Affiliation:
1Department of Internal Medicine, Emory University School of Medicine, Atlanta, GA 30322, United States of America
Email: aturlej@augusta.edu
ORCID: https://orcid.org/0009-0008-6490-3619
Affiliation:
2Division of Clinical Immunology and Rheumatology, University of Alabama at Birmingham, Birmingham, AL 35233, United States of America
3Section of Rheumatology, Birmingham VA Medical Center, Birmingham, AL 35233, United States of America
Email: agaffo@uabmc.edu
ORCID: https://orcid.org/0000-0001-7365-7212
Explor Musculoskeletal Dis. 2024;2:279–292 DOI: https://doi.org/10.37349/emd.2024.00056
Received: March 14, 2024 Accepted: April 18, 2024 Published: July 19, 2024
Academic Editor: Jürgen Braun, Ruhr Universität Bochum, Germany
The article belongs to the special issue Calcium Pyrophosphate Deposition Disease
Calcium pyrophosphate deposition disease (CPPD) is a cause of inflammatory arthropathy that increases in prevalence with increasing age, presents in acute and chronic forms, and is characterized by the finding of positively birefringent crystals on polarized microscopy of synovial fluid. This review finds that although strides are being made in CPPD diagnosis and classification, CPPD remains a poorly understood, unrecognized, and debilitating disease. As a consequence, treatment options usually lack supportive evidence and there has been little progress in novel drug development for the condition. This article aims to discuss the updated evidence on treatment options for CPPD and identifies promising future areas for improvement.
Calcium pyrophosphate deposition disease (CPPD) refers to inflammatory arthritis that occurs predominantly in older adults and is caused by calcium pyrophosphate (CPP) crystals. In the articular cartilage pericellular matrix, pyrophosphate from extracellular ATP complexes with calcium to produce CPP crystals, which then stimulate inflammatory cytokines and mechanical cartilage degradation. The prevalence of CPPD, based on chondrocalcinosis found on imaging (asymptomatic CPPD) in European adults, could be between 4–7%, but this is likely an underestimate [1, 2]. Acute CPP crystal arthritis presents in one or multiple joints such as the knee or wrist as pain, erythema, and edema. It is often associated with constitutional symptoms and elevated inflammatory markers. Due to the similarity of its presentation to gout, it was formerly referred to as pseudogout. On the contrary, chronic CPP crystal arthritis presents as a polyarthritis that is like rheumatoid arthritis in distribution [1, 3]. Diagnosis of CPPD is aided by the presence of rhomboid positively birefringent crystals on polarized microscopy of synovial fluid and often chondrocalcinosis on imaging studies. Recently, there have been strides made in the development of classification criteria by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) and outcome domains that will likely greatly improve research in CPPD, as well as limited management recommendations [4–6]. However, the evidence for management recommendations continues to be scarce. Available treatment strategies for CPPD remain untargeted and many of the existing treatments are repurposed from gout, due to connections in pathophysiology. This review provides background on available therapies for CPPD, summarizes and interprets evidence up until this point, and suggests future directions for research. The published EULAR recommendations for treatment published in 2011 will serve as a frame for our discussion with reviews on other treatment options published since then [6].
The types of CPPD according to EULAR criteria which align with treatment options include (1) asymptomatic CPPD; (2) acute CPP crystal arthritis; (3) chronic CPP crystal arthritis; and (4) osteoarthritis (OA) with CPPD [7, 8]. For asymptomatic radiographic findings of CPPD, sometimes described as chondrocalcinosis, treatment is not necessary. Mere chondrocalcinosis clinically differs from other forms of CPP crystal arthritis and is associated with seronegative rheumatoid arthritis [3]. For acute CPP crystal arthritis, treatments focus on addressing symptoms secondary to the crystal-induced inflammation by using treatment strategies common to the ones for gout flares such as intra-articular glucocorticoid, non-steroidal anti-inflammatories (NSAIDs), colchicine, or systemic glucocorticoid rather than addressing crystal formation itself. Although randomized controlled studies on all of these options for CPPD have been scarce, this review highlights the progress that has been made with new studies including a recently published controlled trial in which colchicine was compared to oral prednisone and a study that compared loading dose colchicine to non-loading dose for acute CPP crystal arthritis [9, 10]. For chronic CPP crystal arthritis, which requires more long-term anti-inflammatory management, low-dose NSAIDs with gastroprotection or low-dose colchicine were recommended as initial therapy by EULAR, but low-dose glucocorticoids, methotrexate, hydroxychloroquine, and biologics have been investigated for chronic CPP crystal arthritis with data mixed regarding treatment response. Although biologics such as anakinra or tocilizumab are not mentioned by the recent EULAR recommendations, we highlight a study by Damart et al. [11] which shows biologics are currently used off-label in refractory cases of CPP crystal arthritis or cases in which all of the prior treatments are contraindicated or ineffective, drawing on evidence from one randomized controlled trial and other small studies [12, 13]. There are also other forms of CPPD such as “tophaceous pseudogout” or tumoral CPPD in which there is a deposit of CPP crystal and this can be treated surgically [14]. For patients with OA and CPPD, EULAR recommends maintaining the same therapies and objectives as if they had isolated OA [6]. Overall, in this review, we discuss updates to evidence published after the 2011 EULAR guidelines regarding all types of CPPD. We emphasize treatment challenges in the setting of comorbidities and the importance of addressing underlying conditions or potentially causative medications. Finally, we discuss the potential of future targeted treatments such as histone-deacetylase inhibitors (HDACis), which are in the early stages of research.
EULAR recommendations for CPPD are subdivided into asymptomatic CPPD, acute CPP crystal arthritis, chronic CPP crystal arthritis, and OA with CPPD. The objective in the treatment of acute CPP crystal arthritis is oriented towards rapid relief in contrast to the treatment of refractory or chronic CPP crystal arthritis which is more focused on prevention. While some treatments such as NSAIDs, colchicine, systemic glucocorticoids, and biologics are part of both acute and chronic management, intra-articular injection tends to belong to acute management and disease-modifying medications like methotrexate and hydroxychloroquine are more often used in chronic management (see Figure 1) [6]. In 2011, EULAR published recommendations that guide therapy for different manifestations of CPPD (Figure 2) [6]. Since then, additional studies have been published, which have expanded evidence for the treatment approach (see Table 1).
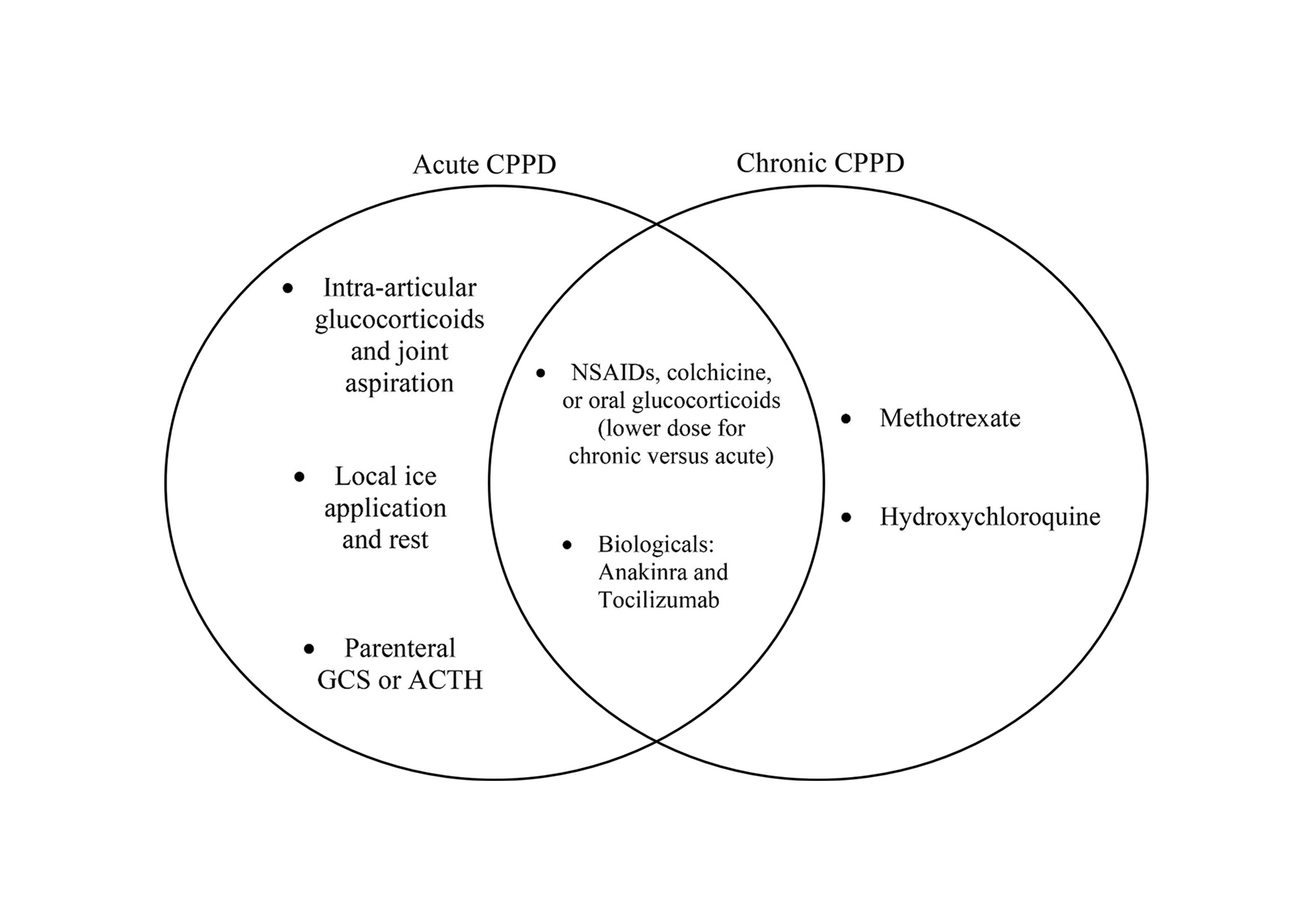
Acute versus chronic CPPD treatment. With some exceptions, this Venn diagram shows the treatments that tend to be used for acute CPPD, chronic CPPD, and both. ACTH: adrenocorticotrophin hormone; GCS: glucocorticoids; NSAIDs: non-steroidal anti-inflammatory drugs; CPPD: calcium pyrophosphate deposition disease
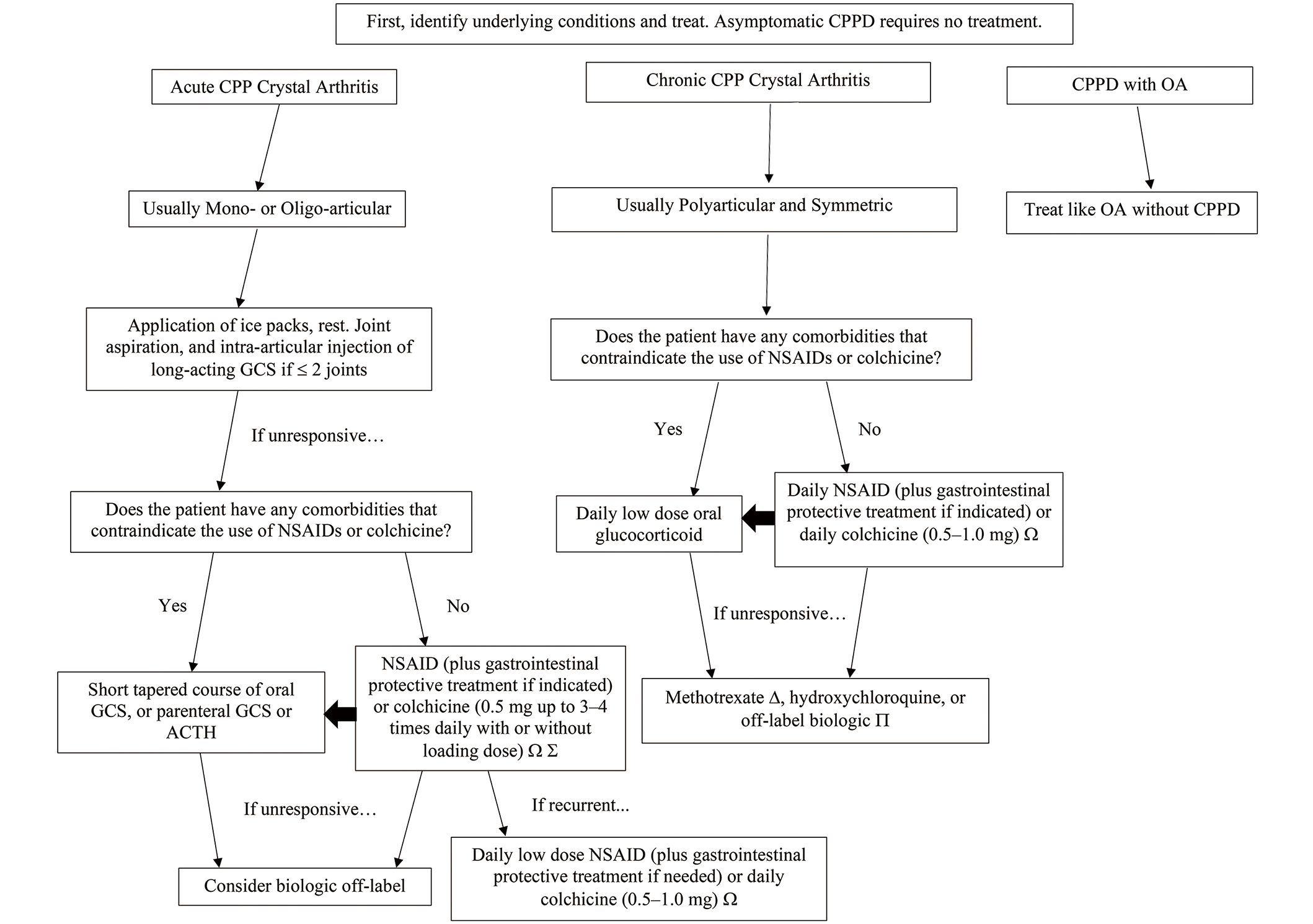
CPPD treatment algorithm is based partly on 2011 EULAR recommendations, with some additions, including biologics [6]. Ω: dosing may be 0.6 mg depending on the country; Ʃ: Laosuksri et al. [10] found no benefit of colchicine loading dose; ∆: Damart et al. [11] found methotrexate had the highest retention rate; ∏: Damart et al. [11] found tocilizumab to have better retention than anakinra. CPP: calcium pyrophosphate; CPPD: calcium pyrophosphate deposition disease; GCS: glucocorticoids; NSAID: non-steroidal anti-inflammatory drug; OA: osteoarthritis; ACTH: adrenocorticotrophin hormone; EULAR: European League Against Rheumatism
Treatments for CPPD with mechanism of action, adverse effects, and supporting evidence
| Therapy | Indication (acute vs. chronic CPP crystal arthritis) | Mechanism | Adverse effects | Examples of literature supporting use in CPPD |
|---|---|---|---|---|
| Rest and ice pack application | Acute | Reduces blood flow and therefore inflammation in the affected area | None known | EULAR recommendations (Zhang et al. [6]), literature on gout |
| Joint aspiration | Acute | Relieves pressure on the distended joint capsule | Septic arthritis, bleeding, neurovascular or tendon damage, others (rare) | EULAR recommendations (Zhang et al. [6]), a small study (O’Duffy [15]), literature on gout |
| Intra-articular glucocorticoid injection | Acute | Locally alters gene expression in a way that has anti-inflammatory effects | Septic arthritis, bleeding, neurovascular or tendon damage, post-injection flare, local skin or fat changes, osteonecrosis, allergy, others (rare) | EULAR recommendations (Zhang et al. [6]), a small study (O’Duffy [15]), literature on gout |
| Oral NSAIDs (with gastroprotection) | Gastrointestinal (ulcer, bleeding, dyspepsia), renal (hypertension, edema, electrolyte disturbance, AKI), cardiovascular, pulmonary, hematologic, hepatic, anaphylaxis or allergy, drug interactions | EULAR recommendations (Zhang et al. [6]), literature on gout | ||
| Oral colchicine | Diarrhea, nausea, vomiting, neuromyopathy, toxicity (cytopenia, liver failure, rhabdomyolysis) | EULAR recommendations (Zhang et al. [6]), a small study on prophylaxis (Alvarellos et al. [16]), RCT comparing colchicine to prednisone (Pascart et al. [9]), RCT on dosing (Laosuksri et al. [10]), retrospective cohort (Damart et al. [11]), literature on gout | ||
| Oral or parenteral glucocorticoids | Acute and chronic (lower dose) | Systemically alters gene expression in a way that led to anti-inflammatory effects, activates anti-inflammatory proteins | Endocrine (HPA suppression, hyperglycemia, weight gain), dermatologic (i.e., Cushingoid striae), cardiovascular (hypertension, edema, etc.), gastrointestinal, bone, and muscle (osteoporosis, myopathy, etc.), neuropsychiatric, ophthalmologic (increased intraocular pressure, cataracts, etc.), immune (i.e., immunosuppression), injection site pain, others | EULAR recommendations (Zhang et al. [6]), RCT comparing oral prednisone to anakinra (Dumusc et al. [12]), RCT comparing prednisone to colchicine (Pascart et al. [9]), study comparing parenteral glucocorticoids to diclofenac (Werlen et al. [17]), study on intramuscular triamcinolone acetonide (Roane et al. [18]), retrospective cohort (Damart et al. [11]) |
| ACTH (parenteral) | Acute | Similar to glucocorticoids, hyperpigmentation | Case series (Daoussis et al. [19]), literature on gout (Siegel et al. [20], Axelrod et al. [21]) | |
| Methotrexate (subcutaneous) | Chronic | Inhibition of dihydrofolate reductase, adenosine-mediated effect, others | Folate deficiency, myelosuppression, teratogenicity and toxicity, pulmonary, injection site pain, others | EULAR recommendations (Zhang et al. [6]), RCT (Finckh et al. [22]), small observational study (Andres et al. [23]) |
| Hydroxychloroquine (oral) | Chronic | Interferes with lysosomal activity and autophagy by accumulating in lysosomes | Ophthalmic (retinopathy), hematologic (i.e., anemia, aplastic anemia, myelosuppression), cardiovascular (sick sinus syndrome), dermatologic, endocrine (weight loss), gastrointestinal, hepatic, hypersensitivity, neurologic, respiratory | EULAR recommendations (Zhang et al. [6]), RCT (Rothschild and Yakubov [24]), retrospective cohort (Damart et. al [11]) |
| Biologics | Acute and chronic | Antagonize interleukin receptors or neutralize interleukin signaling | Immunosuppression, infections, injection site reactions, etc. | Cohort studies and case series (i.e., Damart et al. [11], Lian et al. [13], Latourte et al. [25], etc.), RCT comparing anakinra to prednisone (Dumusc et al. [12]), retrospective cohort (Damart et al. [11]) |
ACTH: adrenocorticotrophin hormone; CPPD: calcium pyrophosphate deposition disease; EULAR: European League Against Rheumatism; HPA: hypothalamic-pituitary-adrenal axis; RCT: randomized controlled trial; CPPD: calcium pyrophosphate deposition disease; NSAID: non-steroidal anti-inflammatory drug; AKI: acute kidney injury
The approach to treating acute CPPD is rapid symptom relief. The treatments are mostly extrapolated from gout treatment and, until recently, lacked significant evidence from controlled trials. The first line set of recommendations in the EULAR guidelines for mono- or oligo-articular attacks of large joints include non-pharmacological measures such as the use of ice packs and temporary rest; but also joint aspiration (which besides diagnostic utility has a therapeutic effect in relieving pressure in distended joint capsules when there are large effusions) combined with intra-articular injection of long-acting glucocorticoids [6]. Although intra-articular glucocorticoid injection has been a common treatment for acute attacks, it has some limitations. Unfortunately, there are no randomized controlled trials evaluating the efficacy of intra-articular glucocorticoids for CPP crystal arthritis. A small case series found intra-articular glucocorticoid injection with aspiration to have the fastest onset of relief, compared to aspiration alone or aspiration combined with NSAIDs [15]. Additionally, in clinical practice, it is only logistically feasible to do this when 2 or fewer joints are affected. Finally, while administering intra-articular therapy, it is important to follow proper techniques to avoid some rare complications [26]. Recurrent acute attacks, polyarticular attacks, and/or chronic CPP crystal arthritis require additional treatment options described next.
EULAR considers oral NSAIDs (with gastroprotective treatment) or colchicine effective options for acute CPP crystal arthritis due to extensive use in gout, despite limited published evidence and their contraindication in patients with comorbidities such as renal insufficiency [6]. NSAIDs inhibit the cyclo-oxygenase enzyme and therefore the conversion of arachidonic acid into prostaglandins and prostacyclins. Although NSAIDs are widely used as an initial treatment, there are no known controlled trials studying NSAIDs for CPPD. Colchicine works by inhibiting microtubules (thus impairing immune cell chemotaxis) and inflammation driven by the NLRP3 inflammasome [27]. EULAR considers oral colchicine effective for acute attacks at the lower dose regimen of 0.5 mg up to three to four times daily with or without a loading dose of 1 mg [6] (in the United States, colchicine is dosed in 0.6 mg tablets and therefore loading dose may be 1.2 mg). For daily prophylaxis of recurrent acute attacks, a lower dose regimen of 0.5–1 mg daily is recommended. Higher doses of colchicine can be dangerous and even fatal (colchicine has a narrow therapeutic index), thus lower dose regimens are preferable [28]. There was previously limited data supporting colchicine use in CPPD including small studies in which it appeared to reduce rates of disease flare in patients receiving the medication [16, 29]. However, studies in recent years have given more insight into colchicine use for acute CPP crystal arthritis. In the recent year, an open-label multi-center randomized study of 95 patients with crystal-proven CPPD added to the data on colchicine by comparing colchicine and prednisone in older hospitalized patients with acute CPP crystal arthritis. The median age was 88 years and 69 (73%) were female. Patients in one group received 1.5 mg oral colchicine on day 1 followed by 1 mg on day 2 while patients in the other group received oral prednisone 30 mg on days 1 and 2. The between-group difference in joint pain at 24 hours measured by visual analogue scale (VAS) was only –1 mm, which showed short-term equivalence between the two drugs. While colchicine caused more incidences of diarrhea, prednisone caused more hypertension and hyperglycemia. Limitations of this study included a lack of placebo and no masking [9]. In terms of colchicine dosing, the results of a recent double-blinded randomized controlled study with 80 patients, 31 of which had CPP crystal arthritis and 49 of which had gouty arthritis, supported the idea that non-loading dose colchicine (1.2 mg in 24 hours given as two 0.6 mg doses) is as effective in reducing pain in patients with acute crystal arthritis as loading dose (2.4 mg in 24 hours given as 1.2 mg loading dose followed by two 0.6 mg doses). There was no significant difference between the group receiving a loading dose versus the group receiving a non-loading dose in mean pain score or the proportion of patients able to achieve 50% pain reduction at 24 hours. Adverse events were also similar between groups [10]. This study therefore suggested non-loading dose colchicine over loading-dose, out of convenience, and it was the first study to compare dosing methods for crystalline arthritis. Because both NSAIDs and colchicine are known to be associated with adverse events and contraindicated in comorbidities commonly present in the patient demographic of CPPD (such as chronic kidney disease, congestive heart failure, liver disease), alternatives for the treatment of acute CPP crystal arthritis are necessary.
Systemic glucocorticoids are an important option for patients with contraindications to NSAIDs or colchicine such as renal disease and either acute CPP crystal (1) mono- or oligo-articular arthritis resistant to intra-articular glucocorticoid injection (plus joint aspiration, cool packs, and rest) or (2) polyarticular arthritis. For these patients, EULAR recommends a short tapering course of oral glucocorticoid, parenteral glucocorticoid, or ACTH (adrenocorticotropin hormone) [6]. Specifics about treatment are also extrapolated from gout but the use of glucocorticoids has been supported by small non-randomized controlled studies showing good clinical response [18]. When compared to the NSAID diclofenac 150 mg daily in one small study (n = 27), intramuscular glucocorticoids (7 mg betamethasone once) and intravenous injections (125 mg methylprednisolone once) achieved a lower number needed to treat to obtain 50% improvement on day 1, suggesting parenteral glucocorticoid can provide quite rapid improvement [6, 17]. However, again, these non-randomized, small studies that are short in duration are only a guidance, albeit supported by a large body of empirical experience, finally leaving this decision to be guided by a risk assessment of comorbid conditions. It is important to note that shorter studies on glucocorticoid use in arthritis may not capture the common side effects associated with frequent or long-term use, including hyperglycemic states, infection, edema, hypertension, and osteoporosis. It is well demonstrated that glucocorticoids can have these adverse effects long-term, leading clinicians to minimize the duration of time patients take them [30]. Due to the side effect profile, the use of steroids in CPPD remains controversial and they are not preferred as long-term or frequent therapy, despite their efficacy.
Like glucocorticoids, ACTH is an alternative for the treatment of acute CPP crystal arthritis that is refractory or polyarticular in patients with conditions contraindicating NSAIDs or colchicine. It may have its effect through stimulation of the release of endogenous glucocorticoids from the adrenals or anti-inflammatory properties of melanocortins themselves. A systematic review by Daoussis et al. [31] summarized studies on parenteral ACTH as a treatment in crystal arthritis (both gout and acute CPP crystal arthritis). Although they described several small prospective and retrospective studies showing that 40 IU or 100 IU ACTH treats gout in patients with comorbidities with efficacy, there were fewer studies that specifically described its use in acute CPP crystal arthritis [20, 21, 31, 32]. One of these retrospective studies included 5 patients with acute CPP crystal arthritis whose flare resolved in an average of 4.2 days of receiving 40 IU ACTH every 8 hours [32]. However, there was only a single study by Daoussis et al. [19] that consisted exclusively of 14 patients with acute CPP crystal arthritis treated with 100 IU of ACTH, which showed rapid symptom improvement within 24 hours. There were no significant side effects (blood pressure, glucose, and potassium changes were measured). As is the case with glucocorticoids, small studies seem to support ACTH use in patients with acute crystal arthritis and comorbidities that preclude the use of NSAIDs or colchicine, keeping in mind the small sample sizes (which are even smaller for CPPD compared to gout). Additionally, its expected marginal benefit compared with systemic glucocorticoids, and its markedly increased cost in many markets relegate ACTH formulations to a distant consideration for acute management.
For the treatment of chronic CPP arthritis, the 2011 EULAR guidelines recommend in order of preference the following systemic therapies: low-dose oral non-steroidal anti-inflammatory drugs with gastroprotective treatment and/or low-dose colchicine 0.5–1.0 mg daily (if no contraindications), low-dose glucocorticoid, methotrexate, and hydroxychloroquine [6]. A retrospective cohort study by Damart et al. [11] describing 194 treatments in 129 patients included 4 main off-label treatments used in chronic CPP crystal arthritis or recurrent/refractory acute CPP crystal arthritis, which were colchicine, methotrexate, anakinra, and tocilizumab, listed in descending order of frequency, as well as lesser used treatments (long-term glucocorticoids, hydroxychloroquine, canakinumab, and sarilumab). This study was impactful because it characterized the heterogeneity of treatments currently being prescribed for chronic CPP crystal arthritis including off-label biologics. Colchicine 0.5–1.0 mg dosed 4 times daily was the most used and usually first line, with lasting efficacy in 33–50% of patients. Hydroxychloroquine was used sparingly overall as a first line. Methotrexate was second-most utilized, most often as a second line after colchicine failure and often co-prescribed with colchicine or glucocorticoids. Anakinra and tocilizumab were second or third-line and sometimes in combination with methotrexate or colchicine. Canakinumab and sarilumab were exclusively used as 3rd or 4th line. Glucocorticoids were used as an add-on therapy. Adverse events led to discontinuation most often in anakinra (31.8% due to serious infections or injection site reactions), followed by tocilizumab (20% due to effects similar to those of anakinra), then colchicine (14.1% due to diarrhea), then methotrexate (4.3%), and none of hydroxychloroquine discontinuations. Rather, the more commonly cited reasons for discontinuing most of these drugs were loss to follow-up or inefficacy, with only 34.5% of treatments still ongoing at 24 months. Median on-drug survival was highest for methotrexate (15.3 months) followed by tocilizumab (12.2 months), then anakinra (10.2 months), then colchicine (9.9 months), with 24-month retention being the highest for methotrexate and significantly higher for tocilizumab compared to anakinra. Overall, in 63.9% of follow-up visits, treatment response was assessed as good on the Likert scale (> 2/4). All outcome measures improved over time (patient global assessment, patient-reported disability level, number of episodes, and physician assessment of disease activity) for those who continued treatment [11].
Contrary to the retrospective study by Damart et al. [11] which suggested that methotrexate improved all outcome measures and had the highest retention for chronic CPP crystal arthritis, prior literature on methotrexate for this indication has shown conflicting results. Small studies initially seemed to support methotrexate use, including an uncontrolled trial of 5 patients with chronic or recurrent acute CPPD in which methotrexate significantly decreased pain measured by VAS within a mean period of 7.4 weeks and another trial of 10 patients in which there was a significant decrease in pain however the drug was discontinued in two patients due to bone marrow suppression or liver enzyme elevation [6, 23]. In contrast, a small randomized crossover trial by Finckh et al. [22] with 26 patients showed no significant effect on the disease activity of methotrexate compared to placebo. The more drastic positive response seen in the recent retrospective cohort study by Damart et al. [11] could possibly be due to its co-prescription with glucocorticoids or colchicine.
Hydroxychloroquine was sparingly prescribed as a first line in the retrospective cohort study by Damart et al. [11] and in the few patients who received it, there were no discontinuations for adverse events (all were due to inefficacy and loss of follow-up). The minimal usage could be due to the limitation in studies for the indication of CPP crystal arthritis. There is mainly one 6-month double-blind controlled trial of 36 patients conducted in 1997 that demonstrated that hydroxychloroquine (100 mg per day titrated to up to 400 mg per day) was effective because it significantly reduced the number of affected joints compared to placebo. Authors Rothschild and Yakubov [24] suggested hydroxychloroquine is effective and safe for chronic CPPD. As with methotrexate, this was the singular randomized controlled trial (RCT) available on hydroxychloroquine for this indication.
Anakinra, an interleukin-1 (IL-1) inhibitor usually administered subcutaneously, was one of the first biologics used off-label for gout and CPPD (it is approved by the Federal Drug Administration (FDA) for rheumatoid arthritis, cryopyrin-associated periodic syndromes, and deficiency of IL-1 receptor antagonist). A recent systematic review included 11 studies with 74 patients who received anakinra, mainly patients with contraindications to standard treatment or refractory disease [33]. The review reported that in patients treated with anakinra 100 mg/day, treatment response was seen in 77% (n = 57/74) of patients. Broken down by acute and chronic CPPD, efficacy was 80.6% (n = 54/67) for patients with acute CPPD and 42.9% (n = 3/7) for those with chronic CPPD [33]. Most of the patients achieved their response within 4 days but treatment duration varied with CPPD chronicity. Because this systematic review included any other biologics used for CPPD between 1980 to 2019, it also referred to two patients treated with infliximab that had resolution of symptoms within 4 months of therapy. There was another review concluding the similar effectiveness of anakinra the following year [34]. This review referred to a singular underpowered randomized controlled double-blinded trial of 15 patients with acute CPP crystal arthritis, 8 of which took anakinra 100 mg daily and 7 of which took 30 mg daily oral prednisone with respective placebos for 3 days, that reported that anakinra had similar effectiveness to prednisone [12]. The trial was stopped early due to poor enrollment and there remain no further randomized controlled trials on anakinra. More recently published on this subject was another retrospective case series characterizing a cohort of 70 patients and 79 cases of CPPD: 12 cases were treated using 100 mg anakinra (in mostly male patients with comorbidities, higher C-reactive protein and creatinine levels that had failed conventional therapies) and 67 cases were treated with conventional therapy (NSAID, colchicine, or intra-articular or systemic glucocorticoid). This case series added to the data that anakinra was rapidly effective with the mean time to a substantial and complete response being 1.7 days and 3.6 days respectively with minimal side effects [13]. Interestingly there were minimal adverse events reported in these prior studies, in contrast to the new retrospective cohort study by Damart et al. [11], which indicated that adverse events were the cause of some anakinra discontinuations.
In terms of other biologics, tocilizumab, an IL-6 inhibitor, was used in a pilot study of 11 patients with chronic or recurrent acute CPP crystal arthritis who had contraindications to other medications. Seven of the 11 patients had failed anakinra or experienced severe adverse events (injection site reactions) [25]. Tocilizumab was given monthly as an intravenous infusion (4–8 mg/kg) or subcutaneous injection (162 mg) and by 3 months all patients reported significant improvement in pain measured by VAS. There were three patients with side effects including dyspnea, lung abscess in a smoker, and non-severe infections.
Both anakinra and tocilizumab were medications prescribed off-label in the recent retrospective cohort study conducted by Damart et al. [11]. Tocilizumab achieved efficacy in the study with significantly longer treatment retention than anakinra and slightly fewer discontinuations due to adverse events (serious infections) compared to anakinra. However, it is important to recognize the limitations of a small retrospective study (only 27 patients and 25 patients received anakinra and tocilizumab respectively). Other biologics were also prescribed in this retrospective cohort by Damart et al. [11] including canakinumab and sarilumab sparingly as 3rd or 4th lines, indicating that European physicians are also utilizing these agents (which are also not FDA-approved for CPPD although canakinumab is FDA approved for gout) [11]. In general, biologics have shown promise for effective treatment of CPPD but rarely have increased risk of infection or injection site reaction. A downside of these drugs also tends to be cost and injectable drug delivery. It would be helpful to have future randomized controlled trials and clinical trial data, considering the only RCT available for a biologic (anakinra) was stopped early.
In some cases of CPPD in which there is OA or deposition of tophi present, surgery may be required. Treatment of CPPD superimposed on OA has been viewed as a therapeutic challenge and it has previously been speculated whether patients with CPPD and OA have worse outcomes following joint replacement compared to patients with only OA. Results of a systematic review by Moret et al. [35] suggest that chondrocalcinosis does not significantly influence postoperative functionality after joint replacement for OA. However, the level of evidence (LOE) of the study was low (level IV) and there is a lack of randomized controlled trials to officially guide the approach to CPPD superimposed on OA. Expert opinion from EULAR as of 2011 is to treat patients with OA and CPPD as if they have OA without CPPD [4, 6].
Unlike the common articular locations for CPPD such as the knee and wrist, “Tophaceous pseudogout” is a variety of CPPD that causes deposition of CPP crystals in the TMJ, which may require surgical treatment. In one case report, the patient with tophaceous pseudogout was treated with eminectomy, condylectomy, and total TMJ reconstruction [14]. The authors of the case report made recommendations for the surgical treatment of advanced tophaceous pseudogout: (1) include tophaceous pseudogout in the differential diagnosis of lesions mimicking neoplastic processes of the TMJ or infratemporal fossa; (2) use of image-guided fine needle aspiration for tissue sampling to aid in pre-operative diagnosis; (3) consider pre-surgical embolization when dealing with a large mass in the infratemporal or peri-articular TMJ region; (4) intra-operative navigation assistance in extensive lesions may be beneficial; (5) consider single-staged alloplastic reconstruction using stock versus custom-fabricated TMJ prosthesis after resection of advanced lesions; (6) continue long-term follow up to rule out recurrence, and (7) rheumatologic referral may be indicated for thorough workup of other commonly involved joints [14]. Other case reports similarly described the reconstruction of the TMJ [36, 37]. CPP masses can occur in other areas, usually associated with repeated trauma. These might also need to be approached surgically.
The older population in which CPPD is prevalent also has a high prevalence of other conditions. As previously mentioned, CPPD superimposed on OA is a treatment challenge but can be treated as if the patient has only OA, keeping in mind there is limited data on this [6]. Treatments for OA include acetaminophen, weight loss, physical therapy, limiting the progression of joint damage, and/or joint replacement. In patients unresponsive to these therapies, NSAIDs and opioids can be used with caution unless contraindicated.
As previously discussed, in patients with CPPD and renal disease, colchicine and NSAID use are contraindicated and patients must resort to oral glucocorticoids, parenteral glucocorticoids, ACTH, or other disease-modifying agents.
Although CPPD can be idiopathic, some cases of CPPD may be associated with underlying disorders such as primary and secondary hyperparathyroidism, hypomagnesemia, hypophosphatasia, hemochromatosis, Gitelman syndrome, previous joint surgery, metabolic risk factors such as obesity and hypertension, and chronic gout [38]. It has been suggested that sudden fluctuations in calcium, such as in the post-parathyroidectomy period, can also instigate CPPD thus it has been suggested to supplement calcium following the procedure [39]. Magnesium carbonate supplementation was studied in CPPD in one small randomized controlled trial [40]. Additionally, mutations in certain genetic loci affecting the ankylosis human (ANKH) protein are associated with familial CPPD, and potential future treatment for this is discussed in another paragraph. Investigating underlying causes in patients, especially those presenting under the age of 60, is particularly important and addressing these underlying conditions should not be underestimated as a treatment option [1]. EULAR cites that treatment of underlying conditions with a LOE of Ib is a higher LOE than most other recommendations [6].
Medications may also underlie some CPPD attacks. Case reports have identified certain medications such as chemotherapies and bisphosphonates potentially associated with CPPD that may be worth avoiding in patients with CPPD. For example, Kim et al. [41] reported recurrent pseudogout a week to 10 days following infusions of nivolumab, an immune checkpoint inhibitor. There have also been reports of CPP crystal arthritis with alendronate, pamidronate, and etidronate [42–44]. Interestingly, a patient that received melanoma-associated antigen A4 (MAGE-A4) directed T-cell receptor (TCR) engineered T cells for fallopian tube cancer developed cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and acute left knee arthritis that was determined to be crystal-proven CPP crystal arthritis. The patient was administered tocilizumab and her pseudogout resolved [45].
A review by Parperis et al. [29] discussed other treatment strategies that have been investigated but not used widely, including radiation synovectomy (destruction of the synovial membrane), laser irradiation, and glycosaminoglycan or hyaluronic acid injections [46, 47]. Although these treatments reported promising results in the small and limited studies, they were not followed up by additional studies.
There is a clear need for randomized controlled trials to determine the most effective therapies for CPP crystal arthritis. Additionally, studies investigating mechanisms of crystal formation and crystal-induced inflammation are needed to identify future therapies. Recent studies linking extracellular phosphate to CPP crystal arthritis suggest that there are mechanisms of inflammation in this disease that are not yet fully understood. Specifically, researchers identified regulator proteins ectonucleotide pyrophosphatase 1 (ENPP1), ANKH, and non-specific alkaline phosphatase (TNAP) that affect levels of extracellular phosphate [48]. HDACis trichostatin A (TSA) and vorinostat (SAHA), which could be potential targeted CPPD therapies in the future, change the expression of these regulator proteins, thus lowering levels of extracellular phosphate and CPP in human primary cultured articular chondrocytes [49]. Currently, most of the clinical indications for HDACis such as SAHA are neoplastic conditions. There are no ongoing clinical trials with HDACis for CPPD in the United States. Theoretically, any substance that could decrease levels of free inorganic phosphate could decrease CPP crystals [50]. Probenecid has been considered as an option to lower free phosphate and studies have looked at nucleoside analogues, but these ideas remain in the basic research phase [51]. Investigating mechanisms of other causes of CPPD could help further elucidate whether addressing those causes would be therapeutic (for example, understanding hypomagnesemia’s role and the efficacy of magnesium supplementation). Additionally, further research could be based on the role of solubility of CPP crystals and the role of supersaturation of crystals leading to crystal deposits. It is notable that bisphosphonates, despite being inhibitors of other pathologic calcification processes, are associated with cases of CPP crystal arthritis [42]. Investigating the mechanism of these processes could lead to a better understanding of the disease and ways to treat it.
With the development and publication of the CPPD classification criteria [4], standardization of patients included in CPPD studies should improve and this could lead to more predictable outcomes of intervention studies in CPPD. In addition, increased recognition of CPPD as a common and disabling arthropathy should increase interest in studies in this area. So far, therapeutic studies in CPPD have been met with limited success. Limited data, extrapolation from gout, and expert opinion suggest that there are several standard therapies in the research of CPPD treatment. For CPP crystal arthritis, local ice application, intra-articular glucocorticoid injection, and joint aspiration for mono- or oligo-articular attacks, NSAIDs, colchicine, oral or parenteral glucocorticoids, disease-modifying medications, and off-label biologicals can be effective treatments for CPPD. Similar to glucocorticoids, biologics provide another option to patients with contraindications or lack of response to NSAIDs and colchicine. However, many of these treatments are used without the support of randomized controlled trials. Certain comorbidities, especially renal disease, may guide the choice of therapy to a degree. Although CPPD with OA may seem like a treatment challenge, expert opinion suggests OA should be treated as usual in patients with CPPD, with orthopedic surgery being an option, but this remains controversial due to the paucity of data [6]. With these therapies in mind, it should be emphasized that there may be underlying causes of CPPD that should be addressed, such as the genetic basis which is under investigation [48]. Finally, there is potential for targeted treatment of CPPD in the future but there are no trials or studies known at this time to be ongoing, which is an opportunity for future research. Although there is now at least one randomized controlled trial on colchicine and anakinra, there is a need to conduct more randomized studies on both old and new CPPD therapies.
ACTH: adrenocorticotrophin hormone
CPP: calcium pyrophosphate
CPPD: calcium pyrophosphate deposition disease
EULAR: European League against rheumatism
FDA: Federal Drug Administration
HDACis: histone deacetylase inhibitors
IL-1: interleukin-1
LOE: level of evidence
NSAIDS: non-steroidal anti-inflammatories
OA: osteoarthritis
TMJ: temporomandibular joint
VAS: visual analogue scale
AJT: Writing—original draft, Writing—review & editing, Visualization. ALG: Conceptualization, Supervision, Writing—review & editing. Both authors read and reviewed final version.
Angelo L. Gaffo is the Editorial Board Member of Exploration of Musculoskeletal Diseases, but he had no involvement in the journal review process of this manuscript. Another author declares that there is no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
John F. Hoy ... Xavier C. Simcock
Michael Schirmer, Johannes Dominikus Pallua
Fernando Perez-Ruiz ... Frédéric Lioté
Maria L. Voulgari, Herbert Kellner
Gamze Dilek ... Kemal Nas
Ebru Atalar, Hatice Bodur
