-
 Special Issue Topic
Special Issue TopicBiosimilars: State of the Art in the Treatment of Rheumatic Diseases
Submission Deadline: July 01, 2024Guest Editor
Prof. Valderilio Feijó Azevedo E-Mail
Federal University of Paraná, Brazil
Research Keywords: Spondyloarthritis, gout, biopharmaceuticals, biosimilars, gut microbioma, psoriatic arthritis, pharmacoeconomics
About the Special Issue
Widespread use of biological medicines has transformed outcomes for many patients with inflammatory conditions such as autoimmune rheumatic disorders (ARDs). These therapies come at a price, however, and as their patents expire, a new class of products, very regulated copies named biosimilars are entering the market. Biosimilars are more affordable for healthcare organisations and enable greater patient access to treatment. Biosimilars are subject to a robust regulatory framework to gain approval worldwide. These products have shown biosimilarity in terms of structure, biological activity and therapeutic equivalence in efficacy, safety, and immunogenicity profile. Since the approval of the first Mab Biosimilar used to treat Rheumatic diseases in 2013, many other biosimilars are available as safe and cost reduced options to treat ARDs. A number of real-world scenarios, of a medical and non-medical nature, may lead to cross-switching between biosimilars of the same reference product. This special issue intend to discuss and point out new challenges for biosimilars in rheumatology.
Keywords: Biosimilars, biologic medicines, biopharmaceuticals, reference products, rheumatic diseases
Call for Papers
Published Articles
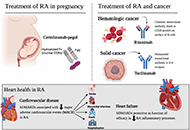 Highly effective treatment options for rheumatoid arthritis afforded by the availability of biosimilarsOpen AccessCommentaryBiosimilars are biologic products that provide equal mechanisms and efficacy to that of their original biologic references. This paper aims to provide a comprehensive overview of the numerous ways b [...] Read more.Leticia A. Shea, Jamshaid S. AhmedPublished: March 03, 2025 Explor Musculoskeletal Dis. 2025;3:100787
Highly effective treatment options for rheumatoid arthritis afforded by the availability of biosimilarsOpen AccessCommentaryBiosimilars are biologic products that provide equal mechanisms and efficacy to that of their original biologic references. This paper aims to provide a comprehensive overview of the numerous ways b [...] Read more.Leticia A. Shea, Jamshaid S. AhmedPublished: March 03, 2025 Explor Musculoskeletal Dis. 2025;3:100787
DOI: https://doi.org/10.37349/emd.2025.100787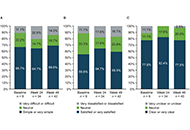 Transition from reference adalimumab to biosimilar SB5 in patients with rheumatoid arthritis: sub-analysis of Spanish patients in the PROPER studyOpen AccessShort CommunicationThis sub-analysis of the PROPER study aimed to evaluate outcomes following the transition from reference adalimumab (ADL) to SB5 (Imraldi™) in routine clinical practice in Spanish patients with rh [...] Read more.Fernando Pérez-Ruiz ... Eugenio Chamizo CarmonaPublished: February 10, 2025 Explor Musculoskeletal Dis. 2025;3:100784
Transition from reference adalimumab to biosimilar SB5 in patients with rheumatoid arthritis: sub-analysis of Spanish patients in the PROPER studyOpen AccessShort CommunicationThis sub-analysis of the PROPER study aimed to evaluate outcomes following the transition from reference adalimumab (ADL) to SB5 (Imraldi™) in routine clinical practice in Spanish patients with rh [...] Read more.Fernando Pérez-Ruiz ... Eugenio Chamizo CarmonaPublished: February 10, 2025 Explor Musculoskeletal Dis. 2025;3:100784
DOI: https://doi.org/10.37349/emd.2025.100784 Efficacy of switching from originator adalimumab to biosimilar adalimumab-AACF in patients with axial spondyloarthritis: a 12-month observational studyOpen AccessOriginal ArticleAim: The use of anti-TNF drugs is well-established for treating axial spondyloarthritis (axSpA). The introduction of biosimilars offers a more accessible alternative, but data on the switching of [...] Read more.Fanny Alcira Reyes Neira ... Andrea Yukie ShimabucoPublished: February 10, 2025 Explor Musculoskeletal Dis. 2025;3:100783
Efficacy of switching from originator adalimumab to biosimilar adalimumab-AACF in patients with axial spondyloarthritis: a 12-month observational studyOpen AccessOriginal ArticleAim: The use of anti-TNF drugs is well-established for treating axial spondyloarthritis (axSpA). The introduction of biosimilars offers a more accessible alternative, but data on the switching of [...] Read more.Fanny Alcira Reyes Neira ... Andrea Yukie ShimabucoPublished: February 10, 2025 Explor Musculoskeletal Dis. 2025;3:100783
DOI: https://doi.org/10.37349/emd.2025.100783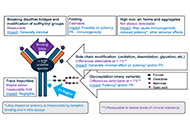 Optimizing development of anti-TNFα biosimilars based on 10 years’ experienceOpen AccessReviewRoutine regulatory requirements for large comparative efficacy trials (CETs) to support marketing approval of monoclonal antibody (mAb) biosimilars have been the focus of extensive debate in the las [...] Read more.Cecil NickPublished: February 08, 2025 Explor Musculoskeletal Dis. 2025;3:100782
Optimizing development of anti-TNFα biosimilars based on 10 years’ experienceOpen AccessReviewRoutine regulatory requirements for large comparative efficacy trials (CETs) to support marketing approval of monoclonal antibody (mAb) biosimilars have been the focus of extensive debate in the las [...] Read more.Cecil NickPublished: February 08, 2025 Explor Musculoskeletal Dis. 2025;3:100782
DOI: https://doi.org/10.37349/emd.2025.100782 Selecting the best-value biosimilar in emerging countriesOpen AccessPerspectiveThe aim of biosimilars is to alleviate the financial burden of biological medicinal products. A most relevant challenge for emerging countries is how to select the best option available. In most cas [...] Read more.Gilberto Castañeda-HernándezPublished: September 14, 2024 Explor Musculoskeletal Dis. 2024;2:423–430
Selecting the best-value biosimilar in emerging countriesOpen AccessPerspectiveThe aim of biosimilars is to alleviate the financial burden of biological medicinal products. A most relevant challenge for emerging countries is how to select the best option available. In most cas [...] Read more.Gilberto Castañeda-HernándezPublished: September 14, 2024 Explor Musculoskeletal Dis. 2024;2:423–430
DOI: https://doi.org/10.37349/emd.2024.00067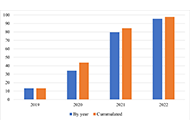 Successful implementation of interchange of biologic medications in chronic arthritis after information of costs to prescribersOpen AccessOriginal ArticleAim: To evaluate the impact of prescription, cost, and switching policy on the rate of switching from reference products to biosimilars. Methods: Analysis of an administrative database for [...] Read more.Fernando Perez-Ruiz ... Amaya de Basagoiti-GorordoPublished: September 10, 2024 Explor Musculoskeletal Dis. 2024;2:384–390
Successful implementation of interchange of biologic medications in chronic arthritis after information of costs to prescribersOpen AccessOriginal ArticleAim: To evaluate the impact of prescription, cost, and switching policy on the rate of switching from reference products to biosimilars. Methods: Analysis of an administrative database for [...] Read more.Fernando Perez-Ruiz ... Amaya de Basagoiti-GorordoPublished: September 10, 2024 Explor Musculoskeletal Dis. 2024;2:384–390
DOI: https://doi.org/10.37349/emd.2024.00064 -
-
Ongoing Special Issues
-
Completed Special Issues