8 results in Exploration of Neuroscience
Latest
Sort by :
- Latest
- Most Viewed
- Most Downloaded
- Most Cited
Open Access
Protocol
Protocol for measuring the benefits of exergames on executive functions under depression through a randomized multi-arm controlled experiment
Eloisa Ruiz-Marquez
Published: December 23, 2024 Explor Neurosci. 2024;3:564–583
This article belongs to the special issue Novel Therapeutic Approaches for the Treatment of Depression

Open Access
Original Article
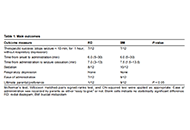
Buccal midazolam vs rectal diazepam administered by parents for continuing and serial epileptic seizures: a randomised controlled trial of parental preferences
Hoong Wei Gan ... William P. Whitehouse
Published: December 20, 2024 Explor Neurosci. 2024;3:559–563
This article belongs to the special issue Advances in Epilepsy Research
Open Access
Perspective
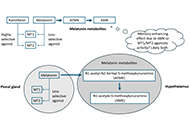
What might melatonin supplementation provide for humans beyond improved onset to sleep?
Leticia A. Shea
Published: November 26, 2024 Explor Neurosci. 2024;3:551–558
This article belongs to the special issue Circadian Rhythm and Melatonin
Open Access
Review
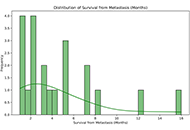
Metastatic glioblastoma multiforme on skin and subcutaneous tissue
Maria Ciscar-Fabuel ... Andreu Gabarros-Canals
Published: October 29, 2024 Explor Neurosci. 2024;3:539–550
This article belongs to the special issue Current Approaches to Malignant Tumors of the Nervous System
Open Access
Original Article
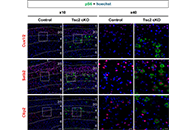
Somatostatin-expressing inhibitory neurons with mTORC1 activation in cortical layers 4/5 are involved in the epileptogenesis of mice
Fumiki Yamashita ... Mari Wataya-Kaneda
Published: October 29, 2024 Explor Neurosci. 2024;3:527–538
This article belongs to the special issue Epilepsy
Open Access
Case Report
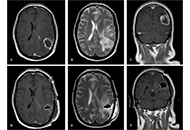
Glioblastoma multiforme recurrence on skin and subcutaneous tissue: a case report
Maria Ciscar-Fabuel ... Andreu Gabarros-Canals
Published: October 16, 2024 Explor Neurosci. 2024;3:520–526
This article belongs to the special issue Current Approaches to Malignant Tumors of the Nervous System
Open Access
Original Article
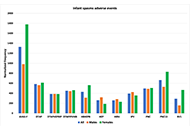
Epilepsy adverse events post vaccination
Darrell O. Ricke
Published: October 16, 2024 Explor Neurosci. 2024;3:508–519
This article belongs to the special issue Epilepsy
Open Access
Original Article
Observing nociceptive detection thresholds and evoked potentials in diabetic patients with and without painful neuropathy
Tom Berfelo ... Jan R. Buitenweg
Published: October 15, 2024 Explor Neurosci. 2024;3:493–507
This article belongs to the special issue Neuropathic Pain

Journal Information
 Previous
Previous