Affiliation:
Department of Radiology, Division of Musculoskeletal Radiology, University of Pennsylvania Health System, Philadelphia, PA 19104, USA
Email: antje.greenfield@pennmedicine.upenn.edu
ORCID: https://orcid.org/0009-0002-3683-8158
Affiliation:
Department of Radiology, Division of Musculoskeletal Radiology, University of Pennsylvania Health System, Philadelphia, PA 19104, USA
Explor Musculoskeletal Dis. 2025;3:100779 DOI: https://doi.org/10.37349/emd.2025.100779
Received: July 30, 2024 Accepted: December 03, 2024 Published: January 19, 2025
Academic Editor: Peter Mandl, Medical University of Vienna (MUW), Austria
The article belongs to the special issue Multifaceted Imaging in Rheumatic and Musculoskeletal Diseases
Musculoskeletal ultrasound has become a valuable imaging tool in the diagnosis and management of rheumatologic disorders. Expertise in recognizing sonographic findings of pathology is the basis for its successful application in clinical use. This article will provide descriptions and a pictorial essay of ultrasound findings in common musculoskeletal manifestations of rheumatologic disorders.
Rheumatologic disorders include a wide spectrum of organ involvement and are associated with joint and other musculoskeletal disease. Traditionally related arthritic processes have been diagnosed and characterized by radiography and soft tissue involvement imaged with magnetic resonance imaging (MRI). In the past two decades, ultrasound has emerged as a valuable imaging technique in assessing joints for presence of early and advanced findings of arthritis [1]. Technical advances of ultrasound transducers have led to sub-millimeter spatial imaging resolution which can be combined with color Doppler imaging to assess presence of hyperemia and ability to perform dynamic multiplanar imaging of the affected site. This offers extended diagnostic capabilities, especially in diagnosing early disease and monitoring of disease during treatment. Ultrasound is an excellent real time imaging guidance tool for interventions such as joint aspirations, tissue sampling and various injection therapies. The European League Against Rheumatism (EULAR) has developed and updated guidelines for performing musculoskeletal ultrasound in the diagnosis and management of rheumatologic conditions [2].
Musculoskeletal ultrasound has been adapted into clinical practice by various clinical specialties including radiologists, rheumatologists, sports medicine, and orthopedic providers. This article will provide a tutorial of ultrasound findings in common musculoskeletal manifestations of rheumatologic disorders.
Ultrasound is an excellent imaging modality to assess soft tissues, especially in the near field, close to the ultrasound transducer. High frequency transducers in current clinical use range typically between 12–18 megahertz (MHz) and provide an image resolution of structures in the sub millimeter range. Ultrahigh frequency transducers in the range of 20–70 MHz can produce image resolution at 50–100 μm [3]. The smallest erosions or osteophytes, tiny calcifications or crystal deposits, soft tissue masses or fluid collections can be identified by ultrasound before detectable with other imaging modalities such as radiography or MRI. Color Doppler ultrasound provides assessment of the vascularity of the examined soft tissues and can detect hyperemia as a sign of active inflammation. It is useful to determine a solid mass from a fluid collection, as fluid is avascular. Ultrasound can be performed in a multiplanar fashion, with the examiner being able to assess structures in real time and dynamically. It becomes an extended physical examination by focusing on a specific position or motion of a structure that is eliciting symptoms [4].
Ultrasound has no contraindications, uses no radiation, and does not require iodinated or gadolinium-based contrast. It is therefore easily applicable to everyone including pregnant women, children and patients with contrast allergies or contraindications to imaging modalities such as MRI.
Ultrasound is limited in evaluating structures located deep from the skin surface due to the loss of imaging resolution. Bones, dense calcifications, and air represent barriers to ultrasound and obscure structures behind them. For example, spinal contents cannot be adequately assessed. While the surface of bone can be evaluated, the bone marrow cannot be examined. Therefore, conditions including bone marrow edema or infiltration are obscured.
Many rheumatologic diseases involve disorders of the musculoskeletal system, including various types of arthritis, tendinopathies, and other soft tissue involvement. Patients present clinically with pain and swelling of the affected anatomic sites during active inflammatory phase of disease and can progress to permanent deformities and dysfunction in the chronic phases of disease.
Findings of joint effusion, synovitis, erosions, and productive changes provide diagnostic clues to the presence and differential diagnosis of arthritic processes. Findings of crystal deposition, soft tissue masses, collections, and evidence of inflammation of bursae, tendons, tendon sheaths and enthesopathy are important additional characteristics to be recognized [5]. Imaging including radiography, MRI and ultrasound are helpful in characterizing these abnormalities [6, 7]. Active inflammatory changes are the dominant features particularly in the early phase of disease and during acute flares [7]. These are well recognized with ultrasound and will be discussed in this article.
Joint effusion refers to abnormal fluid within a joint, causing swelling of the joint. Ultrasound demonstrates abnormal hypo- or anechoic appearing intra-articular fluid stretching or ballooning the joint capsule (Figure 1). The fluid may be simple and appear homogenously black, commonly due to presence of reactive serous fluid. Complex effusions are seen with intrinsic floating echogenic foci in the fluid due to debris, hemorrhage, or white cells. Echogenic lines inside of the joint fluid can be present due to adhesions and septations. A fluid-debris level may be seen. Local figure pressure with probe passage can be applied to test for compressibility of the fluid. This may be helpful in determining presence of fluid versus synovial hypertrophy. The joint capsule can be thickened. Hyperemia as a sign of active inflammation can be recognized by using color Doppler ultrasound to depict increased vascularity of the capsule and periarticular soft tissues.
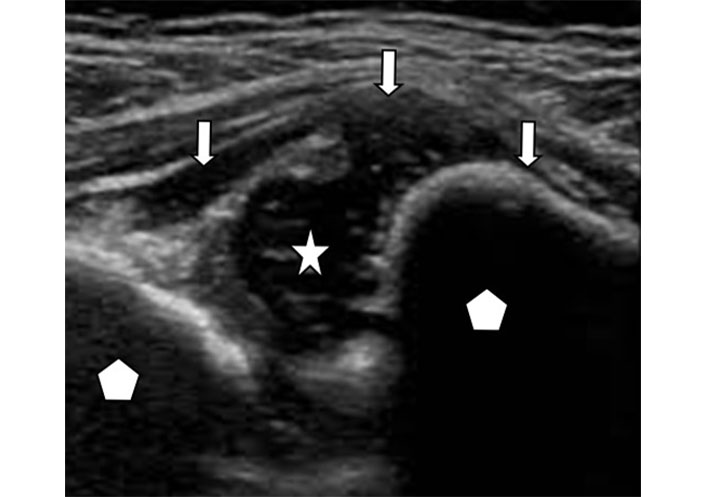
Joint effusion. Ultrasound image of an elbow joint with hypoechoic fluid (star) distending the joint capsule (arrows). The articulating bones (pentagon) are separated by the fluid
Synovitis represents hypertrophy and swelling of the synovial membrane outlining joints, tendon sheaths and bursal sacs in response to an inflammatory process [8]. The normal synovium produces viscous fluid for lubrication. An inflammatory process can cause overproduction and accumulation of fluid resulting in effusion. Ultrasound demonstrates diffuse or lobulated focal hypoechoic thickening of the synovium (Figure 2A). Frondlike hypertrophy of the synovium may be seen in recesses of an affected joint. The abnormal synovium may demonstrate increased vascularity due to acute inflammation as seen with color Doppler ultrasound (Figure 2B) [9]. The ultrasound probe can be used to apply pressure while scanning to assess for compressibility and to differentiate between soft tissue and fluid. Hypertrophic synovium is not compressible as it represents solid soft tissue while effusion is moveable with compression. A consensus-based scoring system of synovitis based on sonographic criteria has been developed by the EULAR-Outcomes Measures in Rheumatology (OMERACT) task force to provide reproducibility and reliability of degree of synovitis [10–12].
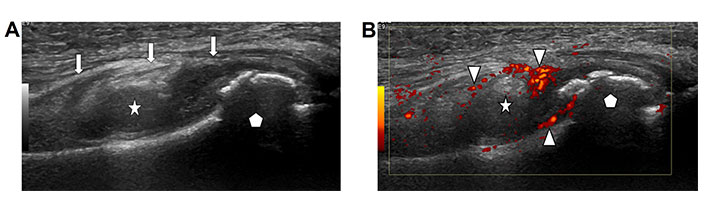
Synovitis. A. Ultrasound image of a joint with mass like synovial hypertrophy (star) distending the joint capsule (arrows). The articulating bone (pentagon) demonstrates an irregular bony surface due to reactive bone production; B. color Doppler ultrasound image of the same joint (bone marked with pentagon) demonstrates hyperemia of the synovium (star) as seen by red coloration of the inflamed synovium (arrow heads)
Erosions are a hallmark finding of inflammatory arthropathies, especially rheumatoid arthritis. Traditionally, the presence and distribution of erosions have been described with radiography and are used in the differential diagnosis of arthritis. However, tiny erosions that occur in the early stages of the arthritis may not be apparent on x-rays but can be seen with ultrasound. Erosions represent a defect of the surface of an articulating bone due to inflammation. Ultrasound demonstrates a focal hypoechoic discontinuity of the normal echogenic cortex of the bone with the appearance of a “bite” out of the bone (Figure 3A). The defect is usually filled with fluid or hypertrophic synovium (Figure 3B) without or with hyperemia (Figure 3C). Most erosions are located at the margins of the hyaline cartilage near the insertion of the synovial membrane and are result of the local synovial inflammation. Note that some erosions, such as those seen in association with gouty tophus, can be periarticular in location. Erosions are signs of bony destruction and are irreversible. The recognition of synovial inflammation with early small erosions or before erosions have developed is crucial in the diagnosis and treatment of arthritis before permanent destructive joint changes have occurred. Chronic erosive changes will lead to loss of articulating bone and remodeling of the joint with resultant deformities and loss of range of motion [13].
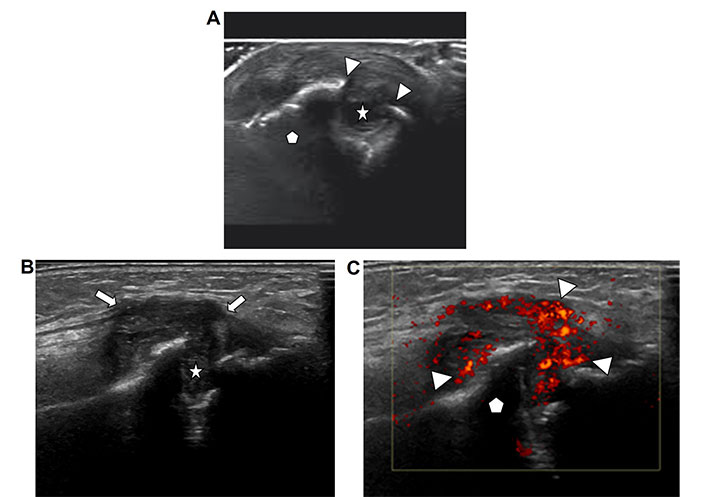
Erosions. A. Ultrasound image of an articulating bone in a joint (pentagon) with discontinuity of the bony surface (arrow heads) due to an erosion (star); B. ultrasound image of a bone (pentagon) with erosion demonstrates hypertrophic hypoechoic synovium (arrows) extending into and filling the erosion defect (star); C. ultrasound image of the same bone with erosion (bone marked with pentagon) as depicted in Figure 3C with added color Doppler ultrasound demonstrates hyperemia of the inflamed synovium as seen by red coloration of the synovium (arrow heads)
Osteophytes are hypertrophic osseous projections from the surface of the bone usually located at joint margins. They cause deformity and limited range of motion of the affected joint. Osteophytes develop in response to chronic degenerative or inflammatory arthropathy. In the spectrum of rheumatic joint disorders, they are often a secondary remodeling response to the destruction of the joint cartilage and subchondral bone by inflammatory changes. In degenerative arthropathies, osteophytes are the dominant finding. Ultrasound demonstrates echogenic irregular projections from the native bony surface resembling the surface of cauliflower (Figure 4A). In advanced disease, the native contour of the articulating bone may not be apparent, and the joint space is obliterated (Figure 4B). Associated synovial hypertrophy, active synovitis and joint effusion can be present [14].
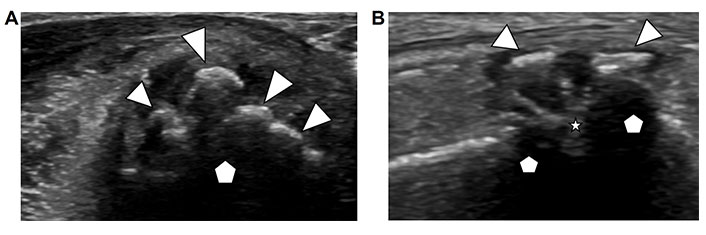
Osteophytes. A. Ultrasound image of an articulating bone in a metacarpophalangeal joint (pentagon) with irregular bony projections from the surface of the native bone (arrow heads); B. ultrasound image of a joint (articulating bones marked with pentagon) demonstrates osteophytes on both sides of the joint (arrowheads) and obliteration of the joint space (star)
Crystal formation occurs in metabolic disorders such as gout and calcium pyrophosphate deposition disorder (CPPD), also known as “pseudo-gout”. The crystals are deposited in joints and periarticular soft tissues. They provoke a local inflammatory response resulting in painful joint destruction and soft tissue swelling. Gout crystals are non-radiopaque and are therefore not well seen on radiographs. CPPD crystals contain calcium and can be detected with radiography.
Ultrasound findings in gout include a thin smooth echogenic coating of the articulating hypoechoic cartilage which has been compared to the appearance of “icing on the cake” or named as “double contour sign” (Figure 5A). Other less specific findings in crystal deposition disorders include synovial hypertrophy with punctate conglomerates of echogenic foci within the synovium (Figure 5B) and associated hyperemia demonstrated as increased vascularity with color Doppler imaging (Figure 5C). These can be seen with gout and pseudo-gout alike. In addition, periarticular erosions in advanced disease and complex joint effusion may be present [15–17].
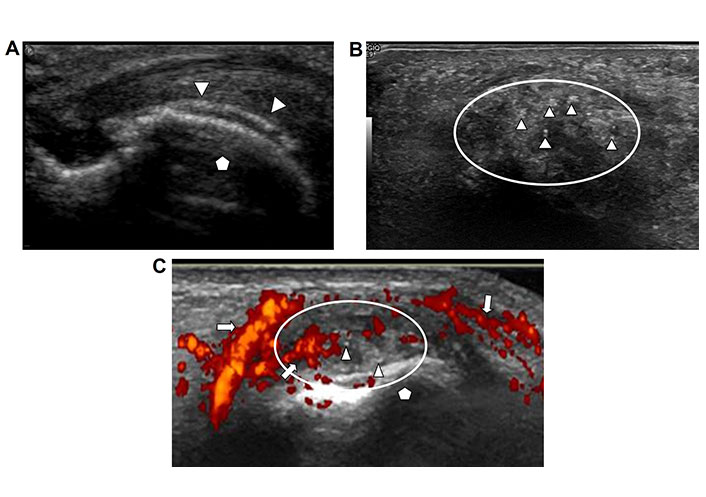
Gout. A. Ultrasound image of an articulating bone in long axis (pentagon). Thin smooth echogenic layer of gout crystals is coating the hypoechoic cartilage (arrow heads) appearing as a “double contour”; B. ultrasound image of periarticular soft tissues with punctate echogenic foci (arrowheads) in a clustered configuration (encircled by oval line); C. ultrasound image of portion of a joint with hypertrophic synovium (circle) adjacent to the articulating bone (pentagon). The synovium is very hyperemic as seen by intense vascularity with color Doppler imaging (arrows). Tiny echogenic foci embedded in the synovium represent crystals deposition in this patient with known gout
CPPD arthropathy most commonly affects the knee and wrist. Plain film radiography and ultrasound are recommended imaging modalities for diagnosis. Typical features of chondrocalcinosis as seen on radiography (Figure 6A) and presence of CPP crystals on synovial fluid polarized light microscopy may provide a definitive diagnosis. However, the sensitivity of radiography is low and there can be overlap with other arthropathies. The 2023 ACR/EULAR CPPD disease classification criteria have been developed including ultrasound imaging characteristics in CPPD arthropathy to improve the diagnostic performance in this disorder [18, 19].
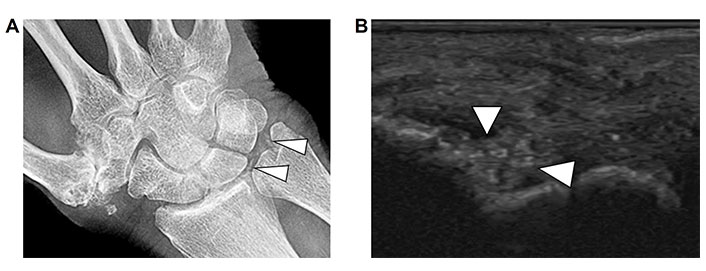
CPPD. A. Radiograph of the right wrist demonstrates punctate calcifications in the triangular fibrocartilage (arrow heads); B. corresponding ultrasound image of the right wrist joint demonstrates punctate echogenic foci in the fibrocartilage consistent with CPP crystal deposition
Ultrasound demonstrates linear and punctate echogenic deposits of calcium within the hyaline or fibrocartilage of joints (Figure 6B) or within tendons. Resultant reactive synovial proliferation, joint effusion and joint destruction may be present [20].
Edema refers to non-specific diffuse fluid retention in the soft tissue which is commonly seen with a wide range of inflammatory conditions. Ultrasound demonstrates hypoechoic loculations or lines separating the soft tissue layers due to extracellular fluid deposition (Figure 7). When involving subcutaneous fat, it separates the fat lobules and creates a typical sonographic pattern that has been termed “cobble-stone pattern”.
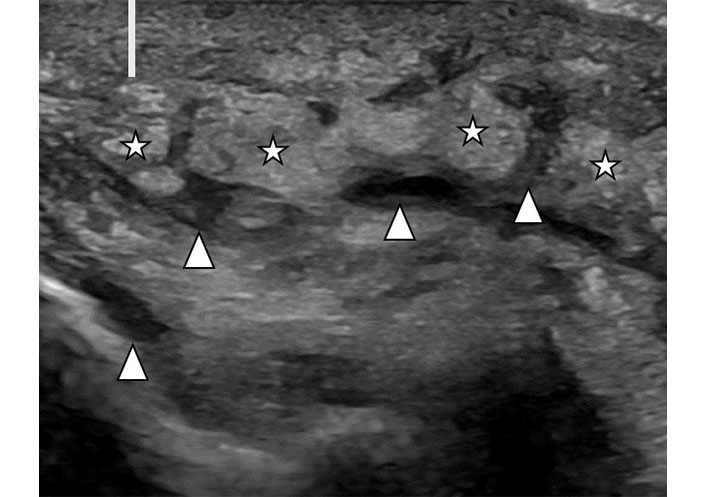
Soft tissue edema. Ultrasound image of subcutaneous soft tissues in a patient with lower extremity swelling. There is diffuse thickening of the skin (line). Subcutaneous fat demonstrates separation of fat lobules (stars) by hypoechoic linear fluid (arrowheads)
Bursitis refers to a painful inflammatory response of bursal sacs functioning as cushions near joints and tendons. These are lined with a synovial membrane which may undergo synovial hypertrophy and synovitis, similar to synovium in joints. Bursitis appears on ultrasound as a focal hypoechoic collection with a defined wall (Figure 8). The wall can be thickened. Synovitis and hyperemia of the surrounding soft tissues may be present. Crystal deposition can be seen if gout or CPPD are present.
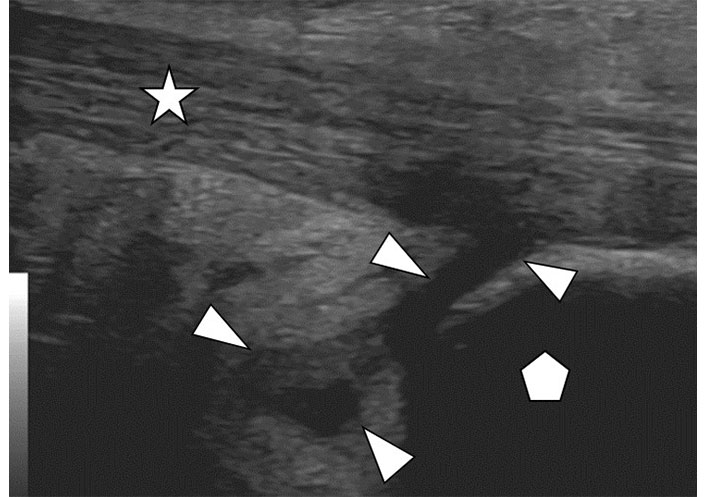
Bursitis. Ultrasound image of retrocalcaneal bursitis. The image depicts the insertion of the distal Achilles tendon (star) at the posterior calcaneus (pentagon). Anterior and adjacent to the tendon is a loculated anechoic-hypoechoic structure (arrowheads) representing fluid in the distended bursa
Soft tissue masses may occur in association with rheumatoid arthritis and are called rheumatoid nodules. If they occur in the setting of gout, they are known as tophi.
These soft tissue masses are seen or may be palpable in periarticular soft tissues or commonly in subcutaneous soft tissues of the extremities (Figure 9A). Ultrasound demonstrates non-specific, round, or oval shaped soft tissue masses with heterogenous echogenicity and intrinsic blood flow (Figure 9B). They may contain echogenic foci if associated with gout (Figure 9C). The diagnosis can be difficult, and the differential diagnosis includes other benign or malignant neoplastic soft tissue masses. If these soft tissue masses occur in isolation and without a known rheumatoid condition, ultrasound guided biopsy can be performed to obtain material for tissue diagnosis. In most cases these soft tissue masses occur with advanced disease and the underlying rheumatoid or crystal deposition disorder is known.
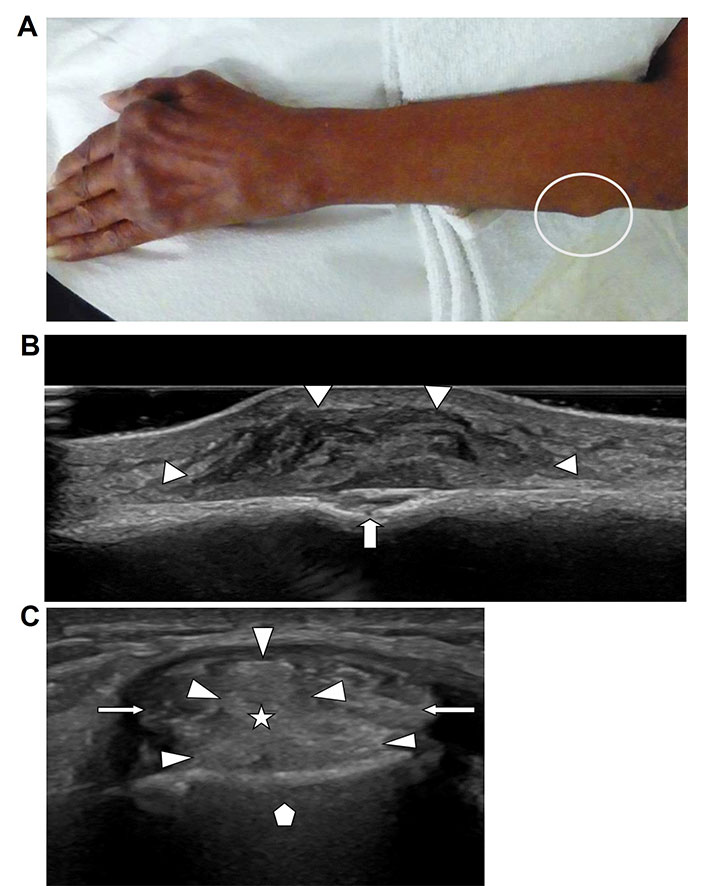
Soft tissue masses. A. Photograph of the forearm of a patient with known chronic rheumatoid arthritis and presence of typical rheumatoid hand and wrist deformities. Visible contour abnormality due to palpable broad-based mass along the proximal ulnar sided forearm (white circle). Biopsy proven rheumatoid nodule; B. ultrasound image of the forearm mass shown in Figure 8A demonstrates a heterogenous solid mass (arrowheads) with well-defined margins, broad-based on the cortical surface of the adjacent ulna with associated cortical erosion (arrow); C. short axis ultrasound image of the 2nd extensor compartment of a wrist in a patient with known gout. The two extensor carpi radialis tendons (arrows) are separated by an echogenic mass (star) with lobulated margins (arrowheads). Tissue diagnosis confirmed a gout tophus
Tendinitis and tenosynovitis refer to a spectrum of inflammatory changes involving tendons and tendon sheaths throughout the body, commonly accompanying rheumatoid disorders. Ultrasound demonstrates affected tendons to be enlarged, hypoechoic and heterogenous in echotexture with loss of the normal organized fibrillar pattern. Intrinsic vascularity of a tendon as seen with color Doppler imaging is indicative of active inflammation. Hypoechoic clefts seen within a tendon are signs of interstitial tears and deposition of inflammatory debris. Calcifications and crystal deposition within tendons present as echogenic foci inside the tendon and near tendinous insertions of bone. The tendon sheaths are lined with a synovial membrane, like joints and bursae which can demonstrate hypertrophy, hyperemia, and overproduction of fluid with fluid distending the tendon sheath (Figure 10A and B) [21].
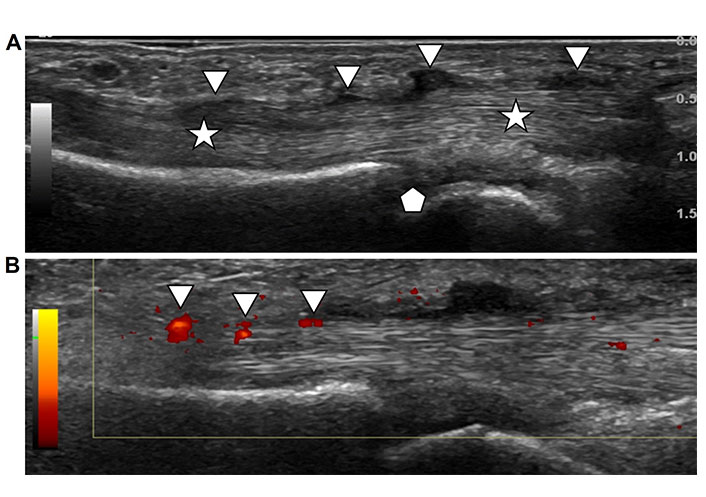
Tenosynovitis. A. Longitudinal ultrasound image of a flexor tendon (stars) at the level of the metacarpophalangeal joint (pentagon) of a hand. The tendon is intact and displays the normal fibrillar pattern of parallel fibers. The tendon sheath is distended by lobulated hypoechoic hypertrophic synovium and fluid (arrowheads); B. same ultrasound image as in Figure 9A with addition of color Doppler demonstrating red color in areas of hyperemia of the thickened tendon sheath and synovium (arrowheads) due to active inflammation
Enthesopathy is a chronic degenerative condition of the entheses of the connective tissues tendons or ligaments and bones. This can be associated with disorders such as gout, ankylosing spondylitis, and psoriatic arthritis. Ultrasound may show hypoechoic soft tissue changes at tendon insertions or fascial insertions like the plantar fascia. Bony spur formation and erosions indicative of reactive bony resorption may be seen (Figure 11). Enthesitis is active inflammation in association with an enthesopathy demonstrating hyperemia and adjacent fluid accumulation with ultrasound.
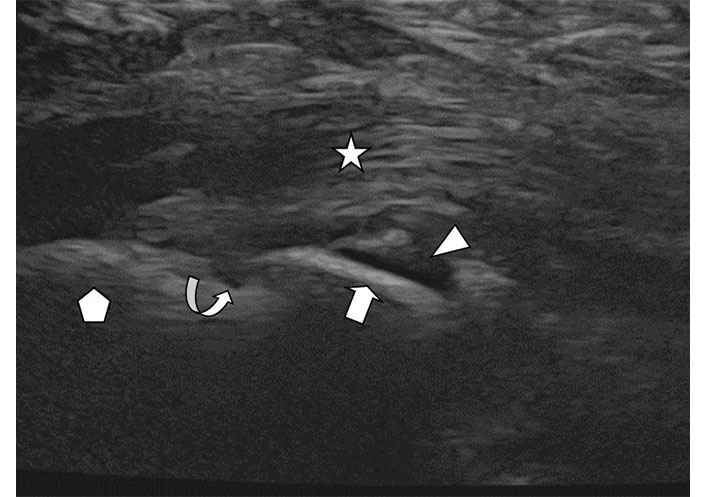
Enthesopathy. Longitudinal ultrasound image of the plantar fascia (star) insertion at the calcaneus (pentagon). There is a calcaneal osseous spur (arrow). Adjacent fluid (arrowhead) and a small hypoechoic erosion (curved arrow) at the junction of the spur with the calcaneus are seen due to local inflammatory changes
Ultrasound is useful as a guidance tool for interventions. While imaging an abnormality in real time the examiner can guide an instrument such as a needle under direct sonographic visualization to the structure (Figure 12A and B) for the purpose of aspiration or biopsy, delivery of injectable medications or other interventions. In rheumatologic conditions this is helpful to obtain material for fluid or tissue diagnosis or to provide targeted cortisone injection therapy of acute synovitis involving joints, tendons, and bursae. These procedures are performed under sterile conditions and with local anesthesia in an outpatient setting [22].
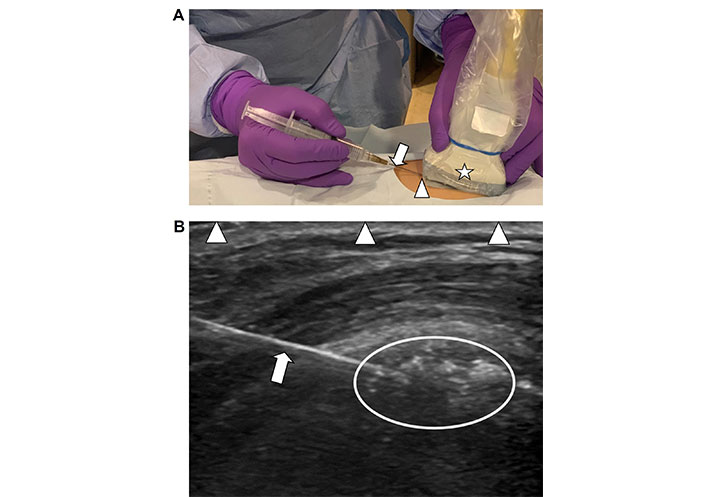
Technique of ultrasound guided needle placement. A. Demonstration of the technique of using a handheld ultrasound transducer (star) to guide a hypodermic fine needle (arrow) into soft tissues to target an abnormality for therapy. The needle is entering the skin at the short end of the transducer (arrowhead) and is guided into the field of view deep to the transducer; B. corresponding ultrasound image of diseased soft tissues with visualization of conglomeration of echogenic amorphous calcifications in a local bursa (encircled area). The needle is seen as a smooth echogenic line (arrow) entering the image from the left side of the transducer and has been placed on an angle to target the depth of calcifications within the soft tissues for lavage. The arrowheads are outlining the interface of the longitudinal axis of the transducer-skin interface which defines the extent of the field of view of the underlying soft tissues
Ultrasound is a versatile imaging tool in the evaluation and treatment of a wide spectrum of rheumatologic conditions. It provides excellent imaging resolution for most musculoskeletal structures and depicts earliest abnormalities such as synovitis and erosion, often before apparent with radiography or MRI. Color Doppler ultrasound provides information about vascularity and aids in the assessment of acute versus chronic inflammation. It is valuable in diagnosis and monitoring disease undergoing treatment. Ultrasound can also be used as imaging guidance for interventional procedures in rheumatologic diseases to deliver medication or obtain samples for diagnosis.
Ultrasound is an operator dependent imaging tool. Expertise and experience of the examiner is the most important factor in utilizing this imaging technique to its fullest potential in clinical practice.
CPPD: calcium pyrophosphate deposition disorder
EULAR: European League Against Rheumatism
MHz: megahertz
MRI: magnetic resonance imaging
OMERACT: Outcomes Measures in Rheumatology
ALG: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. RKT: Validation, Writing—review & editing. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Our IRB at the university of Penn confirmed that the usage of the images in this article does not require IRB approval at our institution.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 5064
Download: 63
Times Cited: 0
Silvia Magni-Manzoni
Janeth Yinh ... Ali Guermazi
Gregory J. Challener ... Minna J. Kohler
Jürgen Braun, Björn Bühring
