Abstract
The gut microbiota and dysbiosis have been implicated in various metabolic diseases and gastrointestinal disorders. Recently, there has been growing evidence suggesting the influence of gut microbiota on neurological disorders, including autism. Although the number of children diagnosed with autism is increasing, the exact cause of the disease remains unknown. Numerous factors, such as genetics, environment, and diet, appear to contribute to its onset. Nevertheless, a degree of general consensus exists regarding the notion that the disease’s progression likely demands the participation of multiple factors. Among the potential causes, the role of the microbiota is particularly intriguing. The gut and brain have extensive connections, with a significant number of neuronal cells in the gut, and autism is often associated with gastrointestinal issues. In this review, the most recent information available on autism and microbiota has been analyzed. Findings of this study indicate that: (1) the microbiota is clearly altered in individuals with autism spectrum disorder (ASD); (2) microbiota transplantation appears to be effective in reducing the severity of autism symptoms; (3) while the microbiota is not solely responsible for the onset of autism, it likely plays a significant role. Considering all the available information, it is suggested that modifying the gut microbiota may have a positive impact on individuals with autism. This opens up possibilities for the use of pre- or probiotics in the treatment of children with ASD, as well as the potential use of fecal microbiota transfer.
Keywords
Autism spectrum disorder, microbiota, fecal microbiota transplantation, bacteria, etiologyIntroduction
The microbiota is a complex community of microorganisms, including bacteria, viruses, yeasts, and fungi. In humans, the microbiota can be found in various parts of the body, such as the mouth, respiratory tract, skin, and vagina [1]. However, the largest microbiota is found in the gastrointestinal (GI) tract.
There are several key points to consider when describing the gut microbiota. Firstly, yeasts make up less than 1% of the microbiota’s living organisms, while bacteria account for over 90% of the microbiota’s composition. Secondly, the intestinal microbiota consists of two main groups of bacteria: the autochthonous bacteria that reside permanently in the gut mucosa, and a transitory microbiota that depends on the type of food consumed.
The intestinal microbiota is estimated to consist of approximately 1 × 1014 microbial cells, which is ten times the number of eukaryotic cells found in the human body. Furthermore, the gut microbiota is known to include more than 100 distinct species in humans, and over 1,000 species have been identified as potential members of the human gut microbiota. Among the most common bacteria present in the human gut microbiota are Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria, Proteobacteria, Synergistetes, and Verrucomicrobia.
Autism spectrum disorder (ASD), also known as autism, is a neurodevelopmental disorder characterized by a range of symptoms. These symptoms primarily involve impaired communication and altered social skills. The key features of ASD include difficulties in initiating and maintaining social interactions, as well as the presence of repetitive and stereotypical patterns of behavior and interests. Additionally, attention deficit-hyperactivity disorder (ADHD) has been observed in 28% to 44% of individuals with ASD. Common comorbidities in ASD patients include sleep dis-orders, anxiety, and depression. Taste perception can also be affected, leading to restricted food preferences [2].
GI distress is a common issue in up to 79% of children with ASD. Chronic constipation, diarrhea, and abdominal pain are the most frequently reported GI problems [3]. Other symptoms such as gastroesophageal reflux, bloody stools, and vomiting are also observed, often indicating GI inflammation. Individuals with ASD have also been found to have a higher prevalence of food allergies and metabolic disorders [4].
The etiology of autism is currently not well understood. However, it is widely accepted that multiple factors contribute to the development of the disorder. More details are available in Steinman’s article [5].
The underlying neurobiology of ASD involves atypical brain development and connectivity. Brain imaging studies have revealed differences in the structure, function, and connectivity of various brain regions in individuals with ASD. These differences can affect social cognition, sensory processing, and the ability to integrate information from different brain areas.
Genetic factors play a significant role in the development of ASD. Studies have shown that there is a strong hereditary component, with certain genes being associated with an increased risk of developing the disorder [6]. However, ASD is a polygenic condition, meaning that it involves the interaction of multiple genes, each contributing a small risk.
Environmental factors also contribute to the etiology of ASD. Prenatal factors, such as maternal infections, exposure to certain medications during pregnancy, or complications during birth, have been associated with an increased risk. Additionally, there has been ongoing research into the potential role of environmental toxins and pollutants, although their exact contribution remains uncertain [7].
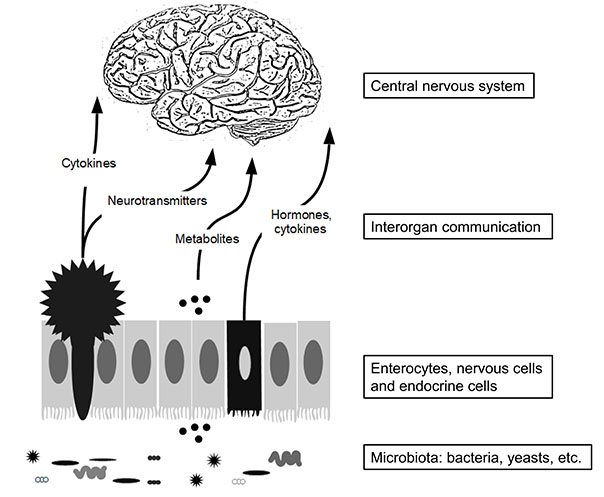
Several studies have reported altered composition of the intestinal microbiota, or dysbiosis, in individuals with ASD [8]. Dysbiosis refers to an unhealthy alteration in the body’s microbiota that can lead to health problems and diseases. In the general population, the use of antibiotics, dietary patterns, and lifestyle factors can induce temporary or long-term changes in the microbiota composition. Conditions like Crohn’s disease and ulcerative colitis, which are common GI disorders, are associated with dysbiosis. In most cases, it is challenging to determine whether dysbiosis is the cause or consequence of the disease. This concept is visually depicted in Figure 1.
Gut microbiota is composed of various bacteria and also contains yeast cells, viruses, etc. The primary cellular composition of the gut epithelium consists predominantly of enterocytes, with the additional presence of nervous cells (star-shaped cells) and endocrine cells (referred to as dark cells) (Figure 1). Altogether these cells contribute to sending messengers to the central nervous system using cytokines, nervous messengers, etc.
It should be mentioned that changes in metabolites derived from gut microbiota in body fluids such as blood and urine, and even in the brain, are an important aspect related to the diagnosis and treatment of diseases [9, 10].
Many publications have addressed the question of a link between microbiota and autism, and among these, three meta-analyses have been conducted. In the present study, the data obtained from these meta-analyses and all reviewed papers published in the last three years on ASD and gut microbiota interactions were compiled. As fecal transplantation has been suggested as a potential treatment for the disease, the analysis focused on the data available in the literature during the same period. A systematic search and review of studies published in the scientific literature in PubMed was followed by a thorough analysis of publications that were considered relevant. Publications that fulfilled the eligibility criteria were selected independently by at least two of the three authors. First, publications were screened by title or abstract to remove irrelevant entries. A critical reading of the selected publication was performed again by at least two of the three authors of this publication. The bibliographic search was performed from the last publication included in the most recent meta-analysis and stopped in November 2022.
The following keywords were used: “autism” and “microbiota” and for the paragraphs concerning fecal transplantation, “fecal transplantation” was added. Only clinical (human) studies and relevant studies were considered.
Alteration of gut microbiota and ASD: meta-analyses and recent publications
Meta-analyses
In July 2019, Xu et al. [11] analyzed nine studies involving 254 individuals with ASD. The gut microbiota of individuals with ASD exhibited a lower abundance of Akkermansia, Bacteroides, Bifidobacterium, Escherichia coli (E. coli), and Enterococcus, a higher abundance of Faecalibacterium and Lactobacillus, and a slightly increased abundance of Ruminococcus and Clostridium. The authors of this study concluded that their meta-analysis suggested an association between ASD and the alteration of microbiota but did not make any hypothesis related to these changes [11].
In another meta-analysis published in 2020, based on 11 clinical trials, the authors found that the gut microbiota of individuals with ASD exhibited a greater abundance of Bacteroidetes and Firmicutes genera, particularly Clostridium, Faecalibacterium and Phascolarctobacterium [8]. The microbiota of individuals also exhibited a lower abundance of Coprococcus and Bifidobacteria. The authors of this study pointed out the possible role of microbiota composition in inflammation and immune system dysfunction. These authors outlined that they were unable to determine whether inflammation alters the microbiota composition or whether it is an alteration of the gut microbiota composition that induces inflammation. They suggested that environmental factors such as diet, lifestyle, exposure to drugs and chemicals, and probiotics could affect gut microbiota composition and influence autism severity.
In a more recent meta-analysis by Andreo-Martínez et al. [12] (2022), 18 studies were selected and analyzed. The authors concluded that ASD can be associated with a lower relative abundance of the Streptococcus and Bifidobacterium genera in children. However, the ultimate conclusion of these studies is that these differences can also be attributed to bias. In fact, this meta-analysis was relatively poorly informative [12].
From these meta-analyses, it can be concluded that (i) the microbiota is altered in patients with ASD, (ii) the association between gut microbiota and severity of autistic symptoms is difficult to identify, (iii) no particular group of bacteria can be identified as fully responsible.
Update on ASD and microbiota composition
Several research articles have been published over the last three years and since the previous meta-analyses. The Autism and Developmental Disabilities Monitoring Network in the United States can be used as a reference because 7,611 papers from the United States were published on childhood ASD from January 1, 2012, to December 31, 2021 [13].
In 2020, Ahmed et al. [14] reported a study including 41 children, in this study, the authors concluded that there was no correlation between the nature of the alteration of the gut microbiota and the severity of autism or GI symptoms [14].
Kandeel et al. [15] focused on Clostridium in ASD patients’ feces. In this study, the number of Clostridium spp. (Clostridium paraputri, Clostridium bolteae, and Clostridium perfringens) was greater in the feces of children compared to healthy controls. Furthermore, the gut microbiota of children with ASD exhibited two types of Clostridia (Clostridium difficile and Clostridium clostridioides) that were not found in healthy children. Healthy children possess in their microbiota Clostridium tertium, a bacterium not found in ASD children [15].
In the work published by Wang et al. [16] after probiotics and fructooligosaccharides (FOS) ingestion in individuals with ASD, they observed an increase in beneficial bacteria (Bifidobacteriales and Bifidobacterium longum (B. longum) in gut microbiota and a decrease in the number of suspected pathogenic bacteria (Clostridium). Concomitantly, they observed a significant reduction in the severity of autism and GI symptoms [16].
Tomova et al. [17] looked at the potential effects of alteration in the composition of the microbiota in individuals with ASD in relation to food intake and food preference [17]. The authors of this study reported that in the ASD group, the microbiota was characterized by the abundance of Dichelobacter, Nitriliruptor, and Constrictibacter, whereas in the control group, Diaphorobacter and Nitratireductor were more abundant. The study also pointed out that fresh fruit and vegetable intake was significantly higher in healthy children than in children with ASD. They also observed that increasing the consumption of fruits and vegetables increased microbiota diversity, but this effect was only observed in the control group. The authors recommended increasing fresh fruit and vegetable consumption for ASD patients, keeping in mind that this may be very difficult to achieve [17].
Zou et al. [18] looked at the composition of the microbiota at the yeast level. The authors reported that Saccharomyces and Aspergillus were more abundant in the feces of individuals with ASD than in controls (59% vs. 40%). A decreased abundance of Aspergillus versicolor has also been observed in patients with ASD [18].
The goal of the study by Khalil et al. [19] was to assess the presence of Clostridium difficile in the feces of children with ASD; however, in this study, no significant differences in both Clostridium difficile detection and toxins A and B production were observed between individuals with ASD and controls [19]. Toxins A and B of Clostridium difficile are proteins produced by the bacterium Clostridium difficile. These toxins play a crucial role in the pathogenesis of the disease. Both toxins cause damage to intestinal epithelial cells, but they act differently on cells. They are responsible for inducing diarrhea and other symptoms associated with Clostridium difficile infection.
Zhang et al. [20] examined the beneficial effect of traditional Chinese medicine on autistic symptoms through its action on the microbiota of patients with ASD [20]. Bu Yang Huan Wu Tang [21] was given to change the composition of the gut microbiota. In this study, it is proposed that (1) a difference in gut microbiota composition exists between control and ASD patients, and (2) the consumption of the herb restores the composition of the microbiota. These intriguing findings are weakened by the total absence of data on ASD symptoms and the low number of individuals enrolled in this study. Microbial fermentation of plant-based fibers can produce different types of short-chain fatty acids (SCFAs) that may have beneficial or detrimental effects on the gut and neurological development of individuals with autism [21].
Zou et al. [22] characterized the nature of the microbiota through the sequencing of the bacterial 16S ribosomal RNA (16S rRNA) gene. The authors reported a decrease in the number of Firmicutes, Proteobacteria, and Verrucomicrobia in the microbiota of children with ASD and an increase in the abundance of Bacteroidetes/Firmicutes [22].
Ding et al. [23], reported that children with ASD have a higher biomass and an increased richness of gut microbiota. They also reported an increase in the relative abundance of unidentified Lachnospiraceae, Clostridiales, Erysipelotrichaceae, Dorea, Collinsella, and Lachnoclostridium, whereas the abundance of Bacteroides, Faecalibacterium, Parasutterella, and Paraprevotella was lower in patients with ASD. The authors also reported that ASD severity may be correlated with the presence of unidentified Erysipelotrichaceae, Faecalibacterium, and Lachnospiraceae [23].
The overall goal of the paper published by Laue et al. [24], was to prospectively characterize the association between the gut microbiome and ASD-related behaviors at the age of three. Gut composition and function were determined by 16S rRNA gene analysis and shotgun metagenomic sequencing, respectively. ASD-related social behavior was assessed using Social Responsiveness Scale (SRS) T-scores. SRS-2 performance was associated with several taxa, including those in the Lachnospiraceae family. Conversely, a higher relative abundance of Adlercreutzia equolifaciens and Ruminococcus was related to poor SRS-2 performance. The authors also reported that two functional pathways, L-ornithine and vitamin B6 biosynthesis, were associated with better social skills at three years old [24].
Hua et al. [25] reported a study carried out on 120 children diagnosed with ASD with or without sleeping disorders. The authors concluded that ASD children with sleep disorders exhibit a decline in the abundance of Faecalibacterium and Agathobacter and a decreased level of melatonin in their gut microbiota [25].
Santocchi et al. [26] explored the effects of probiotics on autism over a 6-month period. The authors observed that in some children without GI symptoms treated with probiotics, there was a significant improvement in ASD symptoms, especially in the social-affect domain, which is not related to the effect of probiotics on GI symptoms [26].
Hazan et al. [27] analyze the gut microbiota profile of one child with ASD and GI disorders in comparison with her triplet siblings. They reported a high Bacteroidetes/Firmicutes ratio, increased relative abundance of Proteobacteria, and lower relative abundance of Actinobacteria. The major limitation of this study was that it included only one individual [27].
Chen et al. [28] addressed the interesting question of the possible impact of the maternal gut microbiota on offspring microbiota and ASD symptoms. The authors of this study reported that the gut microbiota of children with ASD differed from that of healthy children [28].
Needham et al. [29] took another approach to understanding the non-behavioral features of ASD in association with microbiota. These data suggest that oxidative stress is increased in ASD patients. The authors concluded that a connection exists between general metabolism, including GI physiology, and behavioral traits [29].
In 2021, Zhang et al. [30] published an article comparing the composition of fecal samples from 21 adults with ASD and 21 obese adults. They observed that the relative amount of Firmicutes/Bacteroidetes in the gut of ASD individuals was significantly increased compared to that of obese adults. Particularly, Lachnospiraceae and Ruminococcaceae families were more abundant in ASD adults [30]. Even if obesity is found in adult patients with ASD, the rationale for comparing the microbiota between ASD individuals and obese individuals remains unclear.
The study by Fouquier et al. [31] is intriguing and interesting. In this study, they compared the gut microbiota composition between individuals with ASD and controls in two locations in the USA: Arizona and Colorado. The gut microbiome composition differed between individuals in Arizona and Colorado. Their results suggested that GI symptoms were higher in ASD individuals than in neurotypical individuals in Arizona but not in Colorado. The composition of the gut microbiota appears to be associated with ASD but not with GI symptoms. This result suggests that the relationship between microbiota composition and symptoms is unclear or very complex [31].
Ye et al. [32] published an article in which they compared the gut microbiome of 71 boys with ASD in comparison with 18 neurotypical controls. Significant differences were observed between the two groups. At the genus level, a decrease in the relative abundance of Escherichia, Shigella, Veillonella, Akkermansia, Provindencia, Dialister, Bifidobacterium, Streptococcus, Ruminococcaceae, and several others were observed in the ASD cohort, the abundance of Eisenbergiella, Klebsiella, Faecalibacterium, and Blautia was significantly increased. These results have not been discussed, and no attempt has been made to correlate these data with clinical aspects [32].
In 2021, Laghi et al. [33] published an article in which they realized a metabolomics analysis of the fecal microbiota in ASD patients. In this study, 59 molecules were identified and quantified in several groups of individuals: children with and without GI symptoms, and children with low vs. high autism severity [according to Autism Diagnostic Observation Schedule (ADOS) scores]. No links were identified between the severity of autism, intestinal symptoms, and the relative abundance of the analyzed microorganisms. According to the authors of this study, the results of their study suggest that “fecal metabolome discriminates the severity of autism (SA) and intestinal microorganisms mediate the link between metabolome and SA regardless of GI symptomatology” [33].
Ha et al. [34] investigated the fecal microbiota of Korean children with ASD to determine the gut bacterial profiles associated with ASD. This study was carried out using fecal samples obtained from 54 children with ASD and 38 age-matched control children. The authors reported that the composition of the gut microbiota differed between individuals with ASD and the controls. They also reported that SCFAs are more abundant in children with ASD. These authors reported lower Bacteroides levels and higher Bifidobacterium levels in the ASD group, they also found a significant decrease in the relative abundance of Bacteroidetes and an increase in the abundance of Actinobacteria in ASD patients. The authors concluded that these changes may be associated with functional alterations, including genetic information processing and amino acid metabolism [34].
In a remarkable work by Yap et al. [35], the authors reported the results of a metagenomics study performed on the stool of 247 ASD patients. The authors did not find any major link between gut microbiota composition and disease severity. Instead, they suggested that limited changes in the microbiota could be associated with a less diverse diet in patients with ASD. They concluded that ASD induced both restricted interest and a less-diverse diet, and subsequently, the lack of variety in the food-induced a minor microbiome composition. The authors concluded that “microbiome differences in ASD may reflect dietary preferences that relate to diagnostic features” [35].
Based on the analysis of 25 samples of fecal microbiota harvested from ASD individuals and compared with 20 controls, Ding et al. [36] identified that GI symptoms are strongly associated with symptoms of ASD. Their study also revealed that significant differences exist between individuals with ASD and controls in the composition of the microbiota (at the family, order, genus, and phylum levels). The authors concluded that there were significant differences in Actinobacteria and Firmicutes between the two groups. However, this study did not attempt to explain the potential link between these changes in the microbiota and disease severity. The association with less diverse foods has not been addressed [36].
A case-control study conducted by Xie et al. [37] on autistic and healthy children showed the potential risk of gut microbial dysbiosis in ASD onset by increasing the relative abundances of Actinobacteria, Proteobacteria, and Escherichia-Shigella, and decreasing the relative abundance of Blautia and some Lachnospiraceae. Such gut microbiota imbalance may disturb functional pathways such as amino acid metabolism, cofactor and vitamin metabolism, and the adenosine monophosphate (AMP)-activated protein kinase signaling pathway. Gut microbiota analysis showed increased relative abundance of Fusobacterium, Ruminococcus torques group, and Bacteroides plebeius DSM 17135, and reduced relative abundance of Ruminococcaceae UCG 013, Erysipelotrichaceae UCG 003, Parasutterella, Clostridium sensu stricto 1, Turicibacter, Clostridium spiroforme DSM 1552, and Intestinimonas butyriciproducens in autistic individuals compared to the control group [37].
In 2022, Chen et al. [38] published an intriguing paper. They studied the alpha and beta compositions of the gut microbiota from 81 individuals with ASD in comparison with 31 controls. The two populations were relatively homogeneous, with comparable (not similar) ages, intelligence quotients (IQs), etc. They characterized the composition of the microbiota and found that both groups showed comparable alpha diversity. However, in individuals with ASD, the authors reported a higher weighted UniFrac distance for beta diversity. Fusobacteria, Fusobacteriaceae, Fusobacteriaceae, Fusobacterium, and Prevotellaceae were more abundant in the gut microbiota of ASD patients. Conversely, they observed that Turicibacter, Erysipelotrichaceae UCG 003, Clostridiaceae 1, Clostridium sensu stricto 1, and spiroforme DSM 1552 were less abundant in the microbiota of ASD patients. Analysis of microbiota suggested that in ASD patients, metabolic pathways involving amino acid metabolism [Valine (V), Leucine (L), Isoleucine (I), and Arginine (R)], arachidonic acid, and energy metabolism were increased. Individuals with autism had worse GI symptoms than those with typically developing controls (TDC). The altered taxonomic diversity in ASD is significantly correlated with autistic symptoms, especially when looking at delinquent behaviors and self-dysregulation. However, they did not find any association between the altered microbial composition and gut symptoms in these patients. They concluded that their findings suggested that “altered microbiota are associated with behavioral phenotypes but not GI symptoms in ASD” [38]. This paper reports on a very well-conducted study.
Guidetti et al. [39] published a paper describing a randomized crossover study aimed at evaluating the effects of probiotics on GI symptoms and behavior in children with autism in 2022. They analyzed the results of a double-blind crossover study (probiotic vs. placebo) experiment using individuals aged 2–16 years old who were diagnosed with ASD. The experimental group was administered the drug every day for up to eight months. The mixture contained 10 × 109 colony-forming units (CFU) active fluorescent units (AFU) of Limoslactobacillus fermentum LF10, Ligilactobacillus salivarius LS03, Lactiplantibacillus plantarum LP01, and a mixture of five strains of Bifidobacterium longum. The total number of participants included in the study was 61. The results presented in this paper suggest that the administration of specific probiotics can partially but significantly reduce the severity of behavioral and GI symptoms, which may affect individuals with ASD. They concluded that “the presence of taxa related to Streptococcus thermophilus, Bifidobacterium longum, Limosilactobacillus fermentum, and Ligilactobacillus salivarius species in fecal samples may represent a huge probiotic intestinal colonization in these AD patients”. They concluded that many aspects of the connection between the gut and brain still need to be investigated [39].
In September 2022, Raghavan and collaborators [40] published a paper, which a randomized pilot clinical study was carried out to evaluate the composition and role of the gut microbiota of subjects with ASD after the food consumption of Nichi Glucan. In this study, 18 subjects with ASD were randomly allocated to a control group (6 subjects) or a group (12 subjects) supplemented with Nichi Glucan. The duration of treatment was 90 days. The authors reported alterations in the composition of the intestinal microbiota after Nichi Glucan treatment and noticed that Enterobacteriaceae was drastically decreased in the treated group; in these individuals, the level of Bacteroides also decreased. No real attempt has been made to correlate these observed changes with the disease, either at the intestinal or nervous levels [40].
What has been new knowledge over the last three years?
All newly available data confirm the possible role of the microbiota in some aspects of ASD. Again, none of these studies were able to identify whether the alteration in gut microbiota was responsible for behavioral ASD symptoms. In some of these studies, the question was how effective fecal microbiota transplantation (FMT) would be in treating both digestive and behavioral disorders. In the following paragraphs, an analysis of the existing literature concerning FMT is conducted.
FMT for ASD patients
What is FMT? The most common amount of transplanted feces was 50–60 g in a volume of 250–300 mL [41]. Usually, for the patient, the procedure involves (1) initial treatment with antibiotics to ensure that pathogenic bacteria are intensely suppressed. (2) Two days before transplantation, an acid pump inhibitor is administered to reduce stomach acidity. (3) The day prior to transplantation, a drink that flushes the bowels is given to remove most of the remaining gut bacteria and antibiotics. (4) At the same time, it is also possible to add a fasting period of one day to enhance cleansing [41]. (5) Donor microbiota can be administered through the lower GI route (e.g., colonoscopy flexible sigmoidoscopy, rectal tube, and retention enema) or through the upper GI route (nasogastric/nasointestinal method or gastroduodenoscopy). According to Choi and Cho [42], colonoscopic FMT is the best way to perform FMT, as it is safe, well-tolerated, and easily performed. FMT through the upper GI route is easy to perform and safe but may be uncomfortable and induce vomiting [42]. Donor microbiota is obtained from healthy individuals. Donors are selected after rigorous screening involving questionnaires, physical examinations, and analysis of medical history. The objective was to eliminate people with infectious diseases, metabolic syndrome, GI disorders, and neurologic problems. The harvested microbiota was tested for the presence of potential bacterial pathogens (e.g., Campylobacter and Salmonella) and potential viral particles, especially norovirus, rotavirus, and other potential parasites. Once selected, the feces are extensively filtered and stored under standardized anaerobic conditions. The final product was frozen in liquid form.
In 2019, the Food and Drug Administration (FDA) published guidelines for preventing infections caused by multidrug-resistant organisms (MDROs) during FMT.
It is common to find that FMT has been used for centuries; however, in the modern period, FMT was developed for diseases of the GI tract, and in 1978, FMT was recognized as efficient for treating Clostridium difficile-resistant diarrhea. It shows an impressive effect, as in over 95% of cases, the cure is quick and stable [43]. Transplantation from a healthy donor stool induced the disappearance of symptoms, especially inflammation, six months after the procedure.
In 2017, Kang et al. [44] published a complete study involving FMT in individuals with ASD. The population involved in this clinical trial was 18 children (aged 7–16 years old) with GI problems. Ten weeks after FMT, significant changes in GI and ASD symptoms were observed; abdominal pain, indigestion, diarrhea, and constipation were reduced in 16 of 18 patients. In addition to GI improvements, ASD-related behaviors also improved after FMT. Following FMT, both microbiota and phages from the donor appeared engrafted. At the same time, children with ASD showed improvements in social skill deficits, irritability, hyperactivity, lethargy, stereotypy, and aberrant speech. In these patients, FMT was well tolerated. The authors of this well-conducted study concluded that FMT is well-tolerated in children with ASD. However, this was not a double-blind study, and the population did not exhibit homogeneous GI symptoms. The follow-up of this initial experiment was conducted over a 2-year period [45]. These initial observations were confirmed. The changes in gut microbiota composition observed at the end of treatment (especially the increase in bacterial diversity and abundance of Bifidobacteria and Prevotella) persisted 2 years later. As an extension of these studies, Qureshi et al. [46] published a report analyzing the metabolite patterns of the feces in both individuals with ASD and FMT, and several metabolites related to mitochondrial metabolism, such as carnitine, betaine, and adenine, in individuals with ASD [46].
In 2020, the same group published a paper concentrating on metabolite modifications induced by FMT in individuals with ASD. The major conclusion of this study was that FMT resulted in major changes in plasma metabolite profiles and minor changes in fecal profiles [44]. Ten plasma metabolites were showing significantly different levels between the ASD and control groups. Among these, nicotinamide riboside levels were found to be significantly lower in patients with ASD, and this level significantly increased after microbiota transfer therapy (MTT). Nicotinamide riboside is a crucial molecule that is involved in many redox reactions. In the amino acid family, inosine monophosphate (IMP) and sarcosine levels were found to be lower in the ASD group and increased after MTT; the authors reported that this increase was strongly correlated with improvements in GI symptoms. Glutamate levels were higher in ASD plasma samples, consistent with clinical features such as anxiety and excitation. At the lipid metabolism level, caprylate and heptanoate levels were much higher in individuals before FMT and returned to normal after FMT. Data regarding glucid metabolism were not clear, in this study the level of galactonate was significantly lower in the ASD group, this result was in contraction with the data provided by Mu et al. [47]. The level of bilirubin was lower in plasma samples from patients with ASD. Altogether, changes and even marked changes were observed in individuals with ASD and FMT restored most of these alterations. Alterations occur principally in lipid, protein, and vitamin metabolisms. When examining fecal metabolites, no significant differences in the levels of hundreds of metabolites assayed in this study were observed. The authors failed to explain why differences in plasma were observed at the metabolome level and not in fecal samples.
A follow-up study by Zhang et al. [48] was proposed and detailed in a paper published by Pan et al. [49]. The initial study involved over 300 individuals, and the coming study will enroll 318 children with both ASD and GI symptoms. This multicenter study is clearly detailed in this publication, but no results are available. The authors concluded their publication by explaining that “the results of this trial will provide high-quality evidence to inform the future clinical application of this new therapy” [49].
In 2021, Li et al. [50] published the results of a clinical trial involving 40 children with ASD (3–17 years old) and GI tract symptoms such as constipation or diarrhea. As a control group, 16 sex-matched and age-matched, typically developing children without GI disorders were included. The study included a 4-week FMT treatment phase and an 8-week follow-up phase. Two routes of administration for FMT were proposed in this trial: 27 children received oral FMT, and 13 children received colonoscopic FMT. In this trial, the authors reported that the gut microbiota alpha diversity was not altered by the treatment, and they concluded that FMT did not affect the overall structure of the gut microbial communities. They also indicated that pre-existing intestinal landscapes could affect the outcomes of FMT. Finally, they divided the participants from the ASD group into two subgroups: responders and non-responders to the FMT. Another explanation could be an inadequate protocol for ASD, but no such explanation has been proposed by the authors of this publication. Concentrating on FMT clinical responders, it is possible to hypothesized that FMT might reduce the abundance of Eubacterium coprostanoligenes and subsequently improve GI symptoms in individuals with ASD. They concluded that they identify “specific bacteria Eubacterium coprostanoligenes that might be associated with therapeutic outcomes, which should be further explored in future FMT trials in ASD patients” [50].
Conclusions
There is clearly increasing data on the relationship between gut microbiota and ASD symptoms at both the behavioral and digestive levels. From published information, it seems that dysbiosis is present in most individuals with ASD, but not all. In most cases, dysbiosis can be considered a negative aspect of disease severity.
Several studies have shown limited or no beneficial effects of prebiotics, probiotics, and symbiotics on the gut microbiota and the symptoms of ASD. After reviewing the literature from January 1, 2010, to October 30, 2020, Mitchell and Davies [51] found limited preliminary evidence of the efficacy of prebiotics, probiotics, or symbiotics in relieving GI distress, improving ASD-associated behaviors, altering microbiota composition, and reducing inflammatory potential [51]. A systematic review by Tan et al. [52] showed that the beneficial effects of probiotics, prebiotics, symbiotics, and fecal microbiota transplants in ASD are limited and inconclusive. Prebiotics and symbiotic combinations were effective only for selective behavioral symptoms. Evidence of the efficiency of FMT in ASD is still limited [52].
Considering dysbiosis as a problem, FMT can appear as a solution, keeping in mind that it may be hazardous. The results published by the FMT suggest that curing dysbiosis is a possible treatment. Another question arises from this information: microbiota is always studied at the bacterial level, (little to) no information on yeast, fungi, and viruses present in the microbiota is available.
In any case, dysbiosis should be examined in individuals with ASD and dysbiosis should be corrected by FMT or more easily using prebiotics.
Abbreviations
| ASD: |
autism spectrum disorder |
| FMT: |
fecal microbiota transplantation |
| GI: |
gastrointestinal |
| SRS: |
Social Responsiveness Scale |
Declarations
Author contributions
JD: Conceptualization, Writing—original draft, Writing—review & editing, supervision. HO and CD: Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.