Affiliation:
1Osakidetza, OSI-EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
2University of the Basque, Medicine and Nursery School, Medicine Department, Country Cruces Teaching Unit, 48903 Barakaldo, Spain
Email: fernando.perezruiz@osakidetza.eus
ORCID: https://orcid.org/0000-0002-5268-1894
Affiliation:
1Osakidetza, OSI-EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
Affiliation:
1Osakidetza, OSI-EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
Affiliation:
1Osakidetza, OSI-EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
Affiliation:
1Osakidetza, OSI-EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
Affiliation:
1Osakidetza, OSI-EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
Affiliation:
1Osakidetza, OSI-EEC, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
ORCID: https://orcid.org/0000-0002-4983-5506
Affiliation:
3Cruces University Hospital, Pharmacy Division, 48903 Barakaldo, Spain
ORCID: https://orcid.org/0000-0003-0894-9539
Explor Musculoskeletal Dis. 2024;2:384–390 DOI: https://doi.org/10.37349/emd.2024.00064
Received: June 05, 2024 Accepted: July 05, 2024 Published: September 10, 2024
Academic Editor: Valderilio Feijó Azevedo, Federal University of Paraná, Brazil
The article belongs to the special issue Biosimilars: State of the Art in the Treatment of Rheumatic Diseases
Aim: To evaluate the impact of prescription, cost, and switching policy on the rate of switching from reference products to biosimilars.
Methods: Analysis of an administrative database for prescription in a rheumatology division. Biosimilars for adalimumab and etanercept were available in 2019. Blinded costs and prescription data were not shared with prescribing physicians until 2021. The rate of prescription, persistence of therapy after switching, and reduction of cost were analyzed from 2019 to 2022. A new etanercept biosimilar was prioritized in 2022, and a new switching wave from biosimilar to biosimilar etanercept was implemented.
Results: Overall switching from 2019 to 2022 comprised 132/135 (97.8%) of patients. The rate of switching increased from 13.3% to 34%, 79%, and 95.5% of patients on reference products during 2019, 2020, 2021, and 2022, respectively. In 2022, after sharing information, the switch comprised 55/135 (40.7%) of overall switching. The rate of persistence on therapy after switching was 86.8% for etanercept and 79.7 for adalimumab. During 2023, a rate of 76.6% switching etanercept reference-biosimilar-biosimilar was achieved. The reduction in the overall biologic budget in 2021 was 19.2% and 29.0% for the patient-year cost.
Conclusions: Information to prescribers may improve switching policies. Persistence on biosimilar medications after switching is as high as previously reported.
The development of biologic medications changed the landscape of the treatment of chronic inflammatory diseases, and especially those involving the musculoskeletal system, but high pricing was considered initially to be a major limiting condition for prescription [1]. The approval of the first infliximab biosimilar some years afterward opened the way to reducing costs and increasing the availability of treatment options and contributing to the sustainability of healthcare systems [2]. Improvement in life expectancy and quality of life of patients with chronic inflammatory rheumatic diseases has been considered to improve supposedly by the accessibility to biologics [3].
In 2019 biosimilars for adalimumab and etanercept became available, and they were prioritized by the Health Department of the Basque Country for initial prescription. In our country, where biologics and all hospital-based prescriptions are facilitated at a null cost for the patient, the reduction of costs is of extreme interest in order to maintain the sustainability of the public health system [2].
Automatic substitution (interchange without prescribing physician and patient approval) is not legally permitted in our country, whereas switching, as interchange suggested by prescribing physicians and approved by patients, is permitted. Therefore, a program to promote a positive attitude of prescribers for switching among prescribing rheumatologists started in 2019 in our rheumatology division.
We used an administrative database of prescriptions of biologics created and regularly updated by the actual chief of the rheumatology division. Prescription data and withdrawals were checked with data held by the pharmacy division. The rheumatology division attends 380,000 reference population. All patients on hospital-pharmacy medications are visited at the university hospital office after clinical evaluation. The database includes prescription data (active medication and dose, previous and current medications), clinical diagnosis for prescription, date of diagnosis, date of active prescription, cause for stopping medications if applicable, and positivity of anti-drug antibodies if tested. No clinical data are included in the database, except for age and gender, and personal data are concealed. The period of study ranged from Jan 2019 to December 2023.
During the period 2019–2020 prescribing rheumatologists were not given information on expense, practices, or policy on biologics. In contrast, information blinded for prescribers regarding the rate of switching, and overall excess of expense due to non-switching during 2019 and 2020 was shared yearly by the chief of division during the periods 2021–2022 and 2022–2023 in order to enhance further commitment to switching. Prescribing physicians were informed that neither they nor the division would receive any benefit either from the pharma industry or from the public health administration for the switching from the reference product to biosimilar or from biosimilar to biosimilar. Patients were given information for switching orally by the prescribing physician, who is always the same physician on any visit for a given patient.
The use of administrative data for scientific purposes, including dissemination of results, was fully approved by the Ethics Committee Board of OSI EEC (CEIC-24/15). The present analysis of data was restricted to patients with an active prescription of biologics susceptible to be switched. Oral consent for switching was obtained from all patients, and annotated in the electronic file.
The analysis was also restricted to anti-tumor necrosis factor (anti-TNF) medications administered subcutaneously that had a biosimilar alternative available. Adalimumab reference product was Humira (Abbvie). From 2019 to date adalimumab biosimilar Imraldi (Samsung Bioepis) was prioritized by the Basque Country Health Department. Etanercept reference product was Embrel (Pfizer). The prioritized etanercept biosimilar in 2019 was Erelci (Sandoz Pharmaceutical). In late 2022, the prioritized etanercept biosimilar changed from Erelci to Benepali (Samsung Bioepis), and a new, still ongoing, wave of interchange was enhanced during 2023 for patients receiving the previously prioritized etanercept biosimilar.
The optimal moment for interchange was suggested for patients fulfilling the following criteria: (1) good clinical control, defined as low disease activity or remission status, for at least a 6-month period; (2) at least 1 year from initiation of biologic reference product; (3) steady dose for the previous 6-month period. Low disease activity was evaluated using Disease Activity Score with 28 joint (DAS28) for peripheral arthritis, and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for axial involvement.
In those patients developing moderate to severe disease after switching from the adalimumab reference product, plasma adalimumab levels were measured following usual clinical practice in the division, and anti-adalimumab neutralizing antibodies were automatically tested by the laboratory when adalimumab levels were below the normal range.
Reduction of cost per year and patient due to switching was evaluated for the period 2020-2021 according to the data facilitated by the economic department of our institution.
Continuous variables are shown as mean ± standard deviation (median, interquartile range). Analysis was made using an institutional license of IBM SPSS V29.0. Means were compared using T-test and Wilcoxon’s corrections if applicable, and proportions were compared using chi-square test, with Fisher’s corrections if applicable.
One hundred and thirty-five patients were on reference product etanercept or reference product adalimumab in early 2019 and therefore were targeted for switching: all adults, 62 women and 73 men, mean age 60 ± 12 years (60, 50–78). The diagnosis was rheumatoid arthritis (63 patients), psoriatic arthritis (31 patients), spondylarthritis, including psoriatic arthritis, ankylosing spondylitis, and non-radiologic axial spondylarthritis (38 patients), and out of age juvenile idiopathic arthritis (3 patients).
Overall, over ninety-five percent (132/135, 97.8%) of patients accepted switching from reference biologic medication to biosimilar from 2019 to 2022. Switching was accomplished in 53 patients receiving Embrel and 79 patients receiving Humira. The mean time on reference biologic product medication from initiation to switching to biosimilar was 4.4 ± 1.2 years (4.0, 2–8). The rate of switching increased through the years: 18/135 (13.3%) in 2019, 41/120 (34.2%) in 2020, 55/69 (79.7%) in the year 2021, and 21/22 (95.5%) in the year 2022. Figure 1 shows graphically the impact of sharing policy, practice, and cost data on the rate of switching from 2019 to 2022, amounting to 40.7% of overall switching in just one year period, 2021.
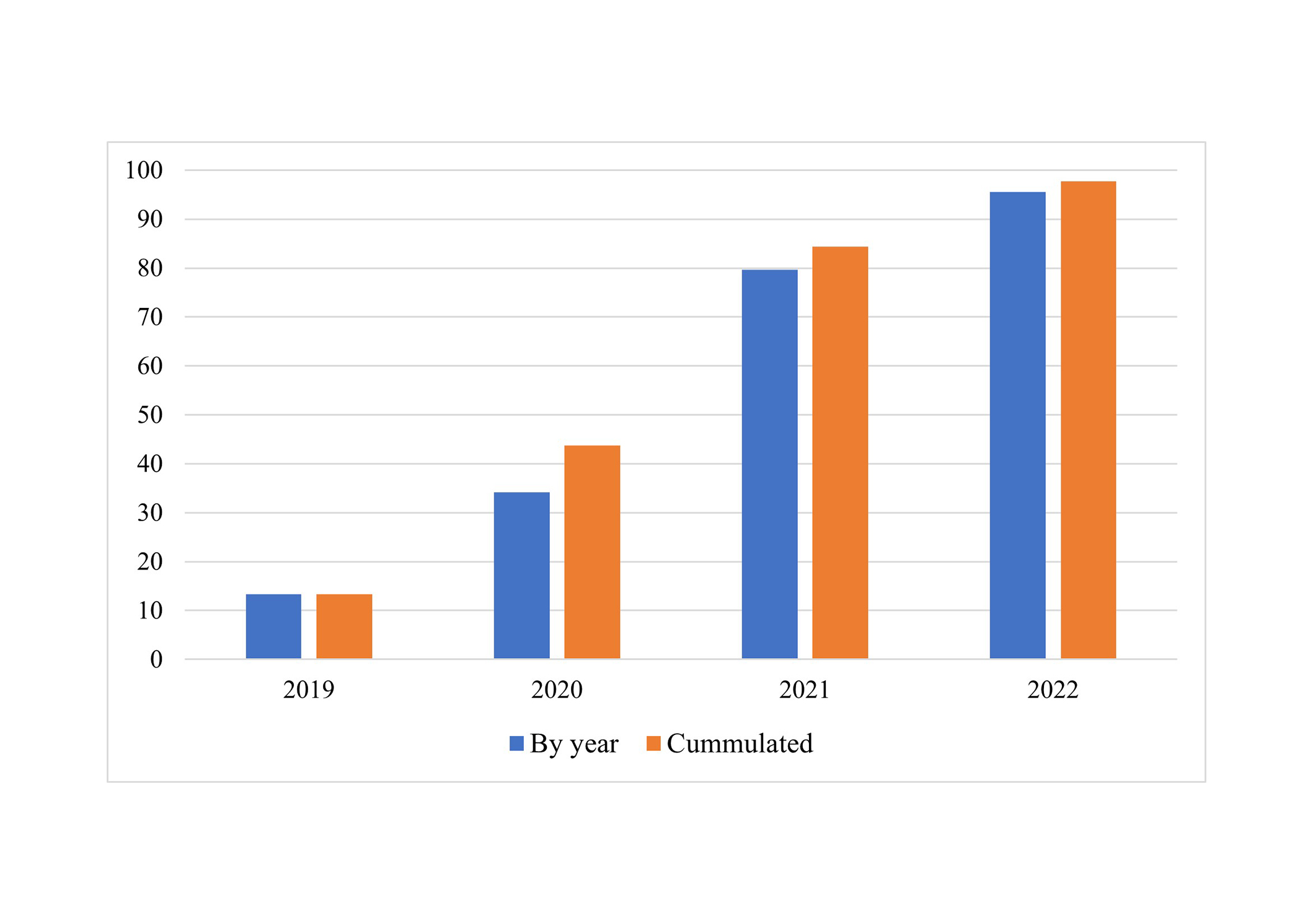
Percentage (%) of switching by year (number of patients switched/patients targeted for switching) and cumulated percentage (%) of patients switched (cumulated number of patients switched/total number of patients targeted for switching)
Persistence on biosimilar from switching to the last observation, restricted to December 2022 for fair exposure comparison. It was overall 109/132 (82.6%) showed no difference between medications: 46/53 (86.8%) for etanercept, and 63/79 (79.7%) for adalimumab (P = 0.29) (Figure 2).
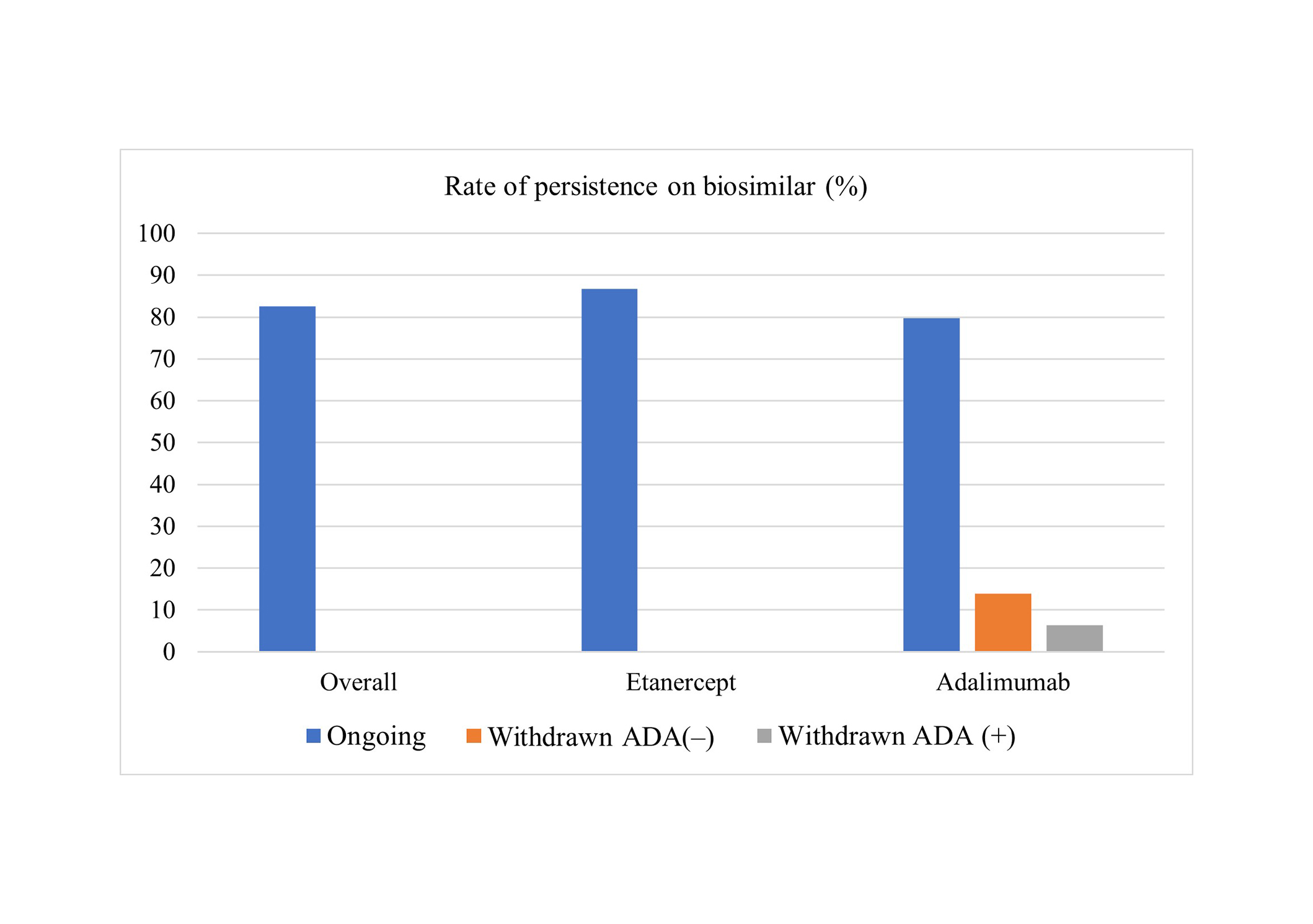
Overall and per medication rate of persistence on biosimilar after switching. Rates of cases with positive anti-adalimumab antibodies (ADA+) and negative anti-adalimumab antibodies (ADA–) are also shown
Anti-adalimumab neutralizing antibodies were detected in 5/16 (31.3%) of patients losing remission or low-disease activity status after switching from reference adalimumab to biosimilar adalimumab (Figure 2). The mean time on reference adalimumab to switching was lower for patients who afterward showed anti-adalimumab antibodies compared to those who did not (1.2 ± 0.6 vs. 3.4 ± 1.3 years, P < 0.01).
During the second wave of switching during 2023 from etanercept biosimilar Erelci to etanercept biosimilar Benepali, 36/47 (76.6%) of patients who met the criteria for switching accepted switching, 34/36 (94.4%) of patients being still on therapy with the second biosimilar during the year 2023. Embrel-Erelci-Benepali (reference-biosimilar1-to-biosimilar2) switching was accomplished in 25 patients, where Erelci-Benepali (biosimilar1-to-biosimilar2) was accomplished in 11 patients who were on biosimilar etanercept Benepali after showing a previous good clinical response to either to adalimumab reference product or adalimumab biosimilar but afterward showing secondary failure because of the development of anti-adalimumab antibodies.
The reduction of cost derived from switching from adalimumab and etanercept reference to biosimilars during 2021 compared to 2020 (change in cost data-sharing policy), was 272,724 euro globally, and 3,050 euro per patient-year. It represented a reduction of 19.2% in global cost and 29.0% in patient-year cost, respectively, of the overall ambulatory hospital-prescribed medication of the rheumatology division.
Biosimilars are biologic medications highly similar in composition, structure, pharmacodynamics, pharmacokinetics, and immunogenicity to a reference originator medication [4, 5].
Interchangeability is the ability to exchange from a biologic, either reference or biosimilar, to another biologic, either biosimilar or reference, based on demonstrated high similarity, and is supported by the World Health Organization (WHO) [4] and regulators as the European Medicine Agency (EMA) [5].
In 2022, WHO further considered that “a comparative efficacy trial may not be necessary if sufficient evidence of biosimilarity can be inferred from other parts of the comparability exercise clinical” [4]. In 2023, further support for the rational of interchangeability was provided by EMA [6].
Benefits of interchange are not restricted to efficiency or cost-saving policies [2], but also centered in increased and early accessibility of any biological medication, and sustainability of health systems [7], and especially for those that benefit the entire population at no cost for the patient, as it is in our country.
Educational interventions on prescribing physicians have been included, among several policies, as mechanisms to increase biosimilar prescription [8]. Our data support that informational interventions, such as sharing cost data with prescribing physicians, may increase switching uptake. Awareness of the incremental cost of maintaining reference products at a higher cost is the most plausible explanation for the increase in the rate of switching comparing 2019–2020 to 2021–2022 periods, although other factors cannot be excluded.
On the contrary, despite increasing scientific evidence on the interchangeability of biologics and specifically supported by WHO and EMA [4–6], the perception of lower cost may infer a potential negative expectation that may lead to increased nocebo effect [9]. Adequate patient information, especially on efficacy, would be associated with patients’ satisfaction using biosimilars [10]. The rate of interchange in our division was extremely high, surpassing 95%. We explain this figure based on: (1) a careful selection of the time for switching; (2) adequate information to patients on safety, efficacy, and then the impact on availability and sustainability; (3) last, but not least, personal implication on the prescribing policy of the division’s physicians.
To this point, we consider it important to emphasize that prescribing physicians were told in advance that no benefit would be derived from the switching policy for either the staff or the division. Incentivization from payers to stakeholders and providers has been considered in the landscape of biosimilars future [11]. Some health organizations have included economic incentives for switching from reference products to biosimilars [12]. Results of such incentivization have been shown to be positive [12], but not to the striking extent of the rate of switching we reached. We have not found similar experiences on the impact of the information of costs reported in the literature, but even economic incentives had a lesser impact, with only a 9.71-point increase [12].
The impact of switching accounted for a substantial reduction in the expense of hospital-based medications for chronic arthritis in the year analyzed, the first one after sharing cost data, and has been maintained during a second wave of switching for etanercept. The savings were observed for the overall and per-patient costs.
There are also studies in our country on the economic impact of prioritizing biosimilars for patients naïve to biologic medications [13, 14] but to our knowledge none on massive switching. Indeed, no such prescriber-induced voluntary massive switching has ever been reported, and it was initially a trend for the prescription of products without available biosimilars [14].
We also show that the rate of withdrawal after switching is relatively small for both etanercept and adalimumab, as expected by the amount of evidence. The presence of neutralizing antibodies explained one-third of the cases showing a lack of clinical effect. To this point, and at least for immunogenic products such as adalimumab, the early switch should be avoided, especially anti-drug antibody testing is not at hand.
The strengths of the study include that it constitutes a prospective one, and the intervention targets were prescribing physicians. Nevertheless, it is not free of limitations: adalimumab levels and anti-adalimumab antibodies were not systematically tested prior to switch, as has been reported in a recent interchange trial [15], but the rate of withdrawal after switching is similar to that reported by other. It is speculative whether it could have detected previous development of anti-adalimumab antibodies while on reference product, but the shorter time on reference product for patients developing antibodies compared to those who did not may suggest so. In addition, as an administrative database for the Head of Division the database lacks clinical or patient-reported outcomes. We had to assume that no clinician would maintain patients on a prescription if they show no proper control of disease, and that failure to properly control clinical activity would enhance a change in therapy.
Also, we cannot exclude that the SARS-Cov2 pandemic environment may have pushed social awareness of increasing costs in the mind of prescribers, therefore supporting the policy of switching. In addition, successful switching through time may have also promoted the rate of switching and induced a bias for better results in the long-term. Finally, we did not retrieve whether patients received information about switching, for better or for worse, from another source.
In conclusions, massive switching from etanercept and adalimumab reference products to biosimilars was possible after sharing cost data, without any meaningful impact on survival on therapy. We have started a second wave of switching from biosimilar to biosimilar etanercept with similar results, and will favor future waves for new coming biosimilars.
EMA: European Medicine Agency
WHO: World Health Organization
The authors thank the positive and comprehensive attitude of most of our patients to switching policy. We also thank all the staff at the hospital Pharmacy Division who helped us through.
FPR: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Funding acquisition. JCA and ABG: Investigation, Validation, Writing—original draft, Writing—review & editing. EGS, JAL, JDE, JASB, MNRZ, and MCMC: Investigation, Writing—original draft, Writing—review & editing. All authors read and approved the final submitted version.
Fernando Perez-Ruiz is a member of the Pharmacy Committee of OSI EEC, member of the Corporative Pharmacy Commission of the Basque Health Service (Osakidetza), and has served as advisor for biosimilars to Amgen and Fresenius-Kabi. Fernando Perez-Ruiz is the Editor-in-Chief of Exploration of Musculoskeletal Diseases, but he had no involvement in the journal review process of this manuscript. The other authors have no conflicts of interest.
The results of the analysis of this administrative database were approved to be released by the Ethics Committee of OSI EEC (CEIC-24/15).
Not applicable.
Not applicable.
The raw data can be shared by authors after application and ethics committee approval.
This study was granted by Cruces Rheumatology Association [R1/2021].
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gilberto Castañeda-Hernández
Fernando Pérez-Ruiz ... Eugenio Chamizo Carmona
Fanny Alcira Reyes Neira ... Andrea Yukie Shimabuco
Leticia A. Shea, Jamshaid S. Ahmed
Lauren N. McGrath ... Steven R. Feldman
