Affiliation:
Department of Pharmacy Practice, Rueckert-Hartman College of Health Professions, Regis University, Denver, Colorado 80221, USA
Email: Lshea@regis.edu
ORCID: https://orcid.org/0000-0002-4861-2626
Affiliation:
Department of Pharmacy Practice, Rueckert-Hartman College of Health Professions, Regis University, Denver, Colorado 80221, USA
ORCID: https://orcid.org/0009-0007-8511-3619
Explor Musculoskeletal Dis. 2025;3:100787 DOI: https://doi.org/10.37349/emd.2025.100787
Received: December 16, 2024 Accepted: February 12, 2025 Published: March 03, 2025
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Biosimilars: State of the Art in the Treatment of Rheumatic Diseases
Biosimilars are biologic products that provide equal mechanisms and efficacy to that of their original biologic references. This paper aims to provide a comprehensive overview of the numerous ways biosimilars are improving care for individuals living with rheumatoid arthritis (RA), from the effective application of biosimilars in treatment-naive RA patients, switching from an original biologic to a biosimilar, to the ability to tailor biologic therapy in respect to mechanisms provided by different biologic classes. Biosimilars provide a significant reduction in cost and provide patients with treatment options that do not exhibit adverse drug reactions (ADRs) as exhibited with methotrexate and other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). Understanding mechanisms to discern patient response to biologic therapies will gain increasing importance as biosimilars with different targeted mechanisms enter the market. Patients who do not respond to one class of biologic medicine now have alternative biosimilars available to support their care. Study results support that patients initiated on biosimilars stay on biosimilars, so it is prudent to remain aware of the biosimilars available and candidates in development.
Biological disease-modifying antirheumatic drugs (bDMARDs), are designed to target specific mediators of the immune system contributing to rheumatoid arthritis (RA) inflammation. They are more selective than conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), while providing a faster onset to symptom relief and remission [1]. Biologic DMARDs represent a significant improvement in the treatment of RA and other autoimmune diseases, however, the costs associated with this class of medication remain a barrier. Biosimilars offer a cost-effective alternative, making this powerful class of medications more accessible by removing financial obstacles to their use.
The bDMARDs are not “one size fits all”. The 2024 updated recommendations by the French Society of Rheumatology for diagnosis and management of RA provide guidance on when select bDMARDs may be preferred over others, such as when individuals become pregnant or wish to become pregnant, in the presence of cardiovascular comorbidities, or those with a history of cancer [2]. Figure 1 provides a depiction of recommendations based on coexisting conditions, as recommended by expert guidelines, corresponding clinical studies, and translational evidence.
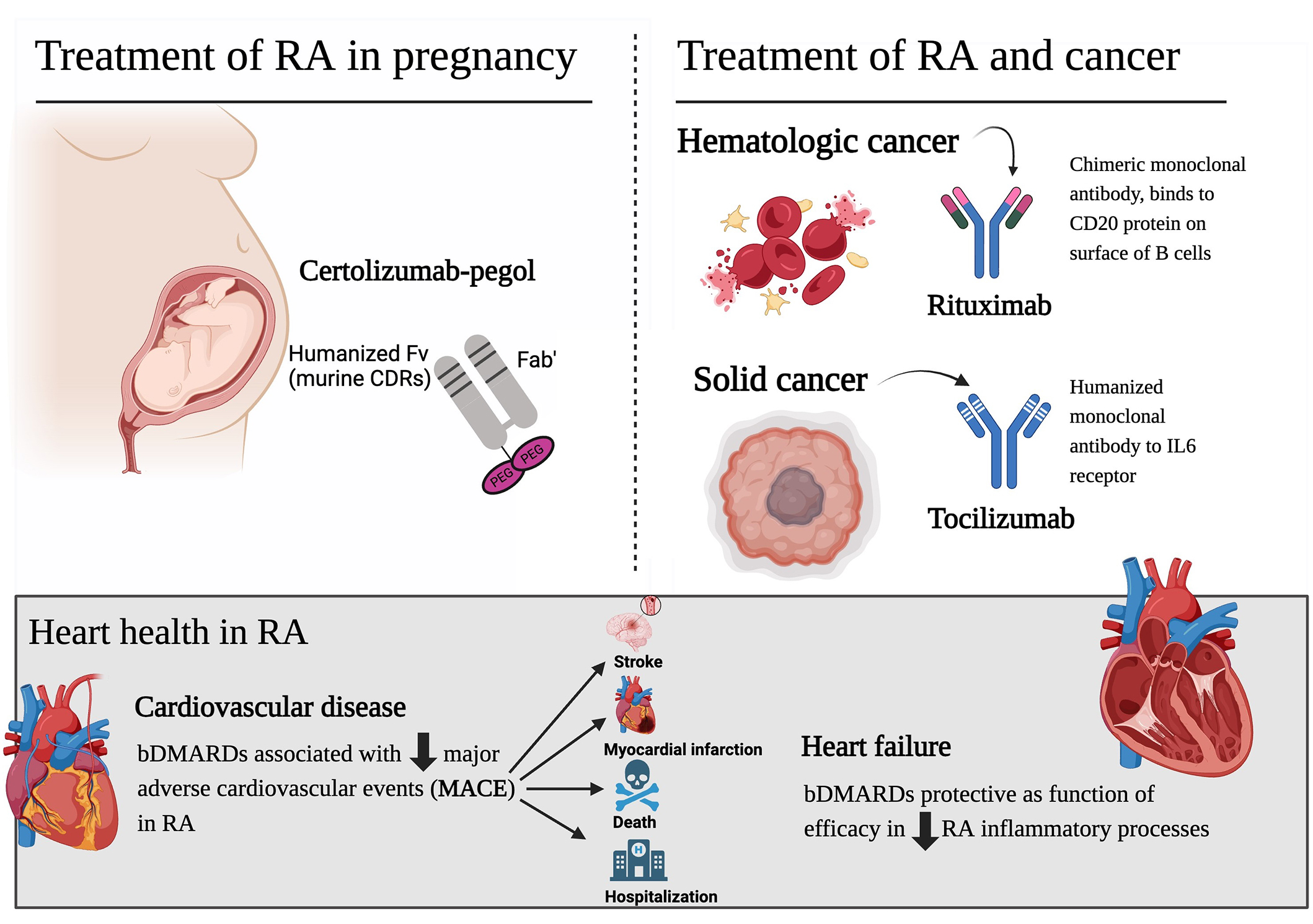
Tailored bDMARDs for RA with coexisting conditions [2–8]. bDMARDs: biological disease-modifying antirheumatic drugs; RA: rheumatoid arthritis; PEG: polyethylene glycol; CDRs: complementarity-determining regions. Downward arrows indicate ‘decreasing’. Created in BioRender. Shea, L. (2025) https://BioRender.com/y00r741
Certolizumab-pegol, the preferred tumor necrosis factor α inhibitor (TNFα-i) in pregnancy, does not have a biosimilar available. Fortunately, there is one currently in development [9]. The lack of a certolizumab-pegol biosimilar is an important deficiency in available biosimilars due to the safety of this biologic in pregnancy and breastfeeding. Certolizumab-pegol lacks the Fc fragment of IgG, which is what binds to the neonatal Fc receptor and is transported to the placenta [3]. IgG antibodies are antibodies that are transferred from the mother to the fetus [3]. When evaluating the placental transfer of TNFα-i therapies, certolizumab-pegol levels were below detection whereas detectable levels were identified with infliximab, adalimumab, and etanercept [3]. The exposure of TNFα-i to the fetus is not ideal, however, TNFα-i therapies are considered safe in pregnancy, with a Food and Drug Administration (FDA) pregnancy level B [3]. The discontinuation of TNFα-i may be more harmful as increased levels of TNFα can negatively impact the pregnancy [3].
In individuals living with RA and a history of cancer, certain targeted therapies may be preferred over others [2]. Rituximab targets the CD-20 antigen on B-cells [10]. It is indicated for the treatment of B-cell (lymphocyte) cancers, so it provides an effective treatment modality for both hematologic cancers and RA. Interleukin-6 (IL-6), a pro-inflammatory cytokine, plays a role in promoting tumor growth and metastasis in various solid cancers [2–9, 11]. Tocilizumab, an IL-6 receptor inhibiting monoclonal antibody, may be beneficial in modulating the tumor microenvironment. However, more research is needed to establish its role in cancer prevention or treatment. The inflammatory processes involved in RA already increase an individual’s risk for cancer, so targeted therapy that effectively results in remission is protective. Importantly, the ideal option for any individual living with RA can only truly be determined between the rheumatologist and their patient.
RA inflammation has systemic consequences leading to accelerated atherosclerosis, increased plaque burden, thrombogenesis, and plaque vulnerability [5, 6]. It is essential to treat to remission to prevent the worsening of disease, disability, and significant cardiovascular morbidity and mortality [5, 6]. A study evaluating biologics and major adverse cardiovascular events (MACE) identified individuals with RA (with high disease activity at baseline) not on bDMARDs were associated with a greater risk of MACE than those with high disease activity and on bDMARDs [7]. The findings indicate that it is not simply the reduction in disease activity that provides cardiovascular protection, as the reduction in risk identified by those on bDMARDs included responders and non-responders [7]. A real-world study performed in Germany identified that TNFα-i in the setting of RA is more likely to be protective than harmful for patients with heart failure (HF) [8]. This is relevant as a clinical trial investigating the TNFα-i, infliximab, as a treatment option for HF resulted in patients in the infliximab arm exhibiting higher incidences of worsening HF and increased HF-associated hospitalizations [12]. The importance is the distinction that the worsening of HF and hospitalizations was demonstrated in trials evaluating infliximab for the treatment of HF, not in individuals with RA and HF [12]. In the setting of RA, TNFα-i therapy has consistently exhibited a cardiovascular risk reduction [5–8, 13, 14].
RA is an autoimmune condition in which early and effective treatment is imperative to arrest the inflammatory processes that manifest well before clinical symptoms present. Inflammation present with RA is often depicted with a picture of a hand showcasing the deleterious effects on the joint composition, but RA inflammation has systemic consequences [5, 6]. The deleterious inflammation present with RA may also contribute to the development of other autoimmune conditions, as overlap in cytokine pathways and immune mechanisms exist [15]. The bDMARDs indicated for RA excel in targeting and inhibiting inflammatory pathways responsible for the manifestation of the disease [1, 16]. Furthermore, bDMARDs have demonstrated superior outcomes when compared to csDMARDs in improving clinical disease activity, physical function, and inflammation identified with magnetic resonance imaging (MRI) [1]. A retrospective cohort identified that methotrexate step-up therapy with csDMARDs demonstrated less efficacy than TNFα-i with methotrexate [17]. Some guidelines do not provide preference for TNFα-i over other bDMARDs [2]. In the United States (US), TNFα-i is recommended as initial step-up therapy for individuals with moderate to severe disease activity that have not responded to maximally tolerated methotrexate therapy [4]. Fortunately, TNFα-i currently have the most biosimilar options in the RA bDMARD artillery [18]. When individuals do not respond to TNFα-i therapy, there are additional biosimilar options available. However, failure to respond to one does not indicate an individual will not attain benefit from a different TNFα-i. These therapies all provide inhibitory action on the cytokine TNFα, however, the mechanisms, affinity, binding, and molecular structures are different [10].
For those that TNFα-i therapy does not result in remission, there are additional bDMARDAs available as biosimilars: rituximab and tocilizumab [18].
While biosimilars are less costly than original reference products, they still represent a significant financial investment. The biosimilars currently available on the market provide equivalent safety and efficacy [19–24]. Because biologics are more complex to identify, develop, and study, biosimilars do not share the same reduction in production cost as seen with generics for synthetic molecules. Biosimilars do not have the extensive research and development costs to re-coup as the original biologic (reference) product but still have an expensive journey in research costs (to ensure the biosimilar has the same efficacy as the reference), and due to complex manufacturing processes.
Figure 2 provides a depiction of when biosimilars were approved by the FDA as a prescription medication, and when launched onto the US market. The launch indicates when the biosimilar is truly available to the public. There may be a significant gap between FDA approval and market launches due to reference product patent extensions.
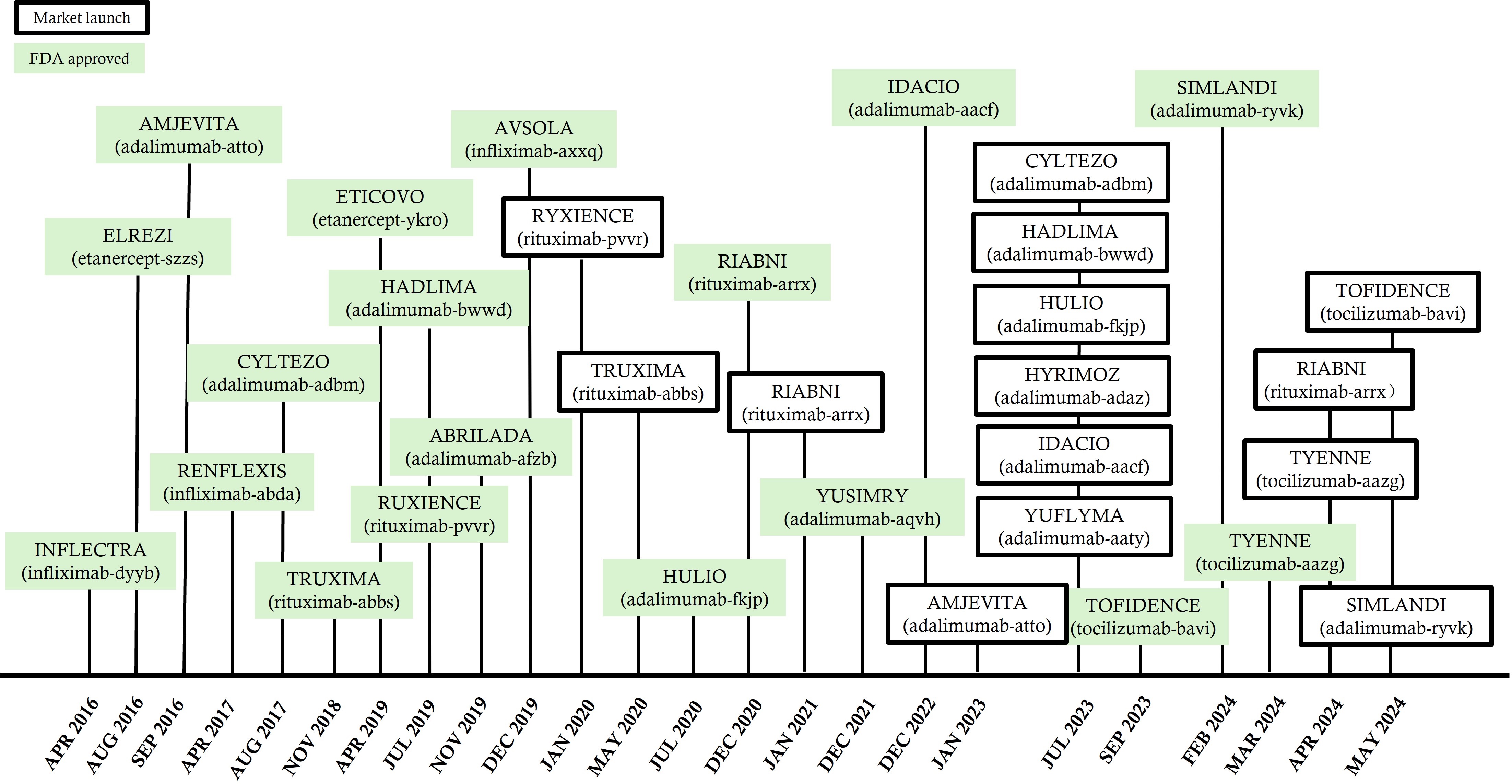
US timeline for biosimilars indicated for RA; approval and launches [18]. RA: rheumatoid arthritis; FDA: Food and Drug Administration
Biosimilars indicated for the treatment of RA available in the US and/or Canada are provided in Table 1. There are several biosimilars available for adalimumab and infliximab. Etanercept biosimilars are available in Canada, but not yet in the US due to patent extensions. Rituximab and tocilizumab biosimilars are also available in both countries, providing additional biologic classes for those not attaining remission/low disease activity with TNFα-i therapy, or those with a history of cancer. You will note that the naming of biosimilars in Canada does not include the 4 letters assigned to biosimilars as required with biosimilars in the US [25].
| Biologic original (reference) | Biosimilar(s) available in the US | Biosimilar(s) available in Canada |
|---|---|---|
| TNFα-i | ||
| Adalimumab (Humira®) |
|
|
| Certolizumab pegol (Cimzia®) | None | None |
| Golimumab (Simponi®) | None | None |
| Etanercept (Enbrel®) | 2 approved but not available |
|
| Infliximab (Remicade®) |
|
|
| CD20 antigen substrate that leads to B-cell lysis | ||
| Rituximab (Rituxin®) |
|
|
| IL-6-receptor inhibitor | ||
| Tocilizumab (Actemra®) |
| Tyenne (tocilizumab) |
| Sarilumab (Kevzara®) | None | None |
| Co-stimulatory inhibitor of T cell activation | ||
| Abatacept(Orencia®) | None | None |
RA: rheumatoid arthritis; TNFα-i: tumor necrosis factor α inhibitor; IL-6: interleukin-6
Although all TNFα-i’s demonstrated remarkable efficacy in treating RA, each biologic exhibits distinct characteristics. Adalimumab is a fully human monoclonal antibody [10]. Etanercept is a fusion protein consisting of two human p75 TNFα receptors (specifically the TNFR2) coupled to the Fc portion of a human antibody [10]. Infliximab is a humanized-chimeric antibody, engineered with a predominant human sequence (75%) and a smaller murine component (25%) [26]. While all three TNFα-i have been shown to be efficacious in RA, etanercept does not exhibit efficacy in the treatment of inflammatory bowel disease (IBD) [26]. When evaluating the differences between the therapies, it has been identified that etanercept exhibits less stable complexes with soluble TNFα, and binds to TNFα with a 1:1 ratio, rather than infliximab which has been shown to bind to all 3 binding sites [26, 27].
Infliximab was the first RA biosimilar to become available in the US in 2016 [18]. A study performed in European countries evaluating cost-savings obtained with the use of the infliximab biosimilar in comparison to the reference reported significant cost reductions ranging from 22.37 million to 25.7 million Euros [28].
Table 2 provides a snapshot of clinical trials and American College of Rheumatology 20% improvement (ACR20) comparisons at 24 weeks for adalimumab and etanercept biosimilars and their reference products. See Table 3 for a description of ACR scoring.
| Biosimilar compared to reference | Patients on biosimilar (n) | Patients on reference (n) | Biosimilar ACR20 (%) | Reference ACR20 (%) |
|---|---|---|---|---|
| PF-06410293 (adalimumab-afzb); Abrilada® | 297 | 300 | 68.7 | 72.7 |
| MSB11022 (adalimumab-aacf); Idacio® | 147 | 145 | 85.7 | 82.8 |
| FKB327 (adalimumab-fkjp); Hulio® | 366 | 362 | 77 | 79.3 |
| BI 695501 (adalimumab-adbm); Cyltezo® | 321 | 318 | 68.8 | 64.5 |
| BI 695501 (adalimumab-adbm); Cyltezo® | 297 | 300 | 83.2 | 77.7 |
| SB5 (adalimumab-bwws); Hadlima® | 271 | 273 | 67.2 | 67.4 |
| ABP 501 (adalimumab-atto); Amjevita® | 264 | 261 | 73.5 | 72.4 |
| SB4 (etanercept-ykro); Eticovo® | 299 | 297 | 80.8 | 81.5 |
| GP2015 (etanercept-szzs); Elrezi® | 186 | 190 | 88.8 | 93.6 |
TNFα-i: tumor necrosis factor α inhibitor; ACR20: American College of Rheumatology 20% improvement
RA assessment & scoring tools [32]
| Assessment tools | Description |
|---|---|
Disease Activity Score-28 (DAS28)
| Scale 0–9.4
|
Clinical trial assessment tool
| Scoring system
|
RA: rheumatoid arthritis; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; VAS: visual analogue scale
Important studies for biosimilars evaluate if efficacy and safety are maintained when individuals switch from the reference (the original biologic) to the biosimilar, or variations thereof in crossover studies. The Spanish RESTART study evaluating the biosimilar MSB11022 (adalimumab-aacf) identified that efficacy and safety were maintained in individuals switched from the reference to the biosimilar [31]. This is consistent with other trials of this adalimumab biosimilar, and the other biosimilars currently available [20, 29–31, 33].
Rituximab is generally not recommended as a first-line biologic in the setting of RA [4]. This biosimilar is often reserved for patients with moderate to severe RA unresponsive to other DMARDs, or those with a history or active hematologic cancer.
The rituximab biosimilar provided a cost reduction of up to 570 million Euros following a budget impact analysis of 1–3 years [34]. A powerful finding reported was the rituximab biosimilar enables significantly more individuals to receive rituximab treatment for those with RA and cancer, with estimates between 7,531 to 47,696 more patients (depending on the scenario) obtaining access that would not be economically feasible without the cost savings exhibited with the biosimilar [34].
Tocilizumab is often reserved for moderate-severe RA that has not responded to other forms of RA therapy, such as methotrexate+/– TNFα-i [35]. Interestingly, several trials evaluating tocilizumab have identified this bDMARD to be effective as monotherapy. The AMBITION (Actemra versus Methotrexate double-Blind Investigative Trial In mONotherapy) demonstrated tocilizumab monotherapy provided superior efficacy to methotrexate monotherapy, with Disease Activity Score-28 (DAS28) remission rates of 33.6% in the tocilizumab cohort versus 12.1% in the methotrexate cohort [36]. See Table 3 for a description of DAS28. The ACT-RAY study evaluated the difference in efficacy of tocilizumab monotherapy versus tocilizumab + methotrexate [37]. Results for the combination cohort included increased incidences of adverse drug reactions (ADRs) while not demonstrating superiority to tocilizumab monotherapy [37]. Elevated liver enzymes (alanine aminotransferase, ALTs) were reported as threefold the upper limit of normal in 7.8% of the combination cohort versus 1.2% for monotherapy [37]. The ADACTA trial evaluated tocilizumab monotherapy versus adalimumab monotherapy in the setting of RA in which patients were intolerant to methotrexate therapy [38]. Tocilizumab exhibited superior efficacy over adalimumab in the primary endpoint of DAS28, with a difference in DAS28 between the two groups of –1.5 [95% confidence interval (CI): –1.8 to –1.1; p < 0.0001] [38]. A follow-up study was performed to evaluate the cost-effectiveness exhibited between tocilizumab monotherapy and adalimumab monotherapy [39]. The mean cost associated with administration and achieving DAS28 < 2.6 was $45,868 for tocilizumab, compared to $244,174 for adalimumab [39]. Tocilizumab also yielded significant cost savings in achieving ACR20, ACR50, and ACR70 responses, with the latter resulting in a difference of $56,253 for tocilizumab and $143,136 for adalimumab monotherapy [39]. Tocilizumab monotherapy is a potent and economically viable bDMARD for individuals living with RA who have not achieved adequate disease control with other therapeutic options.
Current guidelines indicate that methotrexate is generally first line for patients with RA, except for those with low disease activity at diagnosis, in which current ACR guidelines recommend consideration of hydroxychloroquine prior to methotrexate [4]. Yes, methotrexate is an effective first-line medication. However, methotrexate is associated with many ADRs [40]. One ADR rarely mentioned because it is aesthetic rather than leading to additional poor outcomes, such as pulmonary toxicity, is methotrexate-induced alopecia [41]. Łukasik et al. [41] performed a study to truly evaluate the incidence of hair loss in patients initiating methotrexate for RA and found that hair loss occurred in almost 30% of the patients. This is a significant difference from the 1–3% incidence commonly reported [42]. The discussion of hair loss, or alopecia, had to be stated because it is rarely mentioned and is worthy of consideration in and of itself. However, what is well-known about methotrexate is the significant ADR profile [40]. When considering the importance of treating RA to remission, and the 20–30% reported discontinuation within the first year of methotrexate due to adverse events [40], it is easy to understand how RA will progress to a worsening state for many individuals. Methotrexate is an affordable option that is known to provide a level of efficacy necessary to halt disease progression, however, not without the cost of significant ADRs [40, 41]. Biologic therapies offer potent treatment options for RA that enhance efficacy while simultaneously preserving or improving patients’ quality of life.
Molecular signatures can aid in the determination of those that may respond better to one therapy over another. Due to cost restraint and insurance barriers, obtaining a molecular signature may not lead to any change if the recommended therapy is not on the insurance formulary. However, with biosimilars being less expensive than reference biologics, there is hope that future molecular signature guidance may provide actionable information to support personalized and improved treatment of RA.
Rivellese et al. [43] performed a precision medicine randomized clinical trial that included a comprehensive analysis of molecular signatures and the development of machine learning classifications to identify complex mechanisms that may lead to response or poor response from biologic agents (rituximab and tocilizumab) in the setting of RA. This trial addressed the heterogeneity of the disease down to cell lineages, genes, genetic pathways, and molecular signatures. If ever there was a clinical trial that was a masterpiece, this would be it. Furthermore, they provide evidence to identify when an individual is more likely to respond to tocilizumab in place of rituximab [43]. The authors share that in patients with low synovial B-cell signatures, only 12% of the patients responded to rituximab, whereas 50% of those with low synovial B-cell signatures responded to tocilizumab [43]. These responses align with the distinct mechanisms of action of the biologics: rituximab specifically targets B-cells, whereas tocilizumab acts through a B-cell-independent pathway [43]. Thus, an individual that has an inflammatory disease that exhibits less B-cell activity involved with the inflammation, is less likely to respond to a therapy that targets B-cells. These findings are extremely relevant to current practice as most patients who are indicated for biologic therapy in the treatment of RA must try and fail at least 1 to 2 TNFα-i prior to having coverage for a different biologic. With tocilizumab now available as a biosimilar, there are 2 classes of non-TNFα-i biologics that are available in the biosimilar space, meaning TNFα-i are not the only biologics available at a reduced cost. If tocilizumab is not a preferred next-line agent for an insurance formulary, knowing the patient is likely to fail with rituximab based on a molecular signature can offer insight when working on insurance appeals. Understanding mechanisms to discern patient response to biologic therapies will become more and more important as machine learning is implemented into clinical practice.
A retrospective study evaluating the retention time in individuals treated with original and biosimilar biologics of adalimumab and etanercept identified that the biosimilar for etanercept resulted in improved retention time compared to that of the original biologic (45 months versus 19 months of therapy, p = 0.0265) [44]. A large observational study in Sweden evaluated several of the currently available biosimilars and their original products to determine if differences in retention were identified at 1 year [45]. This study evaluated the reference and biosimilars for adalimumab, etanercept, infliximab, and rituximab [45]. Importantly, they evaluated two distinct scenarios that are important when evaluating biosimilars in post-marketing analysis: (1) treatment retention for those starting and staying on either reference or biosimilar and (2) treatment retention when started on reference and switched to biosimilar [45]. The study identified an intriguing finding, also reported by Larid et al. [44], in which improved retention of the biosimilar for etanercept was exhibited in those that started the etanercept biosimilar and stayed on that therapy [hazard ratio (HR) 0.91 (0.83–0.99)] [44, 45]. In cohorts that had switched from a reference to a biosimilar, no statistically significant differences in therapy retention were identified at 1 year [45].
Biosimilars provide therapeutically equivalent outcomes at a lower price point, addressing the dual challenges of clinical efficacy and economic accessibility in RA. While it would be beneficial to have biosimilars for all current biologics on the market for the treatment of RA, one biologic that is missing and is of particular importance is certolizumab-pegol, as this biologic is the preferred bDMARD for individuals wishing to become pregnant or are pregnant living with RA. It is evident that biosimilars are advantageous for both patients and health systems. Supporting biosimilar approvals enables improved access and care for individuals living with RA, and significant cost reductions for patients and health systems.
ACR20: American College of Rheumatology 20% improvement
ADRs: adverse drug reactions
bDMARDs: biological disease modifying antirheumatic drugs
csDMARDs: conventional synthetic disease modifying antirheumatic drugs
DAS28: Disease Activity Score-28
FDA: Food and Drug Administration
HF: heart failure
IL-6: interleukin-6
MACE: major adverse cardiovascular events
MRI: magnetic resonance imaging
RA: rheumatoid arthritis
TNFα-i: tumor necrosis factor inhibitor
US: United States
LAS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. JSA: Investigation, Writing—original draft.
Both authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 5899
Download: 35
Times Cited: 0
Fernando Perez-Ruiz ... Amaya de Basagoiti-Gorordo
Gilberto Castañeda-Hernández
Fernando Pérez-Ruiz ... Eugenio Chamizo Carmona
Fanny Alcira Reyes Neira ... Andrea Yukie Shimabuco
Lauren N. McGrath ... Steven R. Feldman
