Affiliation:
1Department of Rheumatology, Immunology and Allergy, Health New Zealand, Christchurch 8011, New Zealand
2Department of Medicine, University of Otago Christchurch, Christchurch 8011, New Zealand
ORCID: https://orcid.org/0000-0003-3060-4019
Affiliation:
3Division of Rheumatology and Clinical Immunology, University of Alabama, Birmingham, AL 35294, USA
4Birmingham VA Medical Center, Birmingham, AL 35233, USA
ORCID: https://orcid.org/0000-0001-7365-7212
Affiliation:
1Department of Rheumatology, Immunology and Allergy, Health New Zealand, Christchurch 8011, New Zealand
2Department of Medicine, University of Otago Christchurch, Christchurch 8011, New Zealand
Email: lisa.stamp@cdhb.health.nz
ORCID: https://orcid.org/0000-0003-0138-2912
Explor Musculoskeletal Dis. 2024;2:360–374 DOI: https://doi.org/10.37349/emd.2024.00062
Received: June 05, 2024 Accepted: August 08, 2024 Published: September 02, 2024
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain; George Nuki, University of Edinburgh, UK
The article belongs to the special issue Pharmacological and Non-Pharmacological Management of Gout
Gout is common in people with chronic kidney disease and in general is sub-optimally managed. Lack of evidence due to the exclusion of people with chronic kidney disease from the majority of clinical trials, concerns about adverse effects and conflicting gout management guidelines all contribute to suboptimal management. Herein we review the evidence for the pharmacological treatment of gout, both flares and long-term urate-lowering, in people with concomitant chronic kidney disease.
Despite being a very common association, there has been a lack of evidence to guide therapeutic decision-making in people with gout who also have chronic kidney disease (CKD). The reasons for this are multiple [1] but mainly include the systematic exclusion of those with advanced CKD from gout clinical trials. This gap in knowledge has led to poor clinical outcomes and quality of care for this complex population [2]. The objective of this review is to summarize the latest evidence and progress in the pharmacological treatment of gout in individuals with CKD, with special emphasis on recent developments and scenarios not commonly studied like renal-replacement therapy.
Gout and CKD co-exist frequently, with the association being bi-directional and complex. It is estimated that among individuals with CKD stage 3 or more [defined as an estimated glomerular filtration rate (eGFR) of < 60 mls/min/1.73 m2] the prevalence of gout is approximately 24% [3], while in the general population of the United States, the estimate is approximately 5% [3]. On the other hand, people with gout have advanced CKD very frequently. The reported prevalence of CKD stage 2 (defined as an eGFR < 90 mls/min/1.73 m2) or more in those with gout is over 70% [4] while it is 24% for stage 3 CKD or more [5].
It is unclear if the comorbidities commonly present in gout, including cardiovascular disease, hypertension, and diabetes, or the more frequent use of nephrotoxic medications such as non-steroidal anti-inflammatory drugs (NSAIDs) are the only contributors to the high frequency of advanced CKD. A direct causal effect of elevated soluble serum urate (SU) levels, a necessary risk factor for gout, on renal function has not been established, despite laboratory, epidemiological, and early clinical evidence supporting it [4, 6, 7]. Larger randomized clinical trials of urate-lowering medications have failed to demonstrate an improvement in renal function [8]. Whether crystalized urate affects renal function is unclear [9], as is the potential contribution of urate lithiasis to adverse renal function in most patients [10]. More recent evidence suggests that in people with CKD, hyperechoic renal deposits on ultrasound are associated with higher SU, lower renal urate excretion and ischaemic nephropathy [11] and in a murine study there was no effect of asymptomatic hyperuricaemia on CKD progression unless uric acid (UA) crystalluria was observed [12].
CKD likely contributes to a higher prevalence of gout through its association with elevated SU levels [3]. Approximately 90% of individuals with hyperuricemia are renal under-excretors of urate (30% of urate excretion is gastrointestinal and prior evidence suggests it increases in advanced CKD [13]). Besides age-related decline in renal function, genetic variants in renal handling of urate are important contributors to hyperuricemia [14]. A decline associated with decreased glomerular filtration associated with advanced stages of CKD is likely an additional contributor in these cases. Finally, commonly used medications in people with advanced CKD (mainly diuretics) are known to be important contributors to hyperuricemia and ultimately to gout [15].
Several agents may be used to manage symptoms of gout flares, including colchicine, NSAIDs, glucocorticoids, adrenocorticotrophic hormone (ACTH), and interleukin-1 (IL-1) inhibitors (Figure 1). The American College of Rheumatology (ACR) guidelines recommend the use of colchicine, NSAIDs, and glucocorticoids as preferred agents over IL-1 inhibitors and ACTH. Topical ice is also conditionally recommended [16]. The European Alliance of Associations for Rheumatology (EULAR) recommendations for the management of gout flares suggest colchicine, NSAIDs, and oral or intra-articular steroids as first line options for gout flare management. These options are not ranked in order of preference. Avoidance of colchicine and NSAIDs in people with significant renal dysfunction is recommended. The EULAR recommendations support the use of IL-1 inhibitors when contraindications prohibit the use of the first line agents [17].
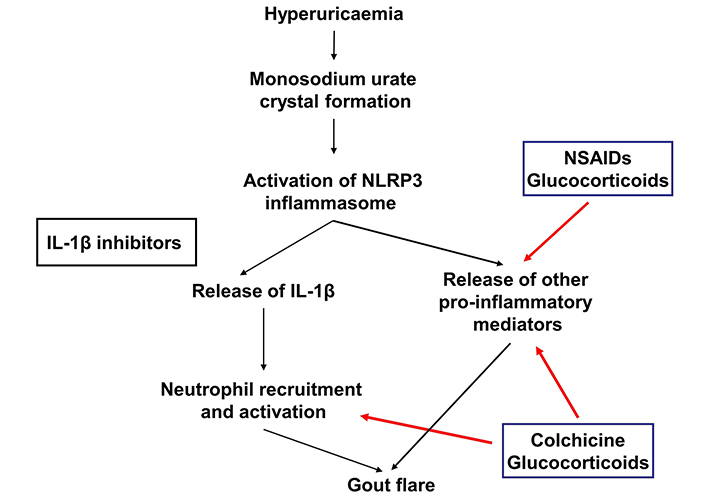
Site of action of medications used to manage gout flares. IL-1β: interleukin-1β; NSAIDs: non-steroidal anti-inflammatory drugs. Red arrow indicates site of action
A previous systematic review which included 33 studies that analysed the efficacy and/or safety of treatments for gout flares stratified according to renal function concluded that there was a lack of evidence to support evidence-based approaches to manage gout flares in people with gout and advanced CKD [18]. The following section will summarize what is known from the literature as well as expert recommendations for the management of gout flares in people with CKD.
Colchicine has been shown to be efficacious when used for the treatment of gout flares as well as for flare prophylaxis. The AGREE study reported that low dose colchicine (1.2 mg followed by 0.6 mg 1 hour later), had similar efficacy compared to a high dose strategy (1.2 mg followed by 0.6 mg every hour for 6 hours) for the treatment of gout flares, with less adverse effects. This study excluded individuals with creatinine clearance (CrCL) < 60 mls/min according to the Cockroft-Gault formula [19]. A recent prospective study of 62 prescriptions for colchicine for crystal-induced arthritis (58 gout flares, one case of calcium pyrophosphate deposition disease, and three cases of combined gout and calcium pyrophosphate deposition disease) in 54 individuals with CKD (stage G4, eGFR 15–30 mls/min/1.73 m2, stage G5, eGFR < 15 mls/min/1.73 m2, or dialysis) reported that low dose colchicine (≤ 1 mg daily) was effective and no serious adverse effects were observed [20]. Colchicine has also been demonstrated to be of benefit for flare prophylaxis when initiating urate-lowering therapy in individuals with gout and an eGFR ≥ 30 mls/min/1.73 m2 [21]. Whether colchicine can reduce the progression of CKD has been the subject of recent study. Colchicine was associated with a lower risk of progression of kidney disease in a nested case control study including individuals with eGFR 15–59 mls/min/1.73 m2, and gout or hyperuricemia, who received treatments that included allopurinol, febuxostat, and colchicine. Whether colchicine itself leads to better renal outcomes cannot be definitively established from this study, and additional studies for example randomized controlled trials are needed to further evaluate this question [22].
Colchicine has a narrow therapeutic index and its clearance is influenced by renal and hepatic function as well as drug interactions. Overall systemic exposure following a single dose of 0.6 mg of colchicine is doubled in individuals with severe renal impairment (eGFR 15–29 mls/min/1.73 m2) compared to those with normal renal function [23]. Cytochrome P450 3A4 and p-glycoprotein are involved in the metabolism and elimination of colchicine, and medications that inhibit these increase colchicine exposure [24]. Therefore, dose reduction is recommended in individuals with impaired renal function or receiving concomitant cytochrome P450 3A4, and p-glycoprotein inhibitors (Table 1).
Recommended dosage of colchicine according to different levels of renal function [25]
| Degree of renal impairment | Colchicine dose |
|---|---|
| Gout flare treatment | |
| Mild impairment (CrCL 50 to 80 mls/min) | 1.2 mg at the first sign of flare followed by 0.6 mg one hour later. |
| Moderate impairment (CrCL 30 to 50 mls/min) | 1.2 mg at the first sign of flare followed by 0.6 mg one hour later. |
| Severe | No adjustment required, but treatment course should be not be repeated more than once every 2 weeks. |
| Dialysis | 0.6 mg as a single dose. Treatment course should not be repeated more than once every 2 weeks. |
| Gout flare prophylaxis | |
| Mild impairment (CrCL 50 to 80 mls/min) | 0.6 mg once or twice daily, max 1.2 mg/day. |
| Moderate impairment (CrCL 30 to 50 mls/min) | 0.6 mg once or twice daily, max 1.2 mg/day. |
| Severe | 0.3 mg/day. |
| Dialysis | 0.3 mg twice per week. |
Additional information: (1) following gout flare treatment with colchicine, wait 12 hours and then resume prophylactic dose; (2) patients with renal or hepatic impairment should not be given colchicine in conjunction with strong CYP3A4 or P-gp inhibitors; (3) treatment of gout flares with colchicine is not recommended in patients with renal impairment who are receiving colchicine for prophylaxis. CrCL: creatinine clearance; P-gp: p-glycoprotein
NSAIDs have shown benefit over placebo for management of gout flares, and similar efficacy has been demonstrated for non-selective NSAIDs vs. selective coxibs, and NSAIDs vs. steroids [26]. Naproxen demonstrated similar efficacy to colchicine for the treatment of gout flares in an open label randomized trial [27].
Although NSAIDs are effective for managing gout flares, their potential to adversely affect renal function is well established. Of particular concern, reduced renal function increases the risk of NSAID-induced acute kidney injury [28]. There is also often a concern in clinical practice that NSAIDs may lead to the progression of underlying CKD. A systematic review of seven observational studies found that while overall NSAIDs use did not increase the risk of accelerated CKD progression, high dose NSAIDs did, although there was no clear definition for what constitutes high [29]. A prospective cohort study including 4,101 people with rheumatoid arthritis registered in the Swiss clinical quality management database evaluated long-term decline in renal function associated with NSAIDs. No deterioration in renal function in NSAID users compared to NSAID naive, among those with eGFR > 30 mls/min/1.73 m2 was identified. However, in people with an eGFR < 30 mls/min/1.73 m2, the decline in renal function was faster in those who used NSAIDs [30].
It is also important to note that the risk of adverse renal outcomes associated with NSAID use in people with pre-existing renal impairment is influenced by a number of factors including comorbidities and other medications, and cautious use of NSAIDs may be considered in selected patients with appropriate monitoring [31]. Specialist societies recommend avoidance of NSAIDs in individuals with severe CKD (eGFR < 30 mls/min/1.73 m2), or those with moderate CKD (eGFR 30–59 mls/min/1.73 m2) taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or diuretics [32]. There is a lack of evidence to guide clinicians regarding the safety of NSAIDs in people with end stage renal disease (ESRD), especially those receiving dialysis however some experts also suggest cautious use of NSAIDs in some people may be appropriate [33].
Oral corticosteroids have been shown to have similar efficacy to NSAIDs for the management of gout flares [34, 35]. Intra-articular, intravenous, and intramuscular corticosteroids have also been shown to be effective [36–38]. Parenteral glucocorticoids are typically recommended over ACTH or IL-1 inhibitors in those unable to take oral medications [16]. Glucocorticoids don’t adversely affect kidney function directly but may lead to adverse effects such as hypertension and hyperglycemia which are risk factors for kidney disease [39, 40]. Glucocorticoids are therefore a useful option when colchicine and NSAIDs are contraindicated and other treatments are not available.
The beneficial effect of ACTH for the treatment of gout flares has been demonstrated in clinical trials, case-series, and case reports; however large randomized controlled trials are necessary to fully determine efficacy and safety [41, 42]. A retrospective study of 181 individuals with gout which included 63 individuals with CKD stages 3, 4, or 5, reported that 77.9% responded to 1 mg synthetic ACTH given intramuscularly. The majority of those who didn’t respond did respond to a second dose. Neither efficacy nor adverse effects were reported according to renal function. Among diabetics an increase in fasting glucose 24 hours post-treatment was observed. Other adverse events included a small number of local skin reactions, a case of flushing, oedema, headache, dizziness, and tachycardia/palpitations [43].
IL-1 inhibitors include canakinumab, rilonacept, and anakinra. There is insufficient evidence regarding these treatments in the context of renal impairment for conclusions about efficacy and safety to be made. Major trials of canakinumab and rilonacept excluded individuals with more severe degrees of renal impairment [18]. Anakinra is predominantly eliminated via renal excretion, and plasma clearance is reduced 50% in people with moderate renal impairment (CrCL 30–49 mls/min), 70% in severe renal impairment (CrCL < 30 mls/min), and 75% in people with ESRD. A reduced dosing frequency to 100 mg every second day rather than daily may therefore be appropriate in people with severe renal impairment or ESRD [44]. A retrospective study including 25 people with CKD stage 4 or 5, and six people with renal transplant, reported that anakinra was effective in all individuals, and did not cause significant changes in renal function. One infection occurred three months following treatment. Most individuals received 100 mg daily. Five individuals received anakinra every 48 or 72 hours and four of these were on haemodialysis and received anakinra on days when dialysis did not occur [45].
Long-term lowering of SU is key to the long-term management of gout. With sustained urate reduction below the recommended target of 0.36 mmol/L (6 mg/dL) gout flares and tophi will resolve [46], albeit with time. Urate-lowering therapies target three central pathways: (1) inhibition of urate production [xanthine oxidase inhibitors (XOI): allopurinol and febuxostat], (2) increased renal excretion of urate (uricosurics: probenecid and benzbromarone), and (3) metabolism of UA to the more water-soluble allantoin (recombinant uricase: pegloticase) (Figure 2).
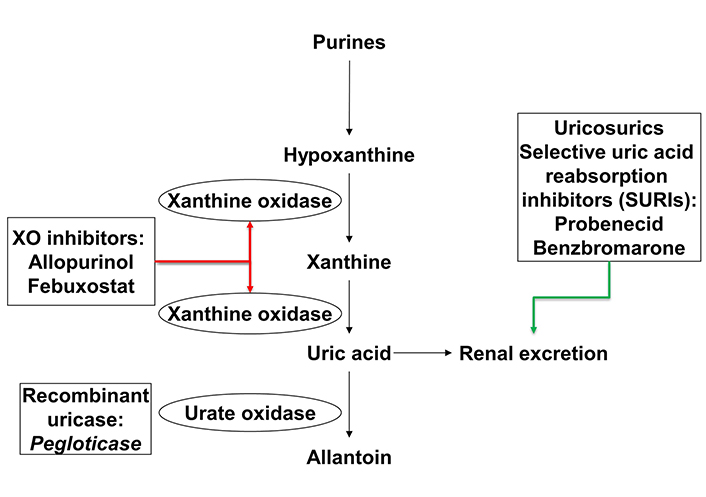
Site of action of urate-lowering therapies. XO: xanthine oxidase. Red arrow indicates inhibition and green arrow indicates and increase
Both the ACR and EULAR gout guidelines recommend allopurinol as the first line urate-lowering therapy, including in those with renal impairment [16, 17]. Switching to febuxostat or adding a uricosuric agent is considered second line while pegloticase is reserved for those individuals who do not tolerate or fail to achieve treatment targets with a XOI or uricosuric. We will discuss the evidence for the efficacy and safety of each of these agents in people with gout and CKD.
The use of allopurinol in people with gout and CKD sadly remains controversial due to concerns over the increased risk of the rare but serious allopurinol hypersensitivity syndrome (AHS) or other serious cutaneous adverse reactions (SCARs) including Steven’s Johnson Syndrome and toxic epidermal necrolysis. These concerns were initially based on a small case series and literature review which highlighted that “the development of this syndrome was associated with the use of standard (200 to 400 mg per day) doses of allopurinol in patients with renal insufficiency” [47]. The authors went on to recommend “Avoidance of allopurinol or use of reduced doses in patients with renal insufficiency according to proposed (dosing) guidelines should be adequate to inhibit uric acid production in most patients and may reduce the incidence of life-threatening allopurinol toxicity”. However, it is important to note that two of the six cases in this series were receiving allopurinol for asymptomatic hyperuricaemia and all but one started on doses above 100 mg daily.
When considering the risk of AHS, it is important to note that it is the starting dose of allopurinol in relation to renal function, not the maintenance dose i.e., the dose required to achieve target SU, that has been associated with AHS [48, 49]. Based on these data a maximum allopurinol starting dose of 100 mg daily is recommended with a lower dose in those with CKD [16, 17]. There is little doubt that the restrictive doses proposed by Hande et al. [47] (Table 2) result in failure to achieve target SU in the majority [50]. However, in those who tolerate allopurinol, monthly dose escalation to achieve target SU has been shown to be safe and effective [51, 52]. In a post hoc analysis, of this dose escalation trial there was no difference in the percentage of participants achieving target SU based on renal function: CrCL < 30 mls/min 64.3%, CrCL ≥ 30 to < 60 mls/min 76.4%, and CrCL ≥ 60 mls/min 75.0% (p = 0.65) [53]. Interestingly the mean allopurinol dose was significantly lower in those with CrCL < 30 mls/min as compared to those with CrCL ≥ 30 to < 60 mls/min or CrCL ≥ 60 mls/min [mean (standard deviation (SD))]: 250 (43), 365 (22), and 460 (19) mg/day, respectively (p < 0.001)]. Finally, the majority of those who do not achieve target SU on CrCL-adjusted doses of allopurinol require an increase of ≤ 200 mg daily to achieve target SU [54]. It is also important to note that a large population-based cohort study reported that neither allopurinol initiation, nor achieving target SU with allopurinol, nor allopurinol dose escalation was associated with increased mortality in people with gout and concurrent CKD [55].
Allopurinol dosing
| eGFR mls/min/1.73 m2 | Allopurinol starting dose [48] | Maintenance (maximum) allopurinol dose recommendations | CrCL mls/min | Maintenance of allopurinol dose according to Hande et al. [47] | |
|---|---|---|---|---|---|
| ACR 2020 gout guideline [16] | EULAR 2016 gout guideline [17] | ||||
| < 5 | 50 mg/week | Maximum dose 800 mg daily including in those with moderate to severe CKD (stage ≥ 3) | Maximum dose should be adjusted to creatinine clearance. Because the dose recommendations in renal disease may slightly differ across countries, the task force recommends following the local summary of product characteristics. | 0 | 100 mg every three days |
| 5–15 | 50 mg twice weekly | 10 | 100 mg every two days | ||
| 16–30 | 50 mg every two days | 20 | 100 mg daily | ||
| 31–45 | 50 mg daily | 40 | 150 mg daily | ||
| 46–60 | 50/100 mg alternate days | 60 | 200 mg daily | ||
| > 60 | 100 mg daily | 80 | 250 mg daily | ||
| - | - | 100 | 300 mg daily | ||
| - | - | 120 | 350 mg daily | ||
| - | - | 140 | 400 mg daily | ||
eGFR: estimated glomerular filtration rate; ACR: American College of Rheumatology; EULAR: European Alliance of Associations for Rheumatology; CrCL: creatinine clearance; CKD: chronic kidney disease. -: no data
In individuals with ESRD receiving dialysis about 25% will have gout [56]. While haemodialysis can clear urate, it may not be sufficient to reduce SU to below the treatment target [57]. In a series of six individuals with gout on hemodialysis the median [interquartile range (IQR)] SU immediately prior to dialysis was 5.9 mg/dL (4.7–8.9 mg/dL). Serum urate dropped precipitously during dialysis and was back to baseline or within 80% of baseline by 42 hours after dialysis [58] suggesting SU should be measured pre-dialysis. However, in another study SU dropped below the point of saturation with hemodialysis and remained low; however, in this study, not all individuals were hyperuricaemic or had gout [59]. Oxypurinol, the active metabolite of allopurinol, is also efficiently removed by dialysis and allopurinol dosing pre-dialysis results in about a 25–35 % reduction in exposure compared to post-dialysis [60]. Thus, for individuals on haemodialysis, allopurinol should be administered post-dialysis and if low doses do not lead to achievement of target SU the dose should be gradually increased.
Febuxostat is a XOI approved by the European Medicines Agency in 2008 and the Federal Drug Administration in 2009 for the management of hyperuricemia in people with gout. Febuxostat is conjugated in the liver and is not dependent on renal function for excretion. Thus, dose reduction is not considered necessary for those with mild to moderate renal impairment.
Virtually all the phase III clinical trials undertaken for regulatory approval excluded individuals with CrCL < 50 or < 30 mls/min [61]. However, more recent studies have been reassuring. In a three-month, phase III, multicenter, double-blind, placebo-controlled study, 1,790 people with a history of gout and normal or mild-to-severely impaired renal function were randomized to receive placebo, febuxostat immediate release (IR) 40 or 80 mg daily, or febuxostat extended release (XR) 40 or 80 mg once daily. Participants were required to have an eGFR ≥ 15 mls/min/1.73 m2 at screening and the protocol pre-specified that at least 30% of participants would have an eGFR ≥ 15–59 mls/min/1.73 m2, with ≥ 85% of these an eGFR ≥ 15–29 mls/min/1.73 m2. Importantly both the IR and XR formulations were well tolerated and effective in participants with mild-to-severe renal impairment [62]. Observational data has also been reassuring. In a retrospective review of 370 people with gout of whom 63 had CKD stage 4–5 CKD (eGFR < 30 mls/min/1.73 m2), but were not yet on dialysis, there were significant reductions in SU after 12 months of febuxostat therapy with no difference in adverse events compared to those with CKD stage 1–3 [63]. Similar to the observation that people with CrCL < 30 mls/min require lower doses of allopurinol to achieve target urate, Kim et al. [64] reported that febuxostat dose requirement is lower in those with CKD stage 4–5 compared to CKD stage 3 [50.0 (16.5) mg daily vs. 60 (19.5) respectively p < 0.01]. A further systematic review and meta-analysis of observational studies of the use of febuxostat in people with gout and CKD stage 4–5 but not receiving dialysis concluded that febuxostat was safe and effective in lowering urate [65].
There is relatively little data on the efficacy and safety of febuxostat in people with gout on dialysis. In a small retrospective study of 63 people with gout on dialysis (45 haemodialysis and 17 peritoneal dialysis), 87.1% achieved target SU < 0.36 mmol/L after receiving febuxostat for three months. The febuxostat dose ranged from 20–80 mg daily with the majority (75.8%) receiving 40 mg daily [66].
Probenecid may be used as monotherapy in those who cannot tolerate a XOI or in combination with an XOI in those who fail to achieve target urate. Probenecid increases renal urate excretion by inhibiting renal reabsorption by the urate anion transporter (URAT) 1. Probenecid is less effective in those with renal impairment [67]. However, a small retrospective study reported that in people with gout and an eGFR between 30 and 50 mls/min/1.73 m2 probenecid can be effective in achieving target urate levels [68].
Probenecid may also be used in combination with allopurinol [69]. Of note allopurinol/probenecid combination therapy results in lower oxypurinol concentrations but a greater urate-lowering effect than when each drug is used alone although the urate-lowering effect is less in those with eGFR < 50 mls/min/1.73 m2 [70, 71].
There is minimal data on the combination of febuxostat and probenecid in people with renal impairment. In a single case report the combination of febuxostat 80 mg daily and probenecid 500 mg twice daily was well tolerated and effective in achieving target SU in a 33-year-old man with an eGFR of 37 mls/min/1.73 m2 [72].
Although not widely available the uricosuric benzbromarone has the advantage that it is effective in people with renal impairment. The limited availability is matched by limited data about its efficacy and safety. Studies have shown that benzbromarone is effective even in those with an eGFR as low as 20–25 mls/min/1.73 m2 [73, 74]. While benzbromarone has been withdrawn in many countries due to concerns about potentially fatal hepatotoxicity, the risk of this is low and it can be useful as monotherapy or in combination with a XOI. Monotherapy doses of benzbromarone of 50–100 mg daily are effective at achieving target SU and may be more effective than allopurinol (300 mg daily) in people with gout who are renal under-excretors of UA [75]. Low dose benzbromarone (25 mg daily) has also been reported to be more effective than low dose febuxostat (20 mg daily) in people with gout who are renal under-excretors of UA [76].
Benzbromarone is also effective in combination with a XOI. In a small observational study, allopurinol also reduced SU levels to within the normal range (defined as < 0.38 mmol/L in men and < 0.33 mmol/L in women in 51.8%, benzbromarone alone in 75%, and combination in 85.7% [77]. A more recent study in people with gout and normal renal function reported that benzbromarone (25 mg/day) in combination with febuxostat (20 mg/day) showed superior urate lowering compared to febuxostat monotherapy [78].
Sulphinpyrazone, an alternative uricosuric, may be effective in gout [79] but there is virtually no data on its use in people with CKD and gout. Sulphinpyrazone has been associated with acute kidney injury typically
Pegloticase, a recombinant uricase, rapidly metabolizes urate to the more water soluble allantoin which can be readily excreted through the kidneys. Pegloticase was FDA-approved for gout in 2010 and is currently considered last line therapy for those who do not achieve target urate or do not tolerate other urate-lowering therapies.
There is limited data on the use of pegloticase 8 mg every two weeks in people with gout and CKD. In a post-hoc analysis of two replicate phase III clinical trials using pegloticase 8 mg every two weeks, which included 103 (49%) people with CKD stage 3 (n = 80) or 4 (n = 23) there was no difference in the percentage of participants who had plasma UA < 6.0 mg/dL for 80% of the time during months 3 and 6 combined by CKD stage (32% CKD 1; 23% CKD 2; 35% CKD 3, and 39% CKD 4) [81]. Importantly there were no differences in adverse effect profiles when stratified by CKD stage [81].
Infusion reactions and loss of efficacy have been limiting factors with pegloticase. Both of these are thought to occur secondary to the development of anti-drug antibodies which accelerate pegloticase clearance [82, 83]. Recently attention has focused on co-administration of immunosuppressive agents in order to prevent the development of anti-drug antibodies thereby prolonging the efficacy of pegloticase therapy. Methotrexate [84, 85], mycophenolate mofetil [86], leflunomide [87], and azathioprine may all improve responder rates and/or persistence with therapy. Clinicians will need to consider the implications of CKD when co-administering with pegloticase.
An open-label phase I study examined the pharmacokinetics and pharmacodynamics of a single intravenous dose of pegloticase 8 mg 3 hours prior to hemodialysis in 12 participants with gout. No significant effect of hemodialysis on the stability of serum pegloticase concentrations, urate-lowering effect or adverse event profile was observed [88]. These data are reassuring but there are no data on people with gout receiving longer term pegloticase.
Gout may be successfully managed in people with CKD although careful consideration of the medications used and doses is required. Gout flares can best be managed with low dose colchicine or corticosteroids. There is increasing evidence that urate-lowering therapies allopurinol and febuxostat are safe and effective in people with CKD. Researchers need to ensure that people with CKD are not excluded from gout trials given the frequency of the two conditions presenting in the same individual and studies should have pre-specified analysis plans based on CKD stage.
ACTH: adrenocorticotrophic hormone
AHS: allopurinol hypersensitivity syndrome
CKD: chronic kidney disease
CrCL: creatinine clearance
eGFR: estimated glomerular filtration rate
ESRD: end stage renal disease
EULAR: European League of Associations of Rheumatology
IL-1: interleukin-1
NSAIDs: non-steroidal anti-inflammatory drugs
SU: serum urate
UA: uric acid
XOI: xanthine oxidase inhibitor
HF: Conceptualization, Writing—original draft, Writing—review & editing. LKS: Conceptualization, Writing—original draft, Writing—review & editing. AG: Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Angelo Gaffo, who is the Editorial Board Member of Exploration of Musculoskeletal Diseases, had no involvement in the journal review process of this manuscript. LKS reports funding from the Health Research Council of New Zealand and royalties from Up-to-Date outside this work. The other author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Benjamin Plotz ... Michael H. Pillinger
Naomi Schlesinger, Dan Kaufmann
Mark D. Russell, James B. Galloway
Robert T. Keenan ... Michael H. Pillinger
Robin Christensen ... Lisa K. Stamp
Edward Roddy ... Christian D. Mallen
Philip L. Riches ... Amrey Krause
Emilie Schurenberg ... Kenneth G. Saag
Enrique Calvo-Aranda ... Marta Novella-Navarro
Orsolya I. Gaal ... Tania O. Crișan
