Affiliation:
1Schwerpunktpraxis für Rheumatologie und Gastroenterologie, 80639 München, Germany
2Krankenhaus Neuwittelsbach, 80639 München, Germany
Email: marialvoulgari@gmail.com
Affiliation:
1Schwerpunktpraxis für Rheumatologie und Gastroenterologie, 80639 München, Germany
2Krankenhaus Neuwittelsbach, 80639 München, Germany
Email: hk@prof-dr-kellner.de
Explor Musculoskeletal Dis. 2024;2:443–460 DOI: https://doi.org/10.37349/emd.2024.00069
Received: March 01, 2024 Accepted: August 22, 2024 Published: September 25, 2024
Academic Editor: Cesar Diaz-Torne, Autonomous University of Barcelona, Spain
The article belongs to the special issue Calcium Pyrophosphate Deposition Disease
Calcium pyrophosphate deposition disease (CPPD), characterized by the presence of calcium pyrophosphate crystals in and around joints, poses diagnostic and therapeutic challenges in rheumatology. This review provides a comprehensive overview of CPPD, focusing on its diagnosis, differential diagnosis, therapeutic challenges, and monitoring, with insights into the association between CPPD and cardiovascular risk. Diagnostics in CPPD rely on identifying CPP crystals in synovial fluid or joint tissues, with imaging modalities such as ultrasound and conventional radiography emerging as valuable tools. The 2023 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria prioritize imaging evidence of CPP crystal deposition and recurrent episodes of acute inflammatory arthritis, aiding in standardized diagnosis. Differential diagnosis includes distinguishing CPPD from gout, osteoarthritis, rheumatoid arthritis, basic calcium phosphate deposition disease, and other inflammatory arthropathies. Therapeutic challenges in CPPD management revolve around symptomatic relief, with no targeted therapy to influence CPP deposition currently available. Management strategies include symptom-directed treatments like NSAIDs, steroids and colchicine. IL-6 inhibition with tocilizumab shows promise for refractory cases. Monitoring CPPD involves assessing joint symptoms, inflammation, and cardiovascular risk factors, with regular clinical evaluation. In conclusion, CPPD presents a complex challenge in rheumatology, requiring a nuanced approach to diagnosis and management. Ongoing research is needed to deepen our understanding of CPPD mechanisms and explore novel therapeutic avenues.
Three types of calcium (Ca)-containing crystals can be observed in and/or around joints: Ca oxalate (CaC2O4), basic Ca phosphate Ca3(PO4)2, and Ca pyrophosphate (CPP) dihydrate crystals (Ca2P2O7). This review concentrates on CPP dihydrate crystal deposition disease, now commonly termed CPP crystal deposition disease (CPPD). The European League Against Rheumatism (EULAR) has advocated for a simplified nomenclature, urging for adoption of CPP crystal as an abbreviation for CPP dihydrate crystal, with CPPD serving as the overarching term encompassing related conditions.
CPPD is a condition frequently encountered in the elderly and often associated with a locally excessive (Ca × PP) product. The mechanisms underlying CPP deposition highlight normal serum pyrophosphate P2O4−7 [pyrophosphate (PP)] levels, contrasting with elevated synovial fluid (SF) concentrations in CPPD [1].
The pathogenesis of CPPD involves the accumulation of CPP crystals in the joint, driven by elevated extracellular PP (ePPi) levels. Increased ePPi, regulated by PP transporter ankylosis human (ANKH) and ENPP1 (which upregulate ePPi) and TNAP (which downregulates ePPi), promotes CPP crystal formation. This process is influenced by factors such as aging, genetic mutations in ANKH and COL genes, and altered enzyme activity that increases inorganic PP. Histone deacetylase inhibitors (HDACis) like TSA and SAHA can reduce CPP formation by modulating ANKH, ENPP1, and TNAP expressions, presenting a potential treatment avenue [2–4]. The role of interleukin 1 (IL-1) activation and potential synovial fibroblast interactions demand a thorough understanding [5, 6]. Associations of CPPD with hypomagnesemia, hypercalcemia, and cartilage damage, as well as the crucial role of alkaline phosphatase and ANKH gene mutations, are crucial aspects to be explored [7–9].
While CPPD is often asymptomatic and incidentally discovered in the elderly, this seemingly benign condition poses diagnostic challenges, with acute CPP crystal arthritis initially termed “pseudo-gout” due to its resemblance to gout. Symptoms of acute CPP crystal arthritis typically resolve within 3–4 days and affect large joints such as the knee, wrist, shoulder, and hip [10]. The rapid onset of acute synovitis, characterized by pain, stiffness, swelling/effusion, and marked tenderness (± erythema), is highly indicative of crystal synovitis. However, these features are not specific to any one type of crystal, so precise diagnosis requires crystal identification. In some cases, ligaments, tendons, bursae, bones, and the spine may also be involved. CPPD in the atlanto-occipital joint, known as crowned dens syndrome, can lead to periodic acute cervico-occipital pain accompanied by fever, neck stiffness, and signs of an inflammatory response in laboratory tests. Chronic CPP crystal inflammatory arthritis, marked by joint pain and swelling, morning stiffness, as well as elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels, mimicking rheumatoid arthritis (RA), adds another layer of complexity, necessitating a differential diagnosis approach based on radiographic features and SF analysis [10, 11].
As CPPD often coexists with osteoarthritis (OA) and may affect various anatomical sites, including tendons and ligaments, this review explores the differential diagnostic challenges posed by these overlapping conditions. It is still unclear, whether these crystals play a direct role in the pathogenesis of OA or if they are merely a consequence of joint degeneration. Moreover, rare tumoral CPP crystal deposits further contribute to the diagnostic intricacies [12, 13]. With an emphasis on both differential diagnostics and therapeutic challenges, this review aims to provide a comprehensive guide for navigating the complexities of CPPD in clinical practice.
The prevalence of radiographic chondrocalcinosis (CC), which is commonly used as an indicator of CPPD disease, varies between 4% and over 10% among older adults, although the exact prevalence of symptomatic CPPD disease is not well established [14]. In patients with known joint disease, CPP crystals were detected in 22.28% of OA cases, 8.28% of RA cases, 3.82% of psoriatic arthritis cases, 2.79% of other spondyloarthropathies, 10% of septic arthritis cases, and 9.18% in various other joint diseases [15]. Age is the most significant risk factor for CC, with prevalence increasing from under 4% in individuals below 70 years to 27% in those over 85 [16]. Other risk factors for CPPD include previous joint injury, hereditary or familial predisposition to CPPD and specific diseases such as hemochromatosis, primary hyperparathyroidism, hypophosphatasia, and hypomagnesemia [7].
A recent study [17] highlighted four major themes in the experiences of patients with CPPD: the diagnostic journey, daily life disruptions, psychological effects, and challenges in disease management. Patients frequently experienced a sudden onset of severe symptoms, leading to initial misdiagnoses and delays in receiving a proper diagnosis. This journey often involved significant pain and confusion, as both patients and healthcare providers struggled to distinguish CPPD from other conditions. The impact of CPPD extended beyond physical symptoms, affecting daily activities, social interactions, and overall quality of life. The unpredictability of flares and chronic pain led to psychological stress, including anxiety and frustration. Patients also faced difficulties in accessing effective treatment and clear guidance on managing their condition, highlighting a need for better-informed healthcare providers and more consistent care strategies. The study underscores the importance of comprehensive care approaches to address both the physical and emotional challenges faced by CPPD patients.
As highlighted in the EULAR guidelines for CPPD diagnosis [10], a conclusive diagnosis is achieved through the identification of CPP crystals in SF or joint biopsy specimens. These crystals, characterized by poor birefringence, are more effectively visualized under regular light compared to polarizing microscopy [18]. In compensated polarizing light microscopy, CPP crystals exhibit positive birefringence and are notably present in the SF of asymptomatic joints, particularly in the knee [19].
While radiographic evidence of characteristic CC is diagnostically valuable, its sensitivity may be limited, with knees und wrists being the most frequently positive joints, and others like the pubic symphysis, wrists, and shoulders less commonly displaying CC [20, 21].
Conventional X-ray radiography (CR) is the most commonly used imaging technique in routine clinical practice (Figures 1, 2, and 3). CR shows calcifications in the intermediate zone, especially of the fibrocartilage, but also of the hyaline cartilage. Additionally, CR may reveal other signs of CPPD-related joint damage, such as joint space narrowing, subchondral cyst formation, osteophyte formation, or erosions of the articular surfaces. These findings can help differentiate CPPD from other joint conditions, such as OA or RA, which may present with similar symptoms but have distinct radiographic features. While CR imaging is a valuable tool for diagnosing CPPD, it may not always detect early or subtle changes associated with the disease.
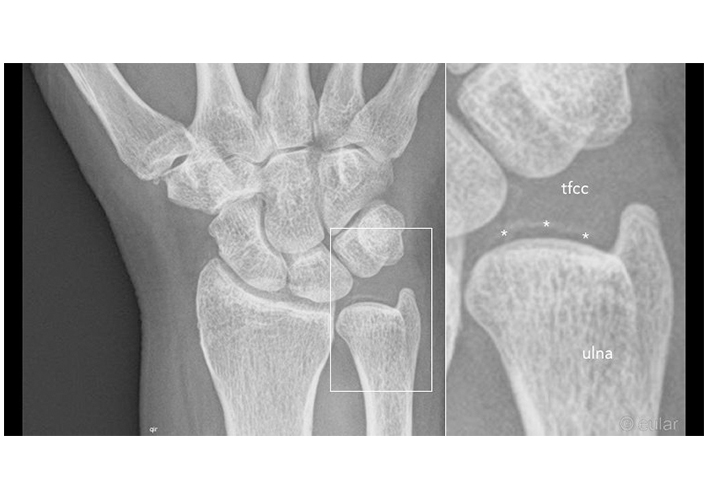
X-ray of the wrist. Calcifications of the tfcc (asterisks) by CPPD. tfcc: triangular fibrocartilage complex. Reprinted from EULAR Imaging Library, with kind permission
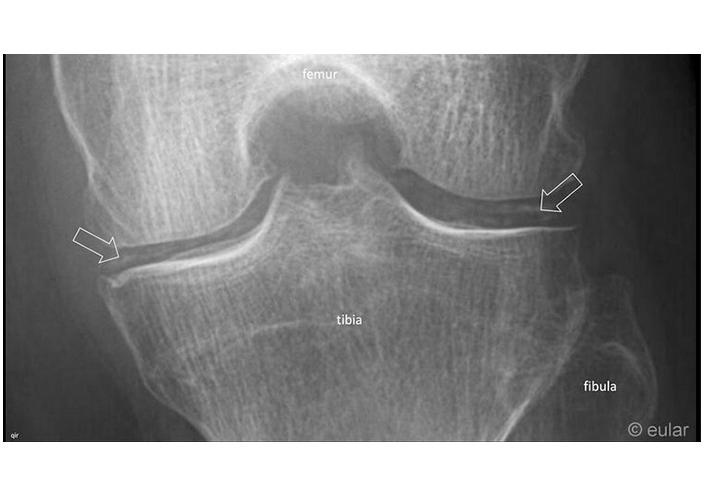
X-ray of the knee. Chondrocalcinosis at the medial and lateral meniscus (arrows). Reprinted from EULAR Imaging Library, with kind permission
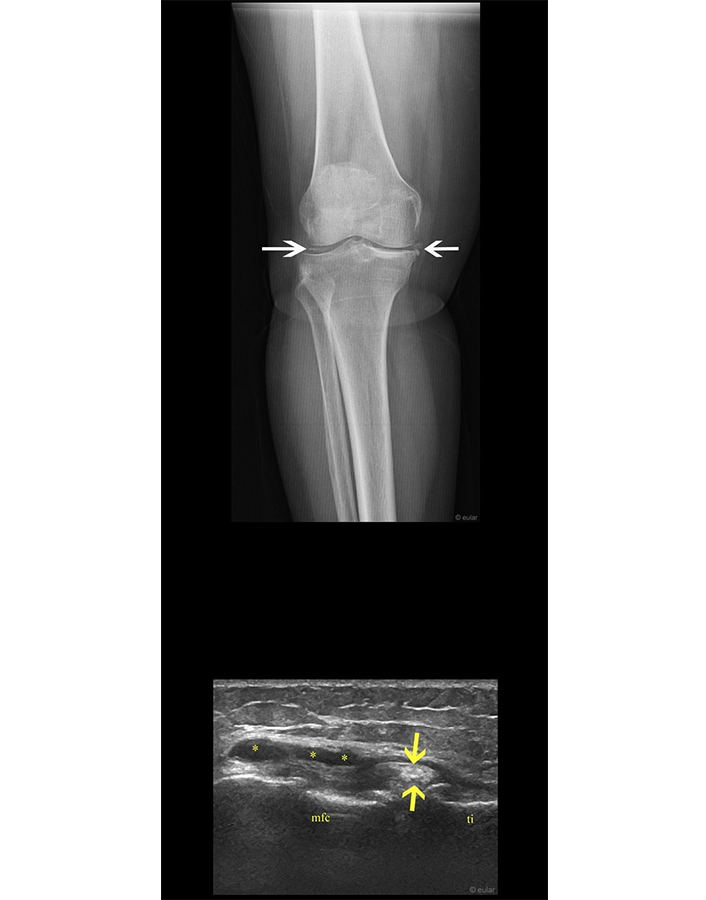
X-ray and ultrasound of the knee. Chondrocalcinosis of the meniscal on radiography (white arrows). Protrusion of the peripheral aspect of the anterior horn of the medial meniscus which shows hyperechoic aggregates (yellow arrows) and well defined lobulated cystic lesions adjacent to the medial meniscus (i.e., parameniscal cyst; asterisks) on ultrasound. mfc: medial femoral condyle; ti: tibia. Reprinted from EULAR Imaging Library, with kind permission
Ultrasound (US) scanning emerges as a promising diagnostic tool for detecting CPP deposits, potentially surpassing radiography in sensitivity, especially in areas like the wrist and the knee [22–24].
US can detect CPP crystal deposits as hyperechoic foci with posterior acoustic shadowing within the soft tissues or joint spaces. These deposits typically present as linear, punctate, or irregularly shaped echogenic structures within the hyaline cartilage, synovium, tendons, or ligaments (Figures 3, 4, 5, and 6).
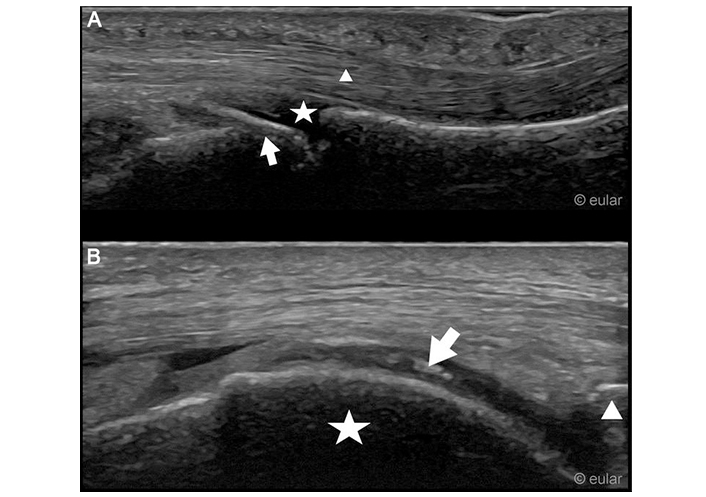
Ultrasound example images. A. Ultrasound of the third metacarpophalangeal joint of the left hand, palmar aspect. Arrow: hook like osteophyte at the head of the metacarpal bone; star: metacarpophalangeal joint; triangle: flexor tendon. B. Medial condyle of the right knee with hyperechoic deposition within the cartilage. Arrow: hyperechoic deposition; star: medial condyle; triangle: upper pole of patella. Reprinted from EULAR Imaging Library, with kind permission
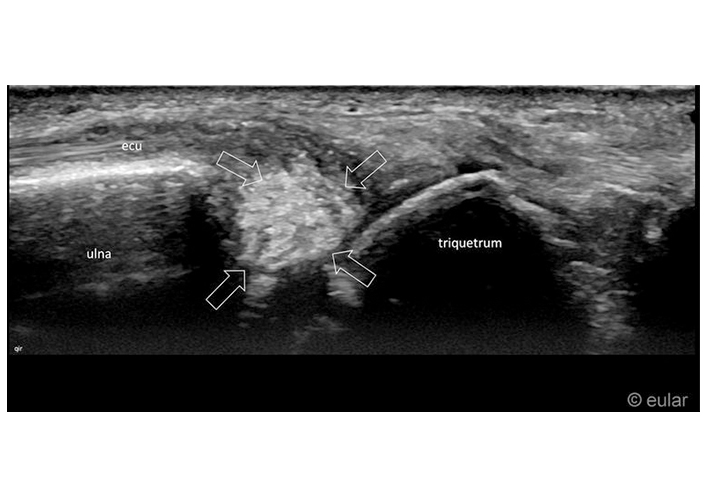
Ultrasound of the wrist. Hyperechoic calcification inside the triangular fibrocartilage complex (tfcc) of the wrist (arrows). ecu: extensor carpi ulnar tendon. Reprinted from EULAR Imaging Library, with kind permission
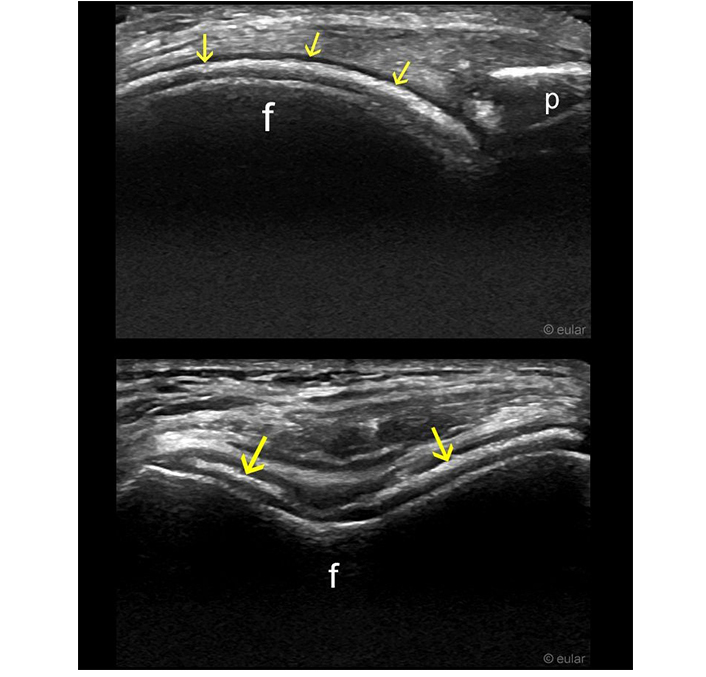
Ultrasound of the knee. Hyperechoic band within the hyaline cartilage visible in longitudinal and transverse planes (arrows). f: femur; p: patella. Reprinted from EULAR Imaging Library, with kind permission
US can also help assess the extent of joint inflammation and synovial hypertrophy associated with acute CPP crystal arthritis. Synovial effusion, synovial thickening, and Doppler signal enhancement indicative of active inflammation can be visualized using US. Bilateral US assessment of knees, wrists and hips had excellent accuracy and good feasibility for the diagnosis of CPPD disease [25].
The OMERACT Ultrasound working group developed and validated a consensus-based US scoring system for CPPD, aiming to standardize assessment methods [26]. The system, based on a four-grade semi quantitative scale, demonstrated high reliability in intra- and inter-reader assessments on both static images and patient examinations. Grades ranged from 0 (no findings) to 3 (deposits occupying > 50% of the examined structure). The system is applicable to the knee and wrist cartilage. This validated scoring system offers a valuable tool for clinical trials and clinical practice, ensuring consistent and comparable evaluation of CPPD extent.
Another study, which explored the US findings detectable at metacarpophalangeal (MCP) joints in CPPD, identified four main types of deposits: within the hyaline cartilage, at flexor digitorum tendons, capsuloligamentous deposits, and intra-articular deposits [27]. These deposits exhibit a diverse appearance on US imaging, including hyperechoic enhancements, linear deposits, and pseudo-double contour signs. The use of high-frequency US transducers enables the detection and characterization of these deposits. Notably, some findings, such as the double contour sign and intra-articular deposits, resemble those seen in gout, highlighting the importance of careful differentiation.
Computed tomography (CT) is more accurate than conventional radiography, especially for the axial skeleton and deep anatomical structures, making it valuable for diagnosing crowned dens syndrome [28].
Dual-energy CT (DECT) has emerged as a promising modality for diagnosing CPPD disease, although current data regarding its efficacy remain equivocal [29, 30]. However, the inclusion of DECT in the 2023 classification criteria for CPPD underscores its growing importance in clinical practice. DECT offers advantages in identifying CPP crystals. Further research and validation studies are needed to elucidate the full potential of DECT in CPPD diagnosis and its impact on patient outcomes. However, DECT has not become established in everyday use yet; its utilization requires an experienced radiologist and appropriate technical equipment.
MRI has been of limited use in imaging CPPD due to its poor visualization of calcifications in articular tissues, often leading to misdiagnosis in conditions like tophaceous CPPD. MRI sequences can miss 75% of CPPD deposits [31]. MRI sensitivity is significantly better at identifying CPPD deposits in the hyaline cartilage of the femoral condyles compared to other internal structures, even when those structures have a higher amount of calcification [32].
The 2023 American College of Rheumatology (ACR)/EULAR classification criteria for CPPD (Figure 7 and Table 1) mark a significant milestone as the first-ever validated criteria for this condition [14]. Derived and validated using established methodology and data from 751 patient profiles, they aim to enhance future observational studies and clinical trials in CPPD disease. The criteria demonstrated high sensitivity and specificity in an independent validation cohort.
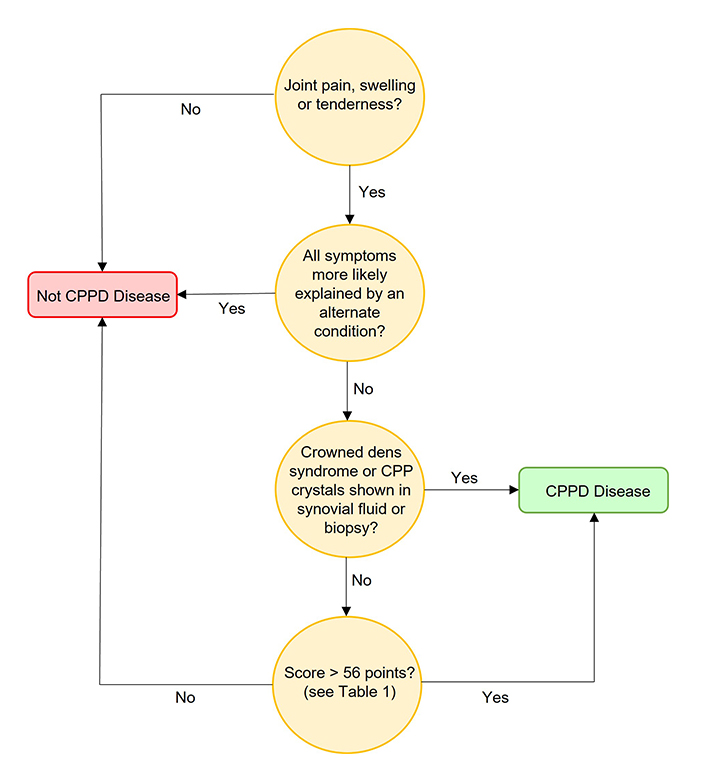
A pathway for the diagnosis of CPPD according to 2023 ACR/EULAR classification criteria [14]
Note. Adapted with permission from “The 2023 ACR/EULAR classification criteria for calcium pyrophosphate deposition disease” by Abhishek A, Tedeschi SK, Pascart T, Latourte A, Dalbeth N, Neogi T, et al. Ann Rheum Dis. 2023;82:1248–57 (https://ard.bmj.com/content/82/10/1248). © Author(s) (or their employer(s)) 2023.
The 2023 ACR/EULAR classification criteria of CPPD [14]
| Criteria | |
|---|---|
| Entry criterion | |
| - Ever had at least one episode of joint pain, swelling, or tenderness (in a peripheral joint or axial joint such as C1/C2 in the case of crowned dens syndrome). | |
| Absolute exclusion criteria | |
| - All symptoms more likely explained by an alternate condition (e.g., rheumatoid arthritis, gout, psoriatic arthritis, OA, etc.). | |
| Sufficient criteria | |
| - Crowned dens syndrome (as characterized by both clinical and imaging features).- Synovial fluid analysis demonstrating CPP crystals in a joint with swelling, tenderness or pain. | |
| Additional classifications | |
| - An individual is classified as CPPD if the entry criterion is met, exclusion criteria are not met, and at least one sufficient criterion is fulfilled.- If none of the sufficient criteria are present, an individual is classified as CPPD disease if the sum of the criteria below is > 56 points. | |
| Scoring guidelines | |
| - Items can be scored if they were ever present during a patient’s lifetime.- If a patient fulfills > 1 item in a given domain, only the highest weighted item will be scored.- Imaging of at least one symptomatic joint by CR, US, CT, or DECT is required. | |
| Domains and levels | Points |
| A. Age at onset of joint symptoms | |
| ≤ 60 years | 0 |
| > 60 years | 4 |
| B. Time-course and symptoms of inflammatory arthritis | |
| No persistent or typical inflammatory arthritis | 0 |
| Persistent inflammatory arthritis (ongoing joint swelling with pain and/or warmth in one or more joints) | 9 |
| 1 typical acute arthritis episode (episode with acute onset or acute worsening of joint pain with swelling and/or warmth that resolves regardless of treatment) | 12 |
| More than 1 typical acute arthritis episode | 16 |
| C. Sites of typical episode(s) of inflammatory arthritis in peripheral joints | |
| 1st MTPJ | -6 |
| No typical episode(s) | 0 |
| Joint(s) other than wrist, knee, or 1st MTPJ | 5 |
| Wrist | 8 |
| Knee | 9 |
| D. Related metabolic diseases (hereditary hemochromatosis, primary hyperparathyroidism, hypomagnesemia, Gitelman syndrome, hypophosphatasia, or a familial history of CPPD disease) | |
| None | 0 |
| Present | 6 |
| E. Synovial fluid crystal analysis from a symptomatic joint | |
| CPP crystals absent on ≥ 2 occasions | –7 |
| CPP crystals absent on 1 occasion | –1 |
| Not performed | 0 |
| F. OA of hand/wrist on imaging | |
| None of the following findings or no wrist/hand imaging performed | 0 |
| Bilateral radio-carpal joints | 2 |
| ≥ 2 of the following: STTJ OA without 1st CMCJ OA; 2nd MCPJ OA; 3rd MCPJ OA | 7 |
| G. Imaging evidence of CPPD in symptomatic peripheral joint(s) | |
| None on US, CT, or DECT (and absent on CR or CR not performed) | –4 |
| None on CR (and US, CT, DECT not performed) | 0 |
| Present on either CR, US, CT, or DECT | 16 |
| H. Number of peripheral joints with evidence of CPPD on any imaging modality | |
| None | 0 |
| 1 | 16 |
| 2–3 | 23 |
| ≥ 4 | 25 |
ACR: American College of Rheumatology; EULAR: European League Against Rheumatism; CPPD: calcium pyrophosphate deposition disease; CPP: calcium pyrophosphate; CR: conventional X-ray radiography; US: ultrasound; CT: computed tomography; DECT: dual-energy computed tomography; MTPJ: metatarsophalangeal joint; OA: osteoarthritis; STTJ: scaphotrapeziotrapezoid joint; CMCJ: carpometacarpal joint; MCPJ: metacarpophalangeal joint
Note. Adapted with permission from “The 2023 ACR/EULAR classification criteria for calcium pyrophosphate deposition disease” by Abhishek A, Tedeschi SK, Pascart T, Latourte A, Dalbeth N, Neogi T, et al. Ann Rheum Dis. 2023;82:1248–57 (https://ard.bmj.com/content/82/10/1248). © Author(s) (or their employer(s)) 2023.
The classification criteria prioritize the presence of CPP crystal deposition as evidenced by imaging, crowned dens syndrome (clinical plus imaging features) or the identification of CPP crystals in SF from a symptomatic joint. Exclusion criteria must be considered, ensuring that another condition does not fully explain the clinical presentation. Patients without these features can be classified using the remaining imaging and clinical criteria, with a particular emphasis on imaging features and recurrent typical episodes of acute inflammatory arthritis.
The crowned dens syndrome must be characterized by both clinical and imaging features. Clinical features include acute or sub-acute onset severe neck pain with elevated inflammatory markers, limited rotation, and often fever. Conditions such as polymyalgia rheumatica and meningitis should be excluded. Imaging features include linear calcific deposits on conventional CT within the transverse retro-odontoid ligament, often resembling two parallel lines in axial views. Calcifications at the atlanto-axial joint, alar ligament, and/or adjacent to the tip of the dens are characteristic. DECT features include a dual-energy index (DEI) between 0.016–0.036.
The criteria underscore the importance of imaging evidence of CPP crystal deposition, particularly in the absence of laboratory evidence of SF CPP crystals. Imaging domains, especially evidence of CPP crystals in a symptomatic joint and evidence in ≥ 4 peripheral joints, carry substantial weight. While imaging modalities more advanced than radiography, like US and CT, offer high sensitivity, their specificity poses challenges. Therefore, evidence of CPPD on all imaging modalities receives nearly equal weight, reflecting the consensus that any modality demonstrating CPP crystal deposition is convincing.
Acknowledging the absence of a practical gold standard for CPPD disease in clinical settings, the criteria prioritize accurate classification regardless of joint aspiration. However, joint aspiration remains important for clinical diagnosis and to exclude conditions like gout and septic arthritis. Recognizing the frequent coexistence of CPPD disease with other rheumatic and musculoskeletal diseases, the criteria exclude from classification only those patients whose symptoms are entirely explained by another condition.
The criteria establish the clinical picture of CPPD disease as an inflammatory arthritis, particularly among older adults, emphasizing acute inflammatory features and a predilection for knee and wrist joints. Despite limitations and challenges related to the heterogeneous nature of CPPD disease, the criteria demonstrate robust validity, providing a valuable tool for identifying patients with symptomatic CPPD disease for inclusion in prospective studies.
Although metabolic predisposition is uncommon, individuals with an onset at a young age (under 55 years) or extensive polyarticular CC should undergo screening for conditions such as primary hyperparathyroidism (serum Ca, parathyroid hormone), haemochromatosis (ferritin), hypomagnesaemia (serum magnesium), and hypophosphatasia (alkaline phosphatase) [10].
CPPD poses a diagnostic challenge due to its varied clinical presentations and overlapping symptoms with other arthropathies. A thorough understanding of the differential diagnosis is essential for accurate identification and management.
First and foremost, CPPD must be distinguished from other crystal-induced arthropathies, particularly gout. Both conditions share similarities, such as acute inflammatory arthritis, but they differ in the type of crystals involved. Gout is characterized by monosodium urate crystals, whereas CPPD involves CPP crystals. As such, joint aspiration with crystal analysis remains a crucial diagnostic step.
Osteoarthritis (OA) is another common condition that can mimic CPPD, especially in elderly individuals. Radiographic evidence of CC, a hallmark of CPPD, may lead to confusion. However, clinical features and crystal identification can aid in differentiation. While OA is primarily a degenerative joint disease, CPPD presents with acute inflammatory episodes. Studies comparing patients with CPPD and OA have revealed higher degrees of inflammation in CPPD, evidenced by increased effusion, synovitis, and SF white blood cell count [33]. Although CPPD and OA share common risk factors such as aging, they may represent distinct diseases with different pathogenic mechanisms. US imaging has proven valuable in identifying crystals and assessing inflammation in CPPD and OA patients.
RA shares some clinical features with chronic CPPD inflammatory arthritis, leading to potential misdiagnosis. Both conditions may involve symmetrical joint involvement and increased acute-phase reactants. However, characteristic radiographic findings, such as CC and absence of erosions in CPPD, help distinguish them. A recent study compares patients diagnosed with CPPD and RA based on real-life data collected from a tertiary care centre [34]. It highlights similarities and differences between these conditions, emphasizing the challenge of differentiating them clinically, especially in the absence of SF analysis. The study reveals a notable co-occurrence of CPPD, CC, and RA, particularly among patients primarily diagnosed with seronegative RA. While CPPD is diagnosed later than RA on average, their onset appears independent, although patients with both conditions develop RA later than those with RA alone. Factors such as age, sex, and symptom onset were explored, with women more commonly diagnosed with CPPD. The study also discusses the clinical features and radiological findings associated with CPPD and RA, indicating similarities in arthritis characteristics and distribution. The limitations of the study, including the retrospective design and potential misdiagnosis, are acknowledged. The importance of reevaluating seronegative RA diagnoses over time is emphasized, given the management differences between RA and CPPD.
Basic Ca phosphate deposition disease (BCPDD), another crystal arthropathy, can present similarly to CPPD. BCPDD involves hydroxyapatite crystals and typically manifests as calcific tendinitis or bursitis. Careful clinical evaluation and imaging studies, such as US or X-rays, can aid in the differential diagnosis.
Inflammatory arthropathies, including ankylosing spondylitis and psoriatic arthritis, may share some features with CPPD, such as inflammatory back pain and joint involvement. Distinguishing between them relies on a comprehensive assessment, including imaging studies, HLA-B27 testing, and consideration of associated clinical features.
Infections, particularly septic arthritis, should be ruled out in cases of acute joint inflammation. The clinical urgency and potential systemic symptoms in septic arthritis contrast with the episodic nature of CPPD attacks. Joint aspiration with culture and analysis is essential for accurate diagnosis.
Metabolic disorders, such as hypophosphatasia, may predispose individuals to CPPD and should be considered in the differential diagnosis, especially in younger patients. Systemic conditions like hyperparathyroidism and hemochromatosis can also contribute to CPPD, necessitating a thorough evaluation of metabolic parameters.
The management of CPPD disease poses challenges, primarily due to the limited understanding of its pathophysiology and the absence of specific targeted therapies [35]. To date, no medication has been demonstrated to directly influence CPP deposition, and consequently, the focus of CPPD management remains symptomatic relief (Table 2). Current evidence on CPPD management is sparse.
Medication for CPP deposition disease
For the management of acute CPPD arthritis, a combination of rest, local ice packs, SF aspiration (allowing for crystal identification), and intra-articular steroid injections has been recommended [35]. While colchicine and nonsteroidal anti-inflammatory drugs (NSAIDs) can be utilized, their tolerance in the elderly population may be limited. The evidence supporting their efficacy is mainly derived from studies on acute gout attacks, emphasizing the need for more specific research on their role in CPPD management.
In cases where traditional treatments are poorly tolerated or ineffective, glucocorticoids, either parenteral or oral (with a gradual tapering regimen), can be considered [36, 37].
The COLCHICORT trial demonstrated that both low-dose colchicine and oral prednisone exhibited equivalent efficacy in reducing joint pain at 24 h among older patients with acute CPP crystal arthritis [38]. However, differences were observed in their safety profiles, with colchicine associated with a higher incidence of diarrhea and prednisone with a higher incidence of hypertension and hyperglycemia.
The 2011 EULAR treatment guidelines also recommend parenteral adrenocorticotropic hormone (ACTH) for patients experiencing polyarticular attacks, citing a retrospective study that demonstrated ACTH as a safe and effective treatment for acute gout and pseudogout, particularly in patients with multiple medical conditions [35, 39]. The adverse effect profile, including hypokalemia, hyperglycemia, fluid retention, and rebound arthritis, was similar to that of corticosteroids.
Chronic CPP crystal inflammatory arthritis may benefit from low-dose colchicine, NSAIDs, steroids, hydroxychloroquine [40], or methotrexate [41]. Low-dose colchicine (1 mg/day) may serve as a preventative measure for patients experiencing recurrent flares [42]. IL-1 inhibitors, targeting crystal-induced inflammation, present a potential alternative for patients unable to tolerate colchicine, NSAIDs, or steroids [43–45]. Anakinra, administered daily via subcutaneous injection, rapidly reduces inflammation and pain within four hours after the first dose, with minimal adverse effects and symptoms alleviated within four days [45–47]. Other IL-1 inhibitors, such as rilonacept and canakinumab, are being explored, though their efficacy in CPP arthritis lacks randomized trial confirmation [48]. The NLRP3 inflammasome inhibitor OLT1177 has also shown promise in early studies, reducing joint pain significantly [49].
Hydroxychloroquine has shown efficacy in a placebo-controlled study [40], whereas methotrexate’s results have been less encouraging in reducing disease activity or pain levels [50].
An open-label study corroborates previous cases where tocilizumab effectively treated CPP arthritis, suggesting that IL-6 inhibition could be a therapeutic option for refractory acute or chronic CPP arthritis, even in cases of previous IL-1 blocker failure or intolerance, opening potential therapeutic avenues in a disease with limited treatment options, yet further randomized controlled trials are necessary to ascertain its efficacy and safety profile [51].
The management of CPPD associated with OA aligns with that of OA without CPPD. Radiation synovectomy has demonstrated improvements in chronic CPPD with OA [1]. However, the risk-benefit profile of these treatments should be approached cautiously, particularly considering the advanced age of most CPPD patients.
Magnesium supplementation has shown promise in reducing CC in a hypomagnesemia patient [52] and may be considered in cases of hypomagnesemia. However, the overall impact of magnesium supplementation on CPPD management warrants further investigation.
Addressing underlying metabolic conditions, such as hyperparathyroidism or hemochromatosis, does not appear to significantly alter the course of CPPD [24]. This suggests that CPPD management primarily revolves around mitigating symptoms rather than modifying the underlying disease processes.
It is noteworthy that asymptomatic CPPD does not necessitate treatment, reinforcing the importance of tailoring management strategies to address symptomatic presentations and improve the overall quality of life for affected individuals [35].
In vitro studies suggest that probenecid, an inhibitor of anion transport, may influence the activity of the ANKH transporter [2, 53]. However, its efficacy in human CPPD has not been tested, highlighting the need for further research into pharmacological interventions targeting the disease mechanisms.
Monitoring CPPD involves assessing joint symptoms, inflammation, and complications, but recent research suggests a potential link between CPPD and cardiovascular risk [54]. A cohort study demonstrated that CPPD was independently associated with an increased risk of incident cardiovascular disease (CVD) events, even after adjusting for traditional cardiovascular risk factors.
The pathophysiological mechanisms underlying the association between CPPD and CVD remain unclear but may involve chronic inflammation, oxidative stress, and endothelial dysfunction, all of which contribute to the development and progression of atherosclerosis. Furthermore, CPP crystals have been shown to promote vascular calcification, potentially exacerbating the atherosclerotic process.
Regular evaluation of joint symptoms and inflammation through clinical assessment, imaging modalities like US, and laboratory tests including CRP and ESR. This includes assessing for acute flares, chronic joint inflammation, and structural damage progression to guide treatment decisions [26, 35].
For cardiovascular risk assessment, screening for traditional cardiovascular risk factors such as hypertension, dyslipidemia, diabetes mellitus, and smoking history is essential [55]. Additional factors associated with increased cardiovascular risk in CPPD, including chronic inflammation, obesity, and metabolic syndrome, should also be considered.
Lifestyle modifications including weight management, regular exercise, and a heart-healthy diet to mitigate cardiovascular risk factors should be recommended [56]. If needed, pharmacological interventions such as statins and antihypertensive medications based on individual cardiovascular risk profiles should be considered.
A collaboration between rheumatologists, cardiologists, and primary care physicians is essential to develop comprehensive monitoring and management plans tailored to the individual patient’s needs. Patient education and shared decision-making is advised to enhance adherence to monitoring and treatment recommendations [56].
In conclusion, CPPD disease presents a complex challenge in rheumatology, necessitating a nuanced approach to both diagnosis and management. The newly established 2023 ACR/EULAR classification criteria for CPPD disease, developed through rigorous methodology, mark a significant milestone, providing a standardized framework for identifying and studying this condition. The criteria prioritize imaging evidence of CPP crystal deposition and recurrent episodes of acute inflammatory arthritis, acknowledging the importance of these features in CPPD disease.
Differential diagnosis remains crucial due to the overlapping clinical presentations with other rheumatic and musculoskeletal diseases. The distinct features of CPPD, such as CC and CPP crystal deposits in various joints and tissues, serve as key diagnostic indicators. The introduction of advanced imaging modalities, like US and CT, has enhanced diagnostic sensitivity, particularly in early CPPD disease.
While the understanding of CPPD pathophysiology is evolving, the management landscape remains predominantly symptomatic. The absence of a specific targeted therapy to influence CPP deposition emphasizes the need for interventions that alleviate symptoms and enhance patients’ quality of life. Magnesium supplementation, addressing underlying metabolic conditions, and judicious use of medications like colchicine and NSAIDs represent current symptomatic management strategies.
The challenging nature of CPPD diagnosis and management calls for ongoing research to deepen our understanding of the disease mechanisms and explore novel therapeutic avenues. The acknowledgment of CPPD’s frequent coexistence with other rheumatic diseases underscores the complexity of its clinical presentation, necessitating a comprehensive and individualized approach to patient care.
Practical recommendations for clinical practice:
Diagnosis: use joint imaging and SF analysis to confirm CPPD.
Management: treat acute symptoms with NSAIDs, colchicine, or corticosteroids; manage underlying conditions.
Long-term care: encourage weight management, exercise, and joint protection; monitor progress.
Patient education: advise on symptom recognition and joint care.
Stay updated: follow new treatments and consider trials.
Multidisciplinary care: collaborate with specialists for comprehensive management.
ACR: American College of Rheumatology
ACTH: adrenocorticotropic hormone
ANKH: pyrophosphate transporter ankylosis human
BCPDD: basic calcium phosphate deposition disease
Ca: calcium
CC: chondrocalcinosis
CMCJ: carpometacarpal joint
CPP: calcium pyrophosphate
CPPD: calcium pyrophosphate deposition disease
CR: conventional X-ray radiography
CRP: C-reactive protein
CT: computed tomography
CVD: cardiovascular disease
DECT: dual-energy computed tomography
ePPi: extracellular pyrophosphate
ESR: erythrocyte sedimentation rate
EULAR: European League Against Rheumatism
IL-1: interleukin 1
MCP: metacarpophalangeal
MCPJ: metacarpophalangeal joint
MTPJ: metatarsophalangeal joint
NSAIDs: nonsteroidal anti-inflammatory drugs
OA: osteoarthritis
PP: pyrophosphate
RA: rheumatoid arthritis
SF: synovial fluid
STTJ: scaphotrapeziotrapezoid joint
US: ultrasound
MLV: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. HK: Validation, Writing—review & editing, Supervision. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Because the figures in the manuscript were obtained and licensed from a public image library [EULAR Imaging Library, https://esor.eular.org/course/view.php?id=41], and the figures are anonymous, ethical approval is not required.
Because the figures in the manuscript were obtained and licensed from a public image library [EULAR Imaging Library, https://esor.eular.org/course/view.php?id=41], and the figures are anonymous, consent to participate is not required.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
John F. Hoy ... Xavier C. Simcock
Michael Schirmer, Johannes Dominikus Pallua
Anna J. Turlej, Angelo L. Gaffo
Fernando Perez-Ruiz ... Frédéric Lioté
Gamze Dilek ... Kemal Nas
Ebru Atalar, Hatice Bodur
