Affiliation:
1Neurosurgery Unit, Hospital Regional del Cusco, Cusco 08000, Peru
2Professional School of Human Medicine, Universidad Andina del Cusco, Cusco 08000, Peru
3Research Unit, Pan-American Student Society of Neurosurgery and Neurosciences Comité de Investigación, Cusco 08000, Peru
Email: johchove@hotmail.com
ORCID: https://orcid.org/0000-0001-5220-1216
Affiliation:
2Professional School of Human Medicine, Universidad Andina del Cusco, Cusco 08000, Peru
3Research Unit, Pan-American Student Society of Neurosurgery and Neurosciences Comité de Investigación, Cusco 08000, Peru
ORCID: https://orcid.org/0000-0002-2822-3922
Affiliation:
3Research Unit, Pan-American Student Society of Neurosurgery and Neurosciences Comité de Investigación, Cusco 08000, Peru
4Faculty of Medicine, Benemérita Universidad Autónoma de Puebla, Puebla, Pu 72410, Mexico
5Mission: Brain BUAP Student Chapter, Puebla, Pu 72410, Mexico
ORCID: https://orcid.org/0000-0002-0267-2738
Affiliation:
3Research Unit, Pan-American Student Society of Neurosurgery and Neurosciences Comité de Investigación, Cusco 08000, Peru
6Faculty of Medical Sciences, Universidad Nacional de Asunción, Asuncion 1535, Paraguay
ORCID: https://orcid.org/0000-0003-0750-6955
Affiliation:
3Research Unit, Pan-American Student Society of Neurosurgery and Neurosciences Comité de Investigación, Cusco 08000, Peru
7Department of Neurosurgery, Hospital de Clínicas-Universidad Nacional de Asunción, San Lorenzo 2160, Paraguay
ORCID: https://orcid.org/0000-0001-6955-6865
Affiliation:
3Research Unit, Pan-American Student Society of Neurosurgery and Neurosciences Comité de Investigación, Cusco 08000, Peru
8Faculty of Medicine and Psychology, Universidad Autónoma de Baja California, Tijuana, BC 22390, Mexico
ORCID: https://orcid.org/0009-0005-3957-3572
Explor Neurosci. 2024;3:175–197 DOI: https://doi.org/10.37349/en.2024.00043
Received: November 21, 2023 Accepted: February 01, 2024 Published: May 13, 2024
Academic Editor: Jinwei Zhang, University of Exeter Medical School, UK
The article belongs to the special issue Epilepsy
Epileptic seizures are prevalent in people with brain vascular abnormalities like arteriovenous malformations (AVMs) and cavernous malformations, greatly affecting their quality of life. The connection between intracranial vascular abnormalities and epilepsy is still under debate. Therefore, investigating epilepsy in individuals with AVMs is a crucial and current research area. This review presents a comprehensive examination of recent developments in epilepsy among individuals with brain AVMs. The authors conducted a detailed analysis of the natural progression, epidemiology, diagnostic methods, therapeutic approaches, and post-treatment outcomes for individuals with epilepsy associated with AVMs.
Epileptic seizures are common among individuals with brain vascular malformations, such as arteriovenous malformations (AVMs) and cavernous malformations, significantly impacting their quality of life. The association between intracranial vascular malformations and epilepsy remains a subject of ongoing discussion. Thus, the study of epilepsy in patients with AVMs represents a current and essential research topic [1–4].
This comprehensive review aims to provide an in-depth analysis of current advancements in understanding epilepsy among individuals with brain AVMs. The initial sections cover fundamental concepts, definitions, natural history, and the epidemiology of epilepsy in AVM patients. Subsequent sections delve into the diagnosis, therapeutic strategies for managing the condition, and the outcomes observed after treating individuals with brain AVMs.
Seizures arise from aberrant and coordinated neural activity within specific cerebral regions or across the entire brain, resulting from irregular neural network formation or disruptions induced by structural, infectious, or metabolic factors. Seizures constitute ephemeral phenomena characterized by precisely defined onsets and conclusions [3].
In the pediatric population, seizures predominantly stem from genetic factors, perinatal insults, or malformations of cortical development [3]. In adults without a familial history of epilepsy, common etiologies include conditions such as encephalitis, meningitis, head injuries, and the presence of brain tumors. In elderly individuals, seizures are often associated with head trauma and brain tumors [3].
As articulated by the International League Against Epilepsy (ILAE), epilepsy clinically manifests through (1) the occurrence of two or more unprovoked seizures with an interictal interval exceeding 24 h, or (2) a solitary unprovoked or reflex seizure when the likelihood of recurrence exceeds 60% in the subsequent decade, or (3) a clinical diagnosis of an epilepsy syndrome [3, 4]. The second criterion deems an individual at risk when brain imaging reveals epileptogenic potential or when epileptiform activity manifests on an electroencephalogram [1]. The classification of epilepsy involves three discernible strata: the nature of seizure onset, the specific epilepsy subtype, and the identification of epilepsy syndrome [1, 2].
Epileptogenic constitutes a nuanced phenomenon involving the conversion of a non-epileptic brain into a condition capable of generating spontaneous and recurrent seizures [5–7]. This process entails the development of brain tissue endowed with the capacity to generate seizures, culminating in the establishment of an epileptic condition and the progression of epilepsy even after diagnosis. In contrast to previous conceptions, palatogenesis extends beyond the timeframe between the epileptogenic trigger and diagnosis, encompassing mechanisms contributing to the ongoing progression of epilepsy [6, 7]. This intricate process is underscored by a delicate balance between excitatory and inhibitory activities within neural networks. Prolonged aberrant neural activity disrupts routine neural processing, exerting deleterious effects on other neural networks [1, 5].
In generalized epilepsies, the networks implicated in epileptogenic typically exhibit widespread distribution, involving bilateral thalamocortical structures [1, 2]. In contrast, focal epilepsies primarily implicate neural circuits within a single hemisphere, often situated in the limbic or neocortical regions [2]. In specific scenarios, such as the absence of seizures and limbic epilepsies in an immature brain, an abnormal increase in inhibition may contribute to a pro-epileptogenic state [1]. While numerous generalized epilepsies are postulated to possess a genetic basis, focal epilepsies have traditionally been associated with structural cerebral abnormalities, particularly in instances of drug-resistant epilepsy (DRE) [1, 8, 9].
The precise pathophysiological mechanisms underpinning the nexus between structural abnormalities and the onset of seizures remain incompletely understood. Seizures are primarily attributed to abnormal activity in cortical neurons, although there may be secondary involvement of glial cells and axons within the white matter [1].
Biomarkers associated with palatogenesis can serve multiple purposes, including predicting the emergence of epileptic conditions, identifying tissue capable of generating spontaneous seizures and determining its extent, measuring the progression of epilepsy post-establishment, and facilitating the cost-effective development of animal models for potential antiepileptogenic drugs and devices. Moreover, these biomarkers offer a means to reduce costs associated with clinical trials for potential antiepileptogenic interventions by including individuals at a heightened risk of developing epilepsy in the trial cohort [7, 10].
The connection between cerebral vascular malformations and epilepsy is still a topic of discussion [11]. In many cases, it is commonly observed that the specific area associated with the vascular malformation is closely linked to the occurrence of focal epileptogenic activity, and the removal of the malformation often results in better seizure control. There are exceptions to this pattern, including situations where vascular malformations are not directly linked to the seizure disorder or are part of a more extensive brain condition characterized by a broader influence on epileptogenesis [12]. Epileptic seizures frequently occur in patients with brain vascular malformations such as AVMs and cavernous malformations, significantly affecting their quality of life [12, 13].
Factors that increase the risk of seizures in patients with AVMs include male gender, younger age, frontal or temporal lobe AVMs, brain cortex-located AVMs, a superficial venous drainage, a superficial temporal lobe AVM nidus, fistulous AVMs, and AVMs with venous stenosis [14–19]. Some studies include a larger AVM (> 3 cm) as an independent predictor of seizures [14, 20].
Seizures in previously ruptured AVMs may result from defects and gliosis caused by intracerebral hemorrhage. However, seizures in unruptured AVMs have a more complex explanation [19]. The tissue surrounding AVMs is particularly vulnerable to hypoperfusion due to the “steal phenomenon” and a lack of vascular autoregulation [21]. The reduced cerebrovascular reserve in the perinidal area can lead to changes in the perinidal cortex induced by hypoperfusion, resulting in chronic ischemia and the development of new blood vessels. These changes promote excitatory pathways in neurons, easily developing epileptogenic mechanisms [20, 22–24]. Localized hypoxia resulting from hypoperfusion can lead to gliosis and trigger seizure activity [19].
Rajeswarie et al. [11] investigated angioarchitectural factors in cerebral vascular malformations related to changes in neuroglial and stromal components in the adjacent cortex to understand the physiopathology of epilepsy in these individuals. The study included 32 patients, with 24 having AVMs and 8 having cavernous malformations [11]. Among the AVM patients (consisting of 18 men and 6 women), 50% experienced seizures as their initial clinical presentation. The most affected areas were the frontal lobe (33.3%) and the temporal lobe (29.2%). The analysis revealed a significantly higher occurrence of hemosiderin deposition and gliosis in the tissue surrounding the lesion in patients with seizures compared to those without seizures (P < 0.05). These findings support the idea that in AVMs, vascular remodeling, chronic bleeding, and microhemorrhages lead to hemosiderin deposition in the surrounding tissue, subsequently triggering gliosis and promoting the development of seizures [11].
AVM patients usually experience their debut through hemorrhage in 45–72% of cases [25–35]. Among these hemorrhages, intraparenchymal bleeding is the most frequent type and can lead to significant neurological impairment associated with the AVM’s location [25]. AVMs can also cause other types of hemorrhages, including intraventricular and subarachnoid hemorrhages [36]. Subarachnoid hemorrhages account for 9% of AVM-related hemorrhages [37, 38].
AVMs in pediatric patients are rare, with a prevalence of 0.014–0.028% [39]. They account for half of intracranial hemorrhages in pediatric patients [40], resulting in neurological deficits in 20.2–40.6% of cases [41, 42].
The estimated average annual bleeding risk for untreated AVMs is 2–4% [25–43]. Unruptured AVMs have a lower rate of 1.3–2.2% while the rate for ruptured AVMs is higher at 4.5–4.8% [37, 42]. In pediatric patients, the annual rate of bleeding from AVMs ranges between 0.9% and 7.14% [40]. The main risk factors for AVM rupture include a history of previous AVM rupture, the AVM’s location in deep brain structures, and exclusive deep venous drainage [42, 44].
The following equation has been proposed to calculate the risk of bleeding [45, 46]: annual rupture risk = 1 – (risk of no hemorrage)life expectancy or rupture risk = 105 – patient age.
Seizures represent the second most common clinical manifestation of AVMs, occurring in 11–35% of cases [15, 18, 25, 36, 40, 47–49]. Some risk factors associated with AVMs presenting with seizures include a history of prior AVM hemorrhage, male gender, and the AVM’s location in the frontotemporal region [36]. Angioarchitectural features, such as AVMs located in the cortex, supplied by the middle cerebral artery, with cortical feeders, no aneurysms, and the presence of varicose veins, also contribute to the risk [17].
Among patients with AVMs, unprovoked epileptic seizures have been observed within 5 years after diagnosis in 26% of cases, 11% at 10 years, and 18% at 20 years of follow-up [50].
Additional symptoms associated with AVMs include headaches, focal neurological deficits due to mass effect, ischemic symptoms resulting from vascular steal phenomena, cognitive dysfunction, learning difficulties, behavioral changes, pulsatile tinnitus, and elevated intracranial pressure [51].
The diagnostic rate for AVMs is estimated at 1–1.42 cases per 100,000 people [44, 52–54]. This rate decreases to 0.84 cases per 100,000 individuals when AVMs are detected due to a hemorrhagic presentation [54]. Autopsies have shown a wide AVM prevalence ranging between 5 cases and 613 cases per 100,000 corpses [55, 56].
The average age at which AVMs are diagnosed ranges between 32 years and 39 years [25–36]. In pediatric patients, AVMs have an estimated diagnostic rate of 0.014–0.028% [40]. No gender differences are observed in either pediatric or adult series [36].
The estimated mortality rate in AVM patients is 0.7–2.9%. Untreated patients have a 50% higher risk of mortality 30 years after diagnosis compared to the general population. For patients with completely occluded AVMs, the risk of mortality 30 years after diagnosis is 13%, as compared to the general population [25].
Individuals with an unruptured AVM who experience a first seizure meet the revised ILAE epilepsy definition due to the increased 5-year probability of having another seizure, up to 58%. There is a potentially higher risk of epilepsy in women and younger patients harboring AVMs, prompting consideration for prescribing antiseizure medications (ASM) [13, 57, 58].
Initiating the delivery of ASM after an initial seizure may postpone the occurrence of second seizures, accelerating the attainment of two years without seizures. However, this strategy does not influence the occurrence of seizures after five years [13].
Options for addressing AVM-related seizures encompass conservative strategies involving ASM and surgical interventions, often implemented in gradual procedures. Invasive management of AVM is usually reserved for seizures resistant to medication [58, 59]. Josephson et al. [60] did not observe a notable distinction in the clinical progression of epileptic seizures between invasive treatment and conservative management of AVM.
In AVM patients experiencing their first seizure without hemorrhage as the initial presentation, healthcare providers typically prefer medical treatment. In these cases, they routinely prescribe ASM unless AVM removal is deemed necessary for treating the seizures. The extent of ASM therapy may vary based on whether the seizures are generalized or focal [59, 60].
No universally standardized pharmacological regimen exists for AVM-related seizures, as recommendations can significantly vary over time. The most effective approach usually entails a multidisciplinary management strategy incorporating the expertise of an epileptologist and neurophysiologist [60].
Patients with AVM-related epilepsy, who have not experienced hemorrhage or functional neurological deficits and have developed epilepsy over a 5-year follow-up, demonstrate a 45% chance of achieving seizure freedom at 2 years after commencing ASM. By the end of the follow-up period, 91% of these patients were actively receiving ASM, with 46% undergoing polytherapy. The probability of attaining seizure freedom with ASM is approximately 60–70% in cases of AVM-related epilepsy [61]. Initiating ASM immediately after diagnosis can prolong the duration until seizure recurrence and decrease the time without seizures to two years [13, 60]. Other studies have reported even more favorable seizure control, with 78% of AVM patients remaining seizure-free for at least one year at their last follow-up visit. Among those with satisfactory seizure control, 64% required only a single ASM [61].
In the context of unruptured AVMs, a randomized trial of unruptured brain AVMs (ARUBA) conducted a comparison between sole medical management and a combination of medical management and interventional therapy. Out of 223 patients, 109 underwent medical management while 114 received interventional therapy. The primary endpoint focused on the time to death or symptomatic stroke, with an examination of seizure data. Both groups exhibited similar rates of seizure presentation, with 41% in the medical management group and 44% in the interventional therapy group. Following an average follow-up of 33.3 months, 37% in the medical management group and 44% in the interventional group continued to experience seizures. The rates of seizures per patient-year were comparable, with 13% for the medical management group and 16% for the interventional group. These findings suggest that AVM-related epilepsy persists irrespective of the chosen treatment approach [62]. It is crucial to acknowledge that the study has limitations, such as patient heterogeneity, absence of treatment standardization, and a short follow-up period [63].
For patients with AVM-related epilepsy who have undergone complete AVM occlusion or successful AVM removal, whether they have pre-existing chronic epilepsy or cases resistant to drugs, it is advisable to continue ASM treatment for a minimum of 1 year, preferably extending to 2 years [60]. Limited information exists in the literature regarding the treatment of DRE in AVM cases [60, 64, 65].
Regarding the discontinuation of ASM, a series involving 75 patients, with a median duration of ASM treatment lasting 12 months (ranging at 3–51 months) and a follow-up of up to 41 months, revealed that 5.3% of patients experienced seizures only after stopping ASM more than 12 months after AVM resection, and 1.3% had an isolated seizure after discontinuing ASM treatment 2 months after AVM resection [66].
More research is necessary to enhance the understanding of the most effective treatment strategies in this clinical context, either through population-based investigations or case-control studies [57]. It is crucial to emphasize that consistently employing a comprehensive treatment approach for individuals with epilepsy has shown favorable outcomes in terms of seizure management [13].
Chen et al. [67] identified the AVM’s location in the cortex as the most robust predictor for AVM-related epilepsy, with 27% of patients having seizures with cortical AVMs. This observation is consistent with earlier literature on the topic [17, 67–69].
AVMs situated in the frontotemporal region of the brain have a higher likelihood of causing seizures compared to AVMs in other cortical regions. However, relying solely on cortical localization remains insufficient to guarantee the diagnosis or prognosis of seizures in the natural course of AVM pathology. The preoperative imaging examination must integrate other characteristics, including AVM hemodynamics, AVM morphology, and the anatomical features of the AVM [70].
Advances in hemodynamic measurement techniques and postprocessing tools for angiography and magnetic resonance imaging (MRI) offer a potential means to enhance the characterization of the morphology and anatomical features linked to AVM-related epilepsy [71].
Angioarchitectural factors assessed through digital subtraction angiography include the origin of feeding arteries from the external carotid artery, middle cerebral artery, or posterior cerebral artery, as well as the presence of superficial venous drainage with venous ectasia [18, 20, 59, 67, 69].
Fierstra et al. [20] proposed that a high-flow shunting vascular system within the AVM may result in the formation of aneurysms. This process generates sufficient wall shear stress to disrupt blood flow, potentially contributing to the occurrence of seizures [72–74]. Considering the characteristics of high-flow AVMs, including venous varix, venous ectasia, and intranidal fistulae, Shankar et al. [19] introduced a scoring system based on these angioarchitectural features. Their findings indicate that higher AVM flow correlates with an increased likelihood of seizures [75]. This scoring system relies on three robust predictors: the AVM’s location (considered cortical when the AVM blood supply originates from the cortical branches of the anterior cerebral artery, middle cerebral artery, or posterior cerebral artery), venous outflow stenosis (defined as a reduction of 50% or more in the diameter of the vein), and the presence of a long pial draining vein (defined as longer than a 3 cm superficial course of the draining vein). Each of these variables is assigned a score of 1 when present [75].
In this system, obtaining a score of 0 demonstrates excellent sensitivity and a high negative predictive value. Surpassing the threshold score of 2 results in overall high diagnostic efficacy, while achieving a perfect score of 3 out of 3 corresponds to the system’s highest positive predictive value and strongest specificity (98%) [75].
Benson et al. [76] observed that various factors correlated with the initial presentation of seizures in AVM patients, including perinidal edema, perinidal T2 MRI blooming, the presence of a venous pouch or varix, the existence of a long draining vein, and large AVM size. These findings align with angiography observations in AVM patients [59, 75, 76].
In terms of AVM location, seizures were associated with AVMs situated in the frontal lobe, motor cortex, and sensory cortex [17]. A significant correlation also exists between epilepsy incidence and damage to both the right precentral gyrus and the right superior longitudinal fasciculus [17]. Zhang et al. [70] emphasized that a high AVM heterogeneity heightened the risk of seizures but did not establish connections between radiomic attributes and the detailed angioarchitecture of AVM. Different authors did not take into account the perinidal regions, potentially overlooking epilepsy induced by gliosis or vascular steal in adjacent brain regions [59, 70, 74].
Blood oxygenation level-dependent (BOLD) cerebrovascular reactivity (CVR) mapping is an emerging hemodynamic imaging technique conducted through functional MRI [77–79]. This technique allows for the quantitative assessment of cerebrovascular reserve capacity on a voxel-by-voxel basis and is capable of quantitatively assessing arterial steal in cerebrovascular pathologies. Arterial steal is observed as an unexpected drop in BOLD signal during vasodilation, resulting in an adverse CVR outcome [77–79]. A recent study employing this BOLD-CVR technique revealed weakened perinidal CVR in conjunction with venous congestion observed in standard angiography. This finding suggests that patients with brain AVMs are more likely to experience seizures [59].
Individuals with epilepsy associated with brain AVMs demonstrate a notable decrease in whole-brain CVR compared to those without epilepsy. This reduction in CVR is observed in both gray and white matter [80].
Sebök et al. [81] reported that a decrease in overall brain CVR, coupled with pronounced hemodynamic alterations like the arterial steal phenomenon and venous outflow restriction, is associated with an increased risk of seizures in patients with brain AVMs. These findings align with current and prior research [59, 81, 82], suggesting that the non-invasive BOLD-CVR method could potentially serve as an imaging marker for predicting epilepsy in individuals with AVMs [81].
Two primary approaches exist for treating AVM-related epilepsy: the conservative approach and the interventional approach. In cases of unruptured AVMs, the preferred choice often involves medical management with ASM [83]. DRE, defined by the ILAE as “epilepsy medically refractory to two trials of ASM, resulting in failure to sustain seizure freedom”, usually necessitates interventional approaches [13]. Conventional interventional therapy is typically aimed at reducing the future risk of AVM-related hemorrhage [84]. The following paragraphs outline the advantages and disadvantages of interventional treatment options, such as (1) microsurgical AVM resection, (2) stereotactic radiosurgery (SRS), (3) endovascular embolization (EVE), (4) or a multimodal intervention [59, 85].
The role of microsurgery in the treatment of AVM-related epilepsy is a topic of ongoing debate, although recent series have shown promising results [59]. Some studies have reported high rates of seizure freedom, ranging from 77% to 91%, following microsurgical intervention, and in some cases, these positive outcomes persisted for up to 10 years [59, 86]. Patients with DRE often experience less favorable outcomes for seizure control after microsurgical resection [59]. Potential better outcomes seem to correlate with extended lesionectomy in comparison to standard AVM resection, but the significance of this finding is limited due to the small amount of available evidence [86].
Microsurgery aims to completely remove brain AVMs, resulting in a change in seizure response as its primary goal [69]. A meta-analysis by Baranoski et al. [87] determined that successful seizure control in AVM-related epilepsy is closely associated with the complete obliteration of AVMs, and microsurgery is more likely to accomplish this outcome. Various surgical series have documented a substantial level of seizure freedom following microsurgical AVM removal, with rates around 77% at 1 year, 79% at 2 years, and 84% at 10 years of follow-up [65, 66, 87–89].
In epilepsy-related AVM surgery, intraoperative neuromonitoring techniques, including electroencephalography, somatosensory-evoked potentials, and intraoperative electrocorticography, appear promising but have been sparingly described in the literature [90, 91]. These tools can identify epileptic foci resulting from secondary epileptogenesis and guide extended lesionectomies to enhance seizure control [90, 91]. Advances in microsurgical technology, neuroimaging, and intraoperative neuromonitoring enable safe microsurgical removal of AVMs located in eloquent motor areas or deep-seated regions. Nevertheless, the treatment-associated morbidity for high-grade AVMs remains elevated [92, 93].
SRS represents a secure alternative to microsurgery and EVE for achieving the elimination of seizures [59]. SRS involves the precise delivery of a single high dose of radiation to a specific intracranial target area, aiming to treat or eliminate a preexisting lesion. While this technology may result in reduced AVM obliteration and a longer time to achieve seizure control, it can lead to a decreased risk of experiencing an epileptic crisis and lower morbidity [85, 94].
A meta-analysis by van Beijnum et al. [92] revealed that, in terms of the case-fatality rate, SRS demonstrated the most favorable outcome with a rate of 0.5 per 100 person-years compared to other procedures. This rate outperformed the rates of 1.1 following microsurgery and 0.96 after EVE. SRS was also associated with the lowest complication rate at 5.1%, whereas microsurgery and EVE had rates of 7.4% and 6.6%, respectively. However, SRS exhibited a lower obliteration rate at 38% compared to microsurgery, which achieved a 96% obliteration rate. This raises questions about the effectiveness of SRS in achieving sufficient seizure control [91].
In other reports on SRS for AVMs, 69% of patients saw an improvement or complete absence of status epilepticus, and 48% experienced a total absence of seizures [92]. The effectiveness of SRS in managing AVM-related epilepsy seems to arise from mechanisms such as neuromodulation of the perinidal nervous tissue, the formation of a gliotic capsule, and vascular modifications [59, 92, 93].
EVE is a minimally invasive procedure in the field of interventional radiology used to intentionally block blood vessels as a treatment for diseased or injured vasculature [94]. Embolizing brain AVMs appear to be beneficial for seizure control by inducing hypoxia in epileptogenic brain tissue, although this might stimulate intranidal angiogenesis and lead to seizure recurrence. AVM patients treated solely with EVE, who continue to experience epileptic seizures, seem to have residual gliotic plaques, perinidal gliosis, and epileptic foci distant from the primary AVM site [95].
EVE serves as an adjunct procedure to other treatment approaches for AVMs. The exploration of EVE as an independent treatment method for AVMs is relatively limited [85]. A study led by Zhang et al. [71] evaluated seizure control in patients who underwent EVE with ethylene vinyl alcohol (Onyx). Complete obliteration and seizure freedom were attained in 51.4% of the AVM patients. Those with complete EVE achieved higher seizure control rates at 57.9% compared to those with partial EVE at 44.4% [71].
In AVM patients, seizures occur in approximately 20–25% of cases, ranking as the second most common initial presentation following intracranial hemorrhage [59, 71]. There is ongoing debate and conflicting evidence about the most effective therapeutic strategy for achieving improvement or remission of seizures in AVM patients [58, 59, 75, 96]. In Table 1, the authors provide a summary of the seizure outcomes following interventional AVM treatments reported since 1992 [14, 48, 65, 71, 86, 94, 97–128].
Surgical series with seizure outcomes in AVM patients undergoing microsurgery, SRS, and EVE since 1992
| Author (publication year) study type | Number of patients (percentage of total AVM oclussion) | Number of patients with seizures prior to procedure (percentage) | Number of patients with seizures who were followed | Follow-up (months) | Unruptured/Ruptured AVMs | Clinical outcome |
|---|---|---|---|---|---|---|
| Microsurgery | ||||||
| Nagata et al. [113] (2006) retrospective cohort | 26 (100%) | 11 (42%) | 11 | NA | 4/7 | Ruptured AVMs: good seizure controlUnruptured AVMs: 50% of recurrent psychomotor seizures after the total AVMs excisionDe novo seizure: 9% |
| Piepgras et al. [114] (1993) retrospective cohort | 280 (NA) | 117 (42%) | 110 | 90 | NA/NA | Seizure-free: 83% (48% without ASM); improved: 13%; same: 2%; worse: 2%De novo seizures: 6% |
| Thorpe et al. [48] (2000) retrospective cohort | 114 (100%) | 53 (46%) | 53 | 48 | NA/NA | Post-AVM surgery seizures: 21% (< 50% of the preoperative rate)De novo seizures: 6.3% (> 12 months post-AVM) |
| Yeh et al. [65](1993) retrospective cohort | 54 (100%) | 54 (100%) | 54 | 57.6 | 54/0 | Excellent postoperative seizure control: 70.4%; good: 18.5%; fair: 9.3%; poor: 1.9%Two patients required a second operation to remove a remote seizure focusDe novo epilepsy: none |
| Englot et al. [86] (2012) prospective cohort | 440 (NA) | 130 (30%) | 117 | 20.7 | NA | Engel class I: 96%Engel class II–IV: 4%De novo seizures: 3% |
| Eliava et al. [97] (2021) retrospective cohort | 160 (NA) | 99 (61.9) | 59 | 59.3 | 99/0 | Engel class I: 84.8%Engel class IIA: 3.4%Engel class IIIA: 6.8%Engel class IV: 5.8%De novo epilepsy: none |
| Ferlisi et al. [98] (2016) retrospective cohort | 110 (NA) | 60 (55%) | 40 | 132 | NA/NA | Engel class I: 77 % (IA: 47%, IB: 0, IC: 22%, ID: 7%)Engel class II: 7%Engel class III: 0Engel class IV: 15%De novo seizures: 56% (43% few seizures and became seizure-free at long follow-up, 13% postoperative epilepsy) |
| von der Brelie et al. [99] (2015) retrospective cohort | 293 (99%) | 126 (43%) | 103 | 147 | 77/26 | DRE: ILAE I: 58.3% (ILAE IA: 45.8%)Chronic epilepsy: 80.5% (ILAE IA: 70.3%) of ILAE ISporadic seizures: 85.7% (ILAE IA: 69.1%) of ILAE IDe novo epilepsy: none |
| Lopez-Ojeda et al. [100] (2013) retrospective cohort | 29 (93%) | 12 (41.3%) | 12 | 28.4 | 0/12 | Engel class I: 75%Engel class III: 8.3%Engel class IV: 16.6%De novo seizures: 11.8% |
| SRS | ||||||
| Andrade-Souza et al. [115] (2006) retrospective cohort | 38 (60.5%) | 27 (71%) | 27 | 42.4 | NA/NA | Engel I: 92.6%Engel II: (3.7%) 83% seizure-free in patients with AVMs smaller than 3 cm3De novo seizures: 2.6% |
| Eisenschenk et al. [116] (1998) retrospective cohort | 100 (64%) | 33 (33%) | 32 | 26 | NA/NA | Seizure-free: 59% Marked reduction of seizure frequency: 19%Seizure remission was most frequent for AVMs of the centrum (83.3%)De novo seizures: NA |
| Falkson et al. [117] (1997) retrospective cohort | 101 (NA) | 24 (24%) | 16 | 60 | NA/NA | Seizure-free: 63% Improved seizure frequency: 94%De novo seizures: NA |
| Gerszten et al. [118] (1996) retrospective cohort | 72 (NA) | 15 (21%) | 13 | 47 | 7/8 | Seizure-free and off anticonvulsant therapy: 85%Significant improvement but continue medication: 3%De novo seizures: 3% |
| Kida et al. [119] (2000) retrospective cohort | 462 (NA) | 79 (17%) | 79 | 24 | 58/21 | Seizures improved in 85.5%, modified in 11.6% and deteriorated in 2.9%Good seizure control: 94.7% of completely obliterated and 77.1% of incompletely obliterated AVMsDe novo seizures: NA |
| Kurita et al. [120] (1998) retrospective cohort | 315 (NA) | 35 (11%) | 35 | 43 | 35/0 | Seizure free: 80%De novo seizures: 4.5% |
| Lim et al. [121] (2006) retrospective cohort | 246 (NA) | 45 (18%) | 43 | 46 | 43/0 | Seizure-free: 53.5%; significant improvement: 23.3%; unchanged: 18.6%; aggravated: 4.6%Complete obliteration in 49%De novo seizures: NA |
| Nataf et al. [122] (2003) retrospective cohort | 57 (61.2%) | 6 (11%) | 5 | 40 | 5/0 | Seizure-free without medication: 80%De novo seizures: NA |
| Silander et al. [123] (2004) retrospective cohort | 26 (NA) | 9 (35%) | 9 | 41 | NA/NA | Seizure-free: 78%De novo seizures: none |
| Steiner et al. [124] (1992) retrospective cohort | 247 (NA) | 59 (24%) | 59 | 24 | NA/NA | Seizure-free without anticonvulsant medication: 18.6%Seizure-free with anticonvulsant medication: 50.8%De novo seizures: 5.8% |
| Sutcliffe et al. [125] (1992) retrospective cohort | 160 (76%) | 48 (30%) | 48 | 24 | NA/NA | Improved epilepsy: 60.4% (worsened transiently in only three of these)De novo seizures: NA |
| Zeiler et al. [126] (2011) retrospective cohort | 69 (87.8%) | 24 (35%) | 20 | 36 | 20/0 | Seizures-free: 95% (55% still on ASM)De novo seizures: 5.7% |
| Bowden et al. [102] (2014) retrospective cohort | 87 (50%) | 36 (41%) | 36 | 64 | NA/NA | Engel class I: 53%De novo seizures: none |
| Mooney et al. [103] (2022) retrospective cohort | 210 (NA) | 35 (17%) | 35 | 43 | 33/2 | Seizure free: 46.7%De novo seizures: 18% |
| Ditty et al. [104] (2017) retrospective cohort | 204 (NA) | 78 (38.2%) | 78 | 37.2 | 69/9 | Engel class I: 80.8%Engel class II–IV: 19.2%De novo seizures: 3% |
| Schauble et al. [105] (2004) retrospective cohort | 285 (NA) | 65 (38.2%) | 65 | 48 | 55/10 | Seizure outcome after the first year: Engel ≤ 4: 73.9%; Engel ≥ 5: 26.1%Seizure outcome after the third year: Engel ≤ 4: 78.4%; Engel ≥ 5: 21.6%De novo seizures: NA |
| Przybylowski et al. [106] (2015) retrospective cohort | 79 (58%) | 76 (96%) | 76 | 78.2 | 60/13 | Engel class IA or IB: 65 patientsEngel class II–IV: 8 patientsDe novo seizures: 7.6% |
| Yang et al. [107] (2012) retrospective cohort | 86 (70%) | 86 (53.4%) | 86 | 89.8 | 86/0 | Seizure-free, off antiepileptic drug: 58.1%De novo seizures: 13.3% |
| Ding et al. [109] (2015) retrospective study | 229 (58%) | 229(100%) | 229 | 65–95 | 208/21 | Seizure improvement: 37.6%; remission: 20%; unchanged: 37.6%; worse: 4.8%De novo seizures: 1.7% |
| Niranjan et al. [110] (2018) retrospective cohort | 155 (78%) | 155(100%) | 155 | 86 | 125/30 | Seizure-free status: 70%; improved: 15%; worsened: 2%De novo seizures: NA |
| Ding et al. [111] (2015) control-case cohort | 175 (63%) | 66 | 66 | 73 | 97/78 | Seizure control: 62 %De novo seizures: 2% |
| EVE | ||||||
| De Los Reyes et al. [127] (2011) retrospective cohort | 20 (NA) | 10 (50%) | 10 | 2.8 | NA/NA | Seizure-free status: 50%De novo seizures: 20% |
| Le Feuvre and Taylor [128] (2007) retrospective cohort | 46 (NA) | 16 (35%) | 16 | 39 | NA/NA | Improved seizures: 56%De novo seizures: none |
| Khumtong et al. [112] (2022) retrospective cohort | 372 (NA) | 105 (28%) | 86(83 followed EVE) | 24 | 75/30 | 2-year seizure-free outcome: 76.2%De novo seizures: NA |
| Zhang et al. [71] (2019) retrospective cohort | 239 (NA) | 68 (29%) | 37 | 37 | 32/5 | Engel class I: 51.4%De novo seizures: 0.6% |
| Lv et al. [94] (2010) retrospective cohort | 109 (NA) | 30 (27.5%) | 30 | 80 | 30/0 | Excellent seizure control: 70%; good:13%; fair: 7%; poor: 10%De novo seizures: none |
| Microsurgery/RSR/EVE | ||||||
| Hoh et al. [14] (2002) retrospective cohort | 424 (NA) | 141 (NA; 33%) | 110 (67/37/6) | 35 | 73/37 | Engel class I: 66%Engel class II: 10%Engel class III: 0.9%Engel class IV: 20%De novo seizures: NA |
| Hyun et al. [101] (2012) retrospective cohort | 399 (NA) | 86 (32/50/4; 22%) | 86 (32/50/4) | 72 | NA/NA | Engel class I: 78%De novo seizures: 2.8% |
| Wang et al. [108] (2013) retrospective cohort | 164 (NA) | 49 (NA; 30%) | 49 (NA) | 38 | 41/8 | No postreatment seizure: 39.6%(58.8% after surgery, 26.7% after SRS)De novo seizures: 19% |
NA: non-available information; ILAE I: free of disabling seizures (A: completely seizure free since surgery; B: non-disabling simple partial seizures only since surgery; C: some disabling seizures after surgery, but free of disabling seizures for at least 2 years; D: generalized convulsions with ASM discontinuation only)
AVM-related seizures treated through AVM microsurgical excision exhibit favorable overall outcomes in terms of seizure freedom. Various AVM microsurgical series report mean overall rates of seizure-free outcomes (Engel scale class I) ranging between 61.54% and 96% [59, 97, 99–102, 125, 126, 128–130]. The average follow-up time across all series was 65.75 months. Microsurgery emerges as the AVM treatment with the highest seizure freedom rates [59, 75, 97, 99–102, 125, 126, 128–130]. A multimodal treatment meta-analyses conducted by Lak et al. [96] described higher rates of seizure control in patients treated with microsurgery compared to SRS and EVE. This meta-analysis included 49 studies involving 2,668 patients, reporting seizure control rates as follows: microsurgery, 65.0%; SRS, 58.5%; and EVE therapy, 48.0% [96]. Hyun et al. [101] reported 78% seizure-freedom outcomes in patients treated with microsurgery while seizure-free outcomes after SRS or EVE were 66% and 50%, respectively. The authors found no significant differences in Class I outcomes between microsurgery, SRS, and EVE (P = 0.1) [101].
Series that report seizure freedom outcomes in patients with partial and total obliteration of AVM volume treated by SRS show a wide range of results, varying from 18.2% to 89% [65, 104, 106, 107, 115–122, 127]. Some of these outcomes indicate a lower seizure control rate (mean of 61%) compared to AVM microsurgery (mean of 79%) [14, 86, 97–101, 112, 114, 129, 130]. The average follow-up time across all series was 58 months. SRS, introduced in the late 1980s as an alternative to microsurgical resection for AVM treatment, has been considered a safe option [14]. Despite its safety, the available literature indicates a lower rate of seizure control compared to microsurgical treatment [14].
Baranoski et al. [87] conducted a meta-analysis of 137 series involving 13,698 AVM patients. The authors concluded that SRS is a more effective treatment strategy than microsurgery and EVE for seizure control in AVM patients. However, this assertion raises questions as it is contingent on the condition that SRS is superior only when achieving complete obliteration of the AVM. The rate of complete obliteration of AVM volume with SRS was only 38%, in contrast to the 96% achieved with microsurgery [87]. Soldozy et al. [59] also highlighted this aspect in their reports.
Zhang et al. [71] reported seizure predictors and outcomes after Onyx embolization in AVM patients. Among 239 patients studied, 28.5% initially presented with seizures. The occurrence of seizures correlated to a history of cerebral hemorrhage, as well as the frontal-temporal and arterial borderzone locations. Among the 37 patients who experienced initial seizures and underwent Onyx embolization, 23 (62.2%) received ASM before the embolization. During the last follow-up, 19 (51.4%) of these 37 patients achieved a modified Engel class I outcome. Among the 23 patients previously treated with ASM, 12 (52.2%) were still using ASM at the last follow-up. Single-factor analysis revealed a significant correlation between arterial borderzone location and a higher modified Engel class outcome (P = 0.046) [71].
Lv et al. [94] reported their experience in addressing seizures associated with brain AVMs in 109 patients without a history of intracranial hemorrhage. The 30 patients with seizure disorders related to brain AVMs underwent endovascular treatment. Post-embolization seizure control after an average follow-up of 80 months was excellent in 21 patients, good in four, fair in two, and poor in three. The study concludes that EVE achieves successful seizure control in AVM patients [94].
Factors linked to pre-operative AVM-related seizures, as documented in the literature, encompass the presence of hemorrhage, gender, localization, normal neurological examination, AVM volume, middle cerebral artery feeding, varix in the venous drainage and the absence of deep venous drainage [17, 86, 98]. Englot et al. [86] identified deep artery perforators as an additional factor associated with post-operative seizures in a series of 416 patients. The univariate Cox regression analysis revealed a significant correlation between post-operative seizures and AVMs with deep artery perforators (Hazard ratio: 4.35) [86]. There was no observed connection between pre-operative seizure factors and post-operative seizure factors [86]. Ferlisi et al. [98] also found no relationship between pre-operative seizure factors and post-operative seizure factors.
Sioutas et al. [131] evaluated the occurrence and risk factors for de novo epilepsy following AVM resection and compared these findings with a nonresection AVM cohort. Additional groups were formed to compare those with and without embolization or rupture in the cohorts. Out of the 536 patients (mean age = 38.9 years ± 19.6 years, 52% females) who underwent AVM resection without a history of seizures, 99 (18.5%) developed new-onset epilepsy, with a 1-year cumulative incidence of 13.8%. Patients with epilepsy had higher rates of intracerebral hemorrhage, while intracerebral hemorrhage was less common in the embolization cohort. Patients in the ruptured cohort were older and more frequently males. Following propensity score matching with 18,588 patients diagnosed with AVM but not undergoing resection, each group comprised 529 patients, and the incidence of de novo epilepsy at 1 year was significantly higher in the AVM resection cohort compared to the nonresection cohort (11.5% vs. 3.4%, P < 0.001) [131].
This examination of 536 patients presents findings indicating that new-onset epilepsy following the resection of brain AVMs has a 1-year cumulative incidence of 13.8%, with a total of 19.4% experiencing de novo epilepsy. The association between intracerebral hemorrhage and postoperative de novo epilepsy varied. The occurrence of de novo epilepsy was notably lower in cases of AVM diagnosis without subsequent resection [131].
Sen et al. [132] retrospectively reviewed patients who underwent AVM resection. Out of the 198 patients in the study period who underwent AVM resection, 111 had supratentorial ruptured and unruptured AVMs without prior seizures. Among them, 21 patients (19%) developed de novo epilepsy. The one-year cumulative rates of developing de novo epilepsy were 9% for the overall cohort and 8.5% for the cohort with ruptured AVMs. Although there were no significant differences between the overall epilepsy and no-epilepsy groups, the de novo epilepsy group in the ruptured AVM cohort was younger (28.7 years ± 11.7 years vs. 35.1 years ± 19.9 years; P = 0.04). The mean time between resection and the first seizure was 26.0 months ± 40.4 months, with the longest onset being 14 years. Subgroup analysis of the ruptured and EVE cohorts did not reveal any significant differences [132].
Patients who developed poorly controlled epilepsy (defined as Engel class III–IV) all had a history of hemorrhage, and half of them had AVMs located in the temporal lobe. The authors concluded that de novo epilepsy after AVM resection occurs with an annual cumulative risk of 9%, potentially with a long-term onset. Younger age may pose a risk factor for patients presenting with rupture. The development of poorly controlled epilepsy may be associated with a temporal lobe location and a delay between hemorrhage and resection [132].
Zhang et al. [70] studied the factors associated with seizures and evaluated the long-term outcome following Onyx embolization in 239 consecutive AVM patients. Among these patients, 68 (28.5%) initially presented with seizures. The occurrence of seizures was associated with a history of cerebral hemorrhage, frontal-temporal location, and arterial borderzone location. Among the 37 patients who initially presented with seizures and underwent Onyx embolization, 23 (62.2%) received ASM before the EVE. At the last follow-up visit, 19 (51.4%) of these 37 patients achieved a modified Engel class I outcome. Of the 23 patients previously treated with ASM, 12 (52.2%) were still using ASM at the last follow-up. Single-factor analysis revealed that the arterial borderzone location significantly correlated with a higher modified Engel class outcome (P = 0.046) [70].
The authors concluded that patients who had experienced a brain AVM hemorrhage, had a frontal-temporal location, and were situated in an arterial borderzone were more prone to seizures. Seizure-free status was not attained in AVM patients located in an arterial borderzone after EVE, although there might be other advantages linked to the procedure [70].
Ding et al. [109] investigated the predictors of seizure outcomes following SRS using their institutional AVM SRS database. Among 229 patients presenting with seizures, the rates of seizure improvement and seizure remission were 57% and 20%, respectively. Multivariate analysis identified independent predictors of seizure improvement, including prior AVM hemorrhage, longer follow-up, and the absence of hemorrhage after SRS. In the case of 778 patients without seizures at presentation, the overall rate of de novo seizures was 1.7%. Multivariate analysis indicated that the absence of de novo seizures was predicted by prior AVM hemorrhage and higher Spetzler-Martin grade. AVM obliteration did not demonstrate a significant association with seizure outcomes after SRS. The authors concluded that SRS provides reasonable rates of seizure improvement for AVM patients presenting with seizures. AVM patients without seizures at presentation harbor a very low risk of de novo seizures after SRS, eliminating the need for prophylactic ASM [109].
In Figure 1 the authors present de novo seizures in reviewed cohorts published since 1992 [48, 65, 71, 86, 94, 97–104, 106–109, 111, 113–115, 118, 120, 123, 124, 126–128].
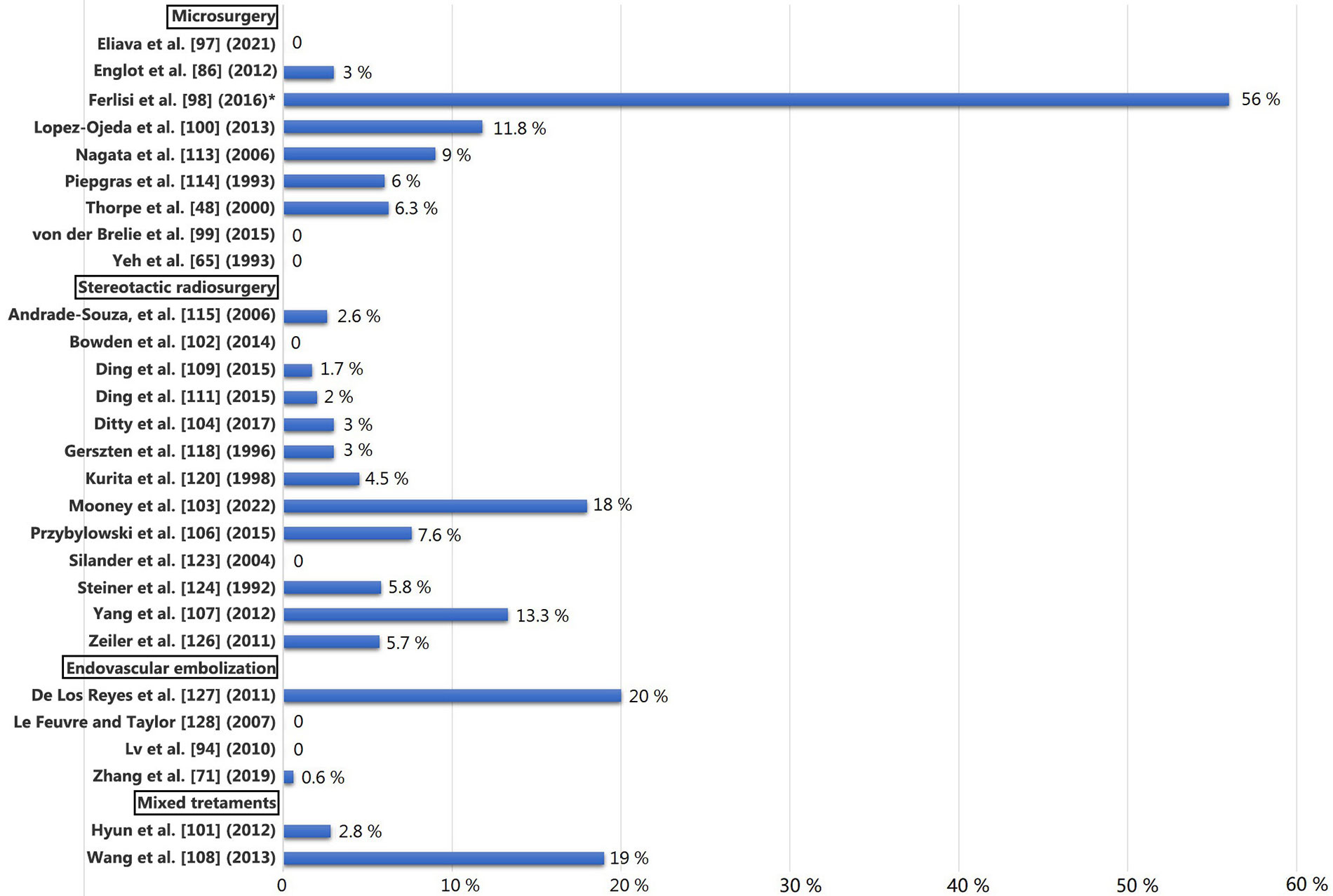
De novo seizures in reviewed cohorts since 1992. * The 43% experienced only a few seizures immediately after surgery, and they were seizure-free at the last follow-up. Only 13% developed new-onset epilepsy after surgical treatment
This review presents a thorough overview of recent developments in comprehending epilepsy in individuals with brain AVMs. This review includes a detailed examination of the natural progression, epidemiology, diagnostic methods, therapeutic approaches, and post-treatment outcomes for individuals with epilepsy related to AVMs.
ASM: antiseizure medications
AVMs: arteriovenous malformations
BOLD: blood oxygenation level-dependent
CVR: cerebrovascular reactivity
DRE: drug-resistant epilepsy
EVE: endovascular embolization
ILAE: International League Against Epilepsy
MRI: magnetic resonance imaging
SRS: stereotactic radiosurgery
JCV: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. UTV, FdJGM, EMM, MGO, and AFVR: Investigation, Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Srilaxmi Vityala ... Swathi Nenavath
Rene Ivan Gonzalez-Fernandez ... Jose Luis Hernandez-Caceres
Kabir Sheikh ... Jeffrey Raskin
Eva Žerovnik
Christine Walker, Chris L. Peterson
Swati Banerjee, Viktor Jirsa
Darrell O. Ricke
Fumiki Yamashita ... Mari Wataya-Kaneda
Jinwei Zhang
