | 2,015 | 380 | No impact of obesity on drug retention; HR 1.08 (95% CI 0.89–1.30). |
| Hilliquin et al. [71], 2021 | Prospective cohort, 12 months | TCZ | 291 | 57 | No difference in retention rates at 12 months in non-obese vs. obese 65.32% vs. 58.85%. |
| Mixed biologics | |||||
| Iannone et al. [62], 2015 | Retrospective review, up to 11 years | CTZ, ETN, IFX, GLM, ABA, RTX | 292 | 66 | 1st TNFi: no significant difference in drug survival between obese/non-obese;2nd TNFi: drug survival is lower in obese, patients vs. normal 43.5% vs. 80%. |
| Rashid et al. [69], 2016 | Retrospective cohort, 6 years | Any bDMARD | 2,171 | 799 | Obesity predictor of need to switch bDMARD; OR 1.51 (95% CI 1.04–2.19). |
ABA: abatacept; ADA: adalimumab; Adj; adjusted; bDMARD: biologic disease modifying antirheumatic drug; BMI: body mass index; CI: confidence interval; CTZ: certolizumab; ETN: etanercept; GLM: golimumab; HR: hazard ratio; IFX: infliximab; OR: odds ratio; RR: risk ratio; RTX: rituximab; SDAI: simplified disease activity index; TCZ: tocilizumab; TNFi: tumour necrosis factor-alpha inhibitor
Studies investigating drug retention in patients receiving treatment with abatacept and tocilizumab did not find any significant impact of obesity on drug retention [54, 70, 71]. No data on drug retention were available for patients receiving rituximab.
Obesity is rising in both the general and RA populations. One study recruiting patients worldwide found 18% of patients were obese [72] although higher rates are seen in the United Kingdom [73]. Obesity is an increasingly common comorbid condition in RA and although less likely to be associated with progressive joint damage [12], patients who are obese often report higher levels of pain with increased tender joint counts compared to patients without obesity [8, 10]
Almost a quarter of our service evaluation cohort were found to be obese, representing a significant proportion of patients on advanced therapies overall. We observed poorer responses to (b/ts)DMARDs, particularly in patients with BMI in the range 35–40, and greater than 40. Patients with BMI > 35 had higher DAS28, ESR, and CRP suggesting higher persistent disease activity. It is possible that the observed higher DAS28 was driven only by increased inflammatory markers rather than clinical signs of joint swelling. We relied on documentation in clinic letters for details of clinical response as clinical records are now archived externally. Our data were limited by ongoing virtual appointments toward the end of the Covid pandemic, which limited the number of eligible patients as BMI was not available for virtual appointments. Although most letters included the DAS28, individual components were not reliably available, particularly for joint counts, and analysis could not be performed. Few patients had confirmation of inflammatory activity by MRI or ultrasound scans. Due to small numbers, we were unable to provide a breakdown of treatment responses by each mode of action, or line of therapy. With a single time point, we were also unable to comment on treatment duration.
Despite recommendations to refer patients with BMI over 40, or over 35 with hypertension, diabetes, or sleep apnoea, most eligible patients were not referred to specialist weight management services. As the notes review only included a short time period, it is possible that some of these patients had declined referral to specialist services in the past. As a result of this service evaluation, local pathways were updated to create closer links with our endocrinologists and weight management dieticians with an aim to signpost patients to appropriate local services either in the community or specialist teams. Patients with obesity were also signposted to holistic approaches such as yoga and swimming alongside medical treatment to aid control of symptoms and improve overall health.
Even with the limitations of our data collection, our finding of higher recorded disease activity in patients with obesity agrees with data from the Swiss registry [66] which demonstrated higher DAS28 in patients with obesity. In our dataset, this seemed to be restricted to patients with BMI over 35 which was not included as a separate category in many of the studies evaluated in our systematic review. Using DAS28 < 4 as a surrogate for EULAR response (assuming DAS28 > 5.1 at treatment initiation), we also saw reduced response rates in patients with higher grades of obesity in agreement with Iannone et al. [62]. We observed DAS28 remission in only one patient with BMI > 35, compared to 35% in non-obese patients. Lower rates of remission were seen in many of the studies in our systematic review of patients with obesity [13, 24, 46, 47, 50]. In contrast to these studies, patients with less severe obesity were still able to achieve remission (47% in the class 1 obesity group). Our results could be skewed by missing data due to virtual appointments during the Covid pandemic, but patients with more severe disease at their last visit would have been more likely to be invited to attend clinic in person. It is also possible that achievement of remission by patients with less severe obesity could have prevented relationships between achievement of remission and obesity being discovered in other cohorts, particularly in cohorts of non-Caucasian patients where BMI cut-offs for obesity are lower [36].
Early reports of responses to infliximab suggested poorer responses to treatment in patients with higher BMI [15]. This has now been reported across all TNFi [13, 24, 46, 47, 50, 61], with an additional impact of obesity on infective and cardiovascular adverse events seen with certolizumab [45]. Obesity appears to have less of an impact on response to treatment with other modes of action biologics [35, 39, 51, 54, 55, 58] or on drug retention with abatacept [54, 70] or tocilizumab [71]. Although weight-based dosing has been suggested as a proposed reason for reduced clinical response to biologics generally, these medications were originally licensed as intravenous preparations only, and similar responses in obese patients are seen with subcutaneous abatacept and tocilizumab. It is possible that these modes of action are less impacted by the obese pro-inflammatory state. However, a number of these studies have shorter follow-up periods and it is possible that responses to these agents may differ in obese patients during longer-term follow-up.
We noted a significantly higher ESR and CRP in patients in higher classes of obesity; this has also been observed in other cohorts [3, 74]. It is possible that these higher values were the predominant drivers of higher disease activity, particularly as tender and swollen joint counts were not always available, although patient global scores were also elevated in these groups.
We did not include targeted systemic DMARDs in our systematic review and this is a clear limitation. However, it has been recently recommended to avoid these drugs in patients with risk factors for cardiovascular and thromboembolic disease, which would include obesity [75]. Due to differing time points of assessment and disease activity measures, we were also unable to perform the meta-analysis.
Obesity is a pro-inflammatory state [3, 4], and can increase the risk of developing RA [76]. Indeed, rising rates of obesity in the general population may be contributing to an increased incidence of RA [76]. Difficulty in the assessment of disease activity in patients who are obese is well recognised and imaging may be helpful in distinguishing inflammatory joint swelling, which can be both under and overestimated [11]. Obesity has also been reported as one of the factors involved in “difficult to treat” RA [77].
It is less clear if weight loss would improve disease outcomes in this patient group. Modest improvement in disease activity was seen in a retrospective cohort study in patients who had lost at least 5kg in weight between visits. In this study, each kilogram of weight loss was associated with a CDAI improvement of 1.15 [78]. However, this needs to be replicated in a prospective cohort.
High rates of obesity are seen in patients with RA. It is therefore important to select therapies that are likely to be most effective in this potentially difficult-to-treat patient group. Based on this review, consideration should be given to choosing a non-TNFi bDMARD in preference to TNFi in patients who are obese, especially those in higher BMI categories. In addition, discussion of the importance of optimal weight management should be discussed with patients at an early stage as weight loss may improve response to treatment and lessen requirements for multiple lines of biologic therapy. Patients should be weighed at least annually, and the consequences of obesity on the severity and response to treatment discussed. For patients with BMI over 40, or over 35 with additional risk factors such as diabetes mellitus or hypertension, consideration should be given to referral to specialist weight management services. For other patients, advice or signposting to other weight loss strategies or lifestyle changes including increased physical activity should be recommended.
BMI: body mass index
CDAI: clinical disease activity index
CI: confidence interval
CRP: C-reactive protein
DAS28: disease activity score 28 joints
ESR: erythrocyte sedimentation rate
EULAR: European Alliance of Associations for Rheumatology
OR: odds ratio
RA: rheumatoid arthritis
SDAI: simplified disease activity index
TNFi: tumour necrosis factor alpha inhibitor
WHO: World Health Organization
The authors acknowledge support from Keri Bramford-Hale for advice and assistance with database searches (CEBIS). We also thank Dr Toby Cox for reviewing the readability of the final version of the manuscript.
ZI: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing. NJG: Conceptualization, Formal analysis, Supervision, Writing—original draft, Writing—review & editing.
The authors declare that they have no relevant conflicts of interest for this work.
The service evaluation was reviewed and registered by the University Hospitals Coventry & Warwickshire NHS Trust Research & Development Department. Ethical approval was not required (Ref SE0353).
Informed consent was not required for this analysis of routinely collected clinical data.
Not applicable.
Data sharing is not permitted within local approvals, but authors are willing to respond to readers’ reasonable questions.
ZI is supported by a National Institute for Health Research (NIHR) Academic Clinical Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gloria Candelas Rodríguez, Virginia Villaverde
Manolya Ilhanli, Ilker Ilhanli
Ozlem Pala ... Joel M. Kremer
Uğur Özkan ... Murat Birtane
Diego Benavent, Chamaida Plasencia-Rodríguez
