Affiliation:
1Department of Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong, China
Email: lhw162@ha.org.hk
Affiliation:
2Department of Orthopaedics and Traumatology, The University of Hong Kong, Hong Kong, China
Affiliation:
6Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong, China
ORCID: https://orcid.org/0000-0001-5132-3035
Affiliation:
8Department of Anaesthesiology and Operating Theatre Services, Queen Elizabeth Hospital, Hong Kong, China
Affiliation:
9Department of Anaesthesiology, Critical Care Medicine, Pain Medicine, Pain Center Lake Starnberg, Benedictus Hospital Feldafing, 82340 Feldafing, Germany
10Clinic for Anesthesiology, Klinikum rechts der Isar, Technische Universität München, 81675 Munich, Germany
ORCID: https://orcid.org/0000-0002-3759-1508
Explor Neurosci. 2024;3:219–230 DOI: https://doi.org/10.37349/en.2024.00046
Received: May 16, 2023 Accepted: November 20, 2023 Published: June 14, 2024
Academic Editor: Janine Gronewold, University Hospital Essen, Germany
The article belongs to the special issue Neuropathic Pain
Aim: The aim of this study was to validate a Chinese version of the painDETECT questionnaire (PD-Q) for the screening and assessment of neuropathic pain (NeP) in a Hong Kong Chinese population.
Methods: The PD-Q was translated and cross-culturally adapted from the original German PD-Q, with forward and backward translation according to standard guidelines followed by cognitive debriefing, and finalized by an expert panel. A multicenter (6-site) observational study was conducted to evaluate the validity of the PD-Q. Patients aged 18 or above with medical conditions giving rise to either neuropathic or nociceptive pain (NoP) provided informed consent to participate in this study. Each patient was evaluated by at least two healthcare professionals for causes of pain, the visual analogue scale (VAS), numeric rating scale (NRS) and the PD-Q.
Results: Hong Kong Chinese adults (n = 151) were given the clinical description of NeP (n = 93), NoP (n = 41), or mixed pain (n = 17). The mean age of study subjects was 58.5 years (age range: 26–90 years); 94 subjects (62.3%) were female. The mixed pain group was only analysed qualitatively, with validation based on the remaining 134 patients. Mean PD-Q scores for patients diagnosed with NeP and NoP were 19.9 [standard deviation (SD) = 6.4] and 12.5 (SD = 6.2) respectively. Receiver operating characteristic (ROC) curves were plotted for the upper/lower boundaries. The upper boundary was calculated on the basis of a neuropathic diagnosis and a nociceptive diagnosis. The cut-off point was > 18 (80% sensitivity, 60% specificity), and area under the ROC curve (AUC) was 0.67 (P < 0.001). The lower boundary was calculated on the basis of a nociceptive and a neuropathic diagnosis. The cut-off point was < 13 (90% sensitivity, 50% specificity), and AUC was 0.79 (P < 0.001).
Conclusions: The PD-Q is a reliable and valid scale to determine neuropathic components of chronic pain in the Hong Kong Chinese population. Validation in a larger Chinese-speaking population worldwide is necessary.
Neuropathic pain (NeP) is currently defined by the International Association for the Study of Pain (IASP) as “pain caused by a lesion or disease of the somatosensory nervous system” [1]. This is in contrast to nociceptive pain (NoP), which is defined by the IASP as “pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors” [1]. Nociplastic pain is defined as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain”. Mixed pain has recently been defined as a complex overlap of the different known pain types (nociceptive, neuropathic, nociplastic) in any combination, acting simultaneously and/or concurrently to cause pain in the same body area. Either mechanism may be more clinically predominant at any point of time and it can be acute or chronic [2]. However, using these definitions as a basis to differentiate between NeP, NoP or mixed pain in clinical practice is problematic. Patients suffering from pain may present with characteristics of NeP despite the absence of clinical evidence or a medical history of somatosensory lesions or disease [3]. The need for evidence of such lesions or disease for a definitive diagnosis of NeP may result in serious under-diagnosis and under-treatment of patients.
Recent reports in Hong Kong Chinese populations indicate that 14.7%–17.1% of chronic pain patients experience pain with neuropathic characteristics [4, 5]. This is consistent with reports from other countries, such as a Danish registry study which showed that more than 20% of rheumatoid arthritis patients had NeP features [6], and a study from Korea which found that 36% of cancer pain patients had NeP components [7]. In addition, a recent global meta-analysis of 20 studies conducted in patients with lower back pain found that 55.8% of patients presented with neuropathic components of pain [8]. These studies indicate that NeP or neuropathic components are an important issue in the treatment of pain, particularly as evidence indicates that NeP is commonly associated with poor response to management and impaired quality of life [3, 9, 10]. Therefore, measures to facilitate the assessment and detection of NeP features are needed during the course of pain management to achieve timely treatment and improved clinical outcomes.
Several clinical questionnaires have been developed as screening tools for identifying NeP, including the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) [11], the Douleur neuropathique 4 questions (DN4) [12], the 6-item identification (ID) pain questionnaire [13], and the painDETECT questionnaire (PD-Q) [14]. Of these, the PD-Q was specifically developed to identify neuropathic components in lower back pain and other types of pain, and offers advantages in that it was designed for the use of non-specialists. It omits the need for physical examination or diagnostic markers in differentiating between predominantly NoP and pain with significant neuropathic characteristics [3, 14]. It also includes a pain drawing, which can help to improve the clinical diagnosis of NeP [15]. The PD-Q was originally developed in German [14], with paper and electronic versions available, but has since been translated and validated in about 30 languages, including English [16], Spanish [17], Japanese [18], Dutch [19], Korean [20], Turkish [21], Hindi [22], Arabic [23] and two Filipino languages (Tagalog and Cebuano) [24]. The PD-Q has been shown to have 85% sensitivity, 80% specificity, and 83% positive predictive accuracy of NeP components in patients with lower back and other types of pain [14, 25]. In addition, the reliability of an English version of PD-Q was investigated by Tampin et al. [16]. With regards to the validity of the German version of PD-Q, most validation studies have been showing lower sensitivity and specificity. In order that the above-mentioned issues be resolved, a cultural adaptation of the PD-Q is considered to be essential. Our panel was of the opinion that the English version may have different perceptions of pain compared with the German language and therefore a translation from German to Chinese will have advantages over a translation from English to Chinese.
It is conceivable that the neuropathic component of a lower back pain and other types of pain exists as a continuum of severity and as such, the PD-Q will be able to delineate such types of pain, provided that a clearly drawn cut-off can be offered and therefore, the validation part will focus on those with marks which fall within the NeP and NoP ranges. Once the cut-off boundaries are set, those with mixed pain will be logically deducted from the scores of the PD-Q.
The Multidisciplinary Panel on Neuropathic Pain (MPNP) was formed in 2001 by local specialists from various disciplines involved in treating NeP to improve awareness and understanding of NeP in Hong Kong. Currently, the panel consisted of four neurologists, two anaesthetists, one orthopaedic surgeon, one neurosurgeon, one rheumatologist, one geriatrician and one specialist nurse in pain medicine. The panel has previously translated the ID Pain questionnaire to Chinese and validated this screening tool in the Hong Kong Chinese population [26]. However, the PD-Q is more comprehensive than the 6-item ID pain questionnaire, with additional questions that cover allodynia and pattern of pain. While a Chinese translation of the painDETECT was conducted in Singapore, the validation study was only in patients with knee osteoarthritis and not previously diagnosed NeP [27]. The Cronbach’s alpha of the 7-item and 9-item questionnaire from that study were 0.60 and 0.58 respectively. The intraclass correlation coefficient of the 7-item and 9-item questionnaire was 0.59 and 0.50, respectively; while the classification consistency of the 9-item questionnaire was 94% [27]. Due to these differences, we have considered our study to be inherently different.
The aim of the current study was to validate a Chinese version of the PD-Q for the assessment of NeP in a Hong Kong Chinese population, which has potential implications for screening and management of NeP in Chinese populations around the world.
The first section rated the pain severity at the present moment and in the last 4 weeks, with a pictorial indication of pain location, and a pictorial indication of pain fluctuations and periodicity. The second section deals with the more qualitative aspect of the pain (such as whether the pain has burning sensation, electric shock). A clear scoring method was provided.
The PD-Q was translated by a physician with Chinese and German mother tongue from the original German version [14] into Chinese with approval from the original copyright owner of the painDETECT, and then back-translated into German by a translator that was blinded to the original German version. This version was cross-checked by the first author of the original development study (RF) and by the expert panel. The expert panel provided suggestions for minor rephrasing of the Chinese translation, based on clinical experience of panel members, to ensure that the wording of the PD-Q would be interpreted consistently and unambiguously the way it should be understood, having regard to the severity of certain pain connotations. The PD-Q was finally reviewed and approved by all members of the expert panel, and the final version of the PD-Q used in this study is presented in Table 1 (summary only).
A partial extraction of the PD-Q (Chinese version)
| Examples from PD-Q |
|---|
| There are 3 questions from which the patient may elect to give a score on a NRS from 0 (none) to 10 (max.), e.g. how would you assess your pain now at this moment, your strongest pain and your pain on average during the past 4 weeks? |
| The questionnaire is accompanied by four pictorial representations of aspects of pain and the patient should mark the picture that best describes the course of pain, e.g. persistent pain with slight fluctuations, persistent pain with pain attacks, pain attacks without pain between them, pain attacks with pain between them. |
| The questionnaire offers a mannequin with front and backside to draw the pain location, e.g. please mark your main area of pain and if it radiates to other regions of your body, please draw the direction in which the pain radiates. |
| There are 7 questions which the patient has to answer by ticking one out of 6 optional boxes (never, hardly noticed, slightly, moderately, strongly, very strongly). The questions relate to 7 specific descriptor items within the painful area: 1. Burning |
A prospective, observational, multicenter study was conducted at six sites in Hong Kong between 2017 and 2019 to validate the PD-Q, and involved Hong Kong Chinese adult patients diagnosed with NeP, or NoP. The study protocol was approved by the respective Hong Kong Hospital Authority cluster or institutional review boards (IRBs) of the six sites participating in the study: Prince of Wales Hospital, Pok Oi Hospital, Hong Kong Sanatorium and Hospital, Queen Elizabeth Hospital, Pamela Youde Nethersole Eastern Hospital and Queen Mary Hospital. Subjects were required to be aged 18 years or older, diagnosed with NeP or NoP, and able to speak and read Chinese. Subjects who were unable to provide written informed consent, or had cognitive impairment or major/severe psychiatric illness, were excluded from the study. Subjects were recruited from a clinical setting, such as a doctor’s clinic, nurse clinic, or medical ward, and written informed consent was obtained for all subjects prior to enrolment in the study. Diagnosis of NeP or NoP for each subject was made by at least two independent physicians, based on detailed medical history, physical examination, and all relevant medical records available, in line with the latest guideline recommendations from the IASP for the first physician reviewer, and based on all relevant medical records available for the second physician reviewer. Cause(s) of pain and demographic data were collected for each subject. The current pain score of study subjects was recorded. The visual analogue scale (VAS) or numeric rating scale (NRS) was utilized based on usual clinical practice in the respective study center; nevertheless, both scales measure pain on a scale from 0–10 and we have pooled the data and calculated a mean VAS or NRS score for each diagnostic group. Subjects were then given the PD-Q for self-completion which was usually done within 10 minutes. Subject PD-Q scores were collected and analysed, and then compared with subject diagnoses to evaluate the validity of the PD-Q in screening on NeP and its capability of differentiating between NeP and non-NeP.
Based on a previous local study using another questionnaire [26], the power calculation for a clinical score with an estimated area under the receiver operating characteristic (ROC) curve (AUC) of 0.61−0.65 will yield a sample size of 150 with α = 0.05 and power of 0.8.
Based on the original validation study for the PD-Q, a score from 0–12 is defined as “unlikely NeP component”, from 13–18 is defined as “ambiguous result, but a NeP component can be present”, and a total score from 19–38 is defined as “likely NeP component (> 90%)” [14]. The validity of the PD-Q was determined by constructing ROC curves for the upper and lower boundaries, and calculating AUC. Optimal cut-off scores, positive predictive score, and negative predictive power at the point of optimal sensitivity and specificity were determined.
This was an observational survey study based on administration of the PD-Q, with no additional risk expected for both subjects and caregivers. The study was conducted in compliance with the Declaration of Helsinki and good clinical practice (GCP), and written informed consent was obtained for each subject prior to enrolment. Informed consent forms were signed by both the subject and responsible investigator. Identifying details for the personal data of both subjects and investigators were redacted, and appropriate measures were taken to preserve confidentiality and safeguard all study data from unauthorized access.
A total of 152 patients were recruited and enrolled in this study, but data for one patient could not be retrieved and was thus excluded. The remaining 151 patients constituted the population of interest. The mean age of all subjects was 58.5 years (age range: 26–90 years), and 94 subjects (62.3%) were female. There were 93 subjects (61.6%) diagnosed with NeP, and mean current pain for this group (n = 91) as determined by a VAS or NRS with increasing pain over a range of 0–10 was 4.75 [range: 0–10, standard deviation (SD) = 2.961]. There were 41 subjects (27.1%) diagnosed with NoP, and mean VAS or NRS scores of current pain for this group (n = 41) was 4.54 (range: 0–9, SD = 2.712). In addition there were 17 subjects (11.3%) diagnosed with mixed pain, including both NeP and NoP components, who were based upon the inclusion criteria (diagnosed with NeP or NoP) excluded from the study. The most common causes of NeP were trigeminal neuralgia (48 subjects, 51.6%), peripheral neuropathy (including diabetes; 14 subjects, 15.0%), central pain (e.g. post-cerebral vascular accident; 11 subjects, 11.8%), and post-herpetic neuralgia (10 subjects, 10.8%). The most common causes of NoP were musculoskeletal pain (19 subjects, 46.3%) and arthritis (17 subjects, 41.5%). The demographic characteristics of study subjects can be found in Table 2. The mixed pain group was also analysed qualitatively although those data had to be excluded in the quantitative analysis of the current study.
Demographic characteristics of study subjects
| Characteristics | Number (percentage) | |
|---|---|---|
| Total study subjects, n | 151 | |
| Mixed pain diagnosis (excluded), n | 17 | |
| Number of female subjects, n (%) | 94 (62.3%) | |
| Mean age, years (range) | 58.5 (26−90) | |
| Most common causes of NeP, n = 93 | ||
| Trigeminal neuralgia | 48 (51.6%) | |
| Peripheral neuropathy (including diabetes) | 14 (15.0%) | |
| Central pain | 11 (11.8%) | |
| Post-herpetic neuralgia | 10 (10.8%) | |
| Others | 10 (10.8%) | |
| Most common causes of NoP, n = 41 | ||
| Musculoskeletal pain | 19 (46.3%) | |
| Arthritis | 17 (41.5%) | |
| Others | 5 (12.2%) | |
| Pain diagnosis | NeP | NoP |
| Subjects, n (%) | 93 (61.6%) | 41 (27.1%) |
| Female subjects, n (%) | 52 (55.9%) | 30 (73.2%) |
| Mean age, years | 61.8 | 53.9 |
| Mean current pain, VAS/NRS score ± SD (range) | 4.75 ± 2.96 (0–10) | 4.54 ± 2.71 (0–9) |
NeP: neuropathic pain; NoP: nociceptive pain; NRS: numeric rating scale; SD: standard deviation; VAS: visual analogue scale
Table 3 presents a summary of PD-Q scores in study subjects. Overall, 65 subjects (48.5%) achieved PD-Q scores > 18, while 32 patients (23.9%) achieved PD-Q scores from 13–18 (inclusive), and 37 patients (27.6%) achieved PD-Q scores < 13. Patients diagnosed with NeP (n = 93) had a mean PD-Q score of 19.9 (SD = 6.4), while patients diagnosed with NoP (n = 41) had a mean PD-Q score of 12.5 (SD = 6.2). The diagnoses of the NeP and NoP groups were explained in the previous section.
Chinese PainDETECT (PD-Q) scores for study subjects
| Analysis of scores | Number | |
|---|---|---|
| Total study subjects, n | 151 | |
| PD-Q score > 18, n (%) | 77 (51.0%) | |
| PD-Q score = 13–18, n (%) | 41 (27.1%) | |
| PD-Q score < 13, n (%) | 33 (21.9%) | |
| Pain diagnosis | NeP | NoP |
| Subjects, n (%) | 93 (61.6%) | 41 (27.1%) |
| Mean current pain, NRS/VAS score ± SD | 4.75 ± 2.96 | 4.54 ± 2.71 |
| Mean PD-Q score, score ± SD | 19.9 ± 6.4 | 12.5 ± 6.2 |
NeP: neuropathic pain; NoP: nociceptive pain; NRS: numeric rating scale; SD: standard deviation; VAS: visual analogue scale
A ROC curve was plotted for the upper and lower boundaries of the cut-off range to ascertain the validity of the PD-Q in assessing NeP. Calculations for the upper boundary were based on a categorization of neuropathic diagnosis versus nociceptive diagnosis, and the cut-off point was set at > 18 based on the following statistical results, rather than being a pre-set value (80% sensitivity and 60% specificity; Figure 1); the AUC was 0.67 (P < 0.001). Calculations for the lower boundary were based on a categorization of nociceptive diagnosis versus neuropathic diagnosis, and the cut-off point was set at < 13 based on the following statistical results, rather than being a pre-set value (90% sensitivity and 50% specificity; Figure 1); the AUC was 0.79 (P < 0.001). Sensitivity for diagnosing NeP increased with higher PD-Q scores, while specificity for diagnosing NoP increased with lower PD-Q scores.
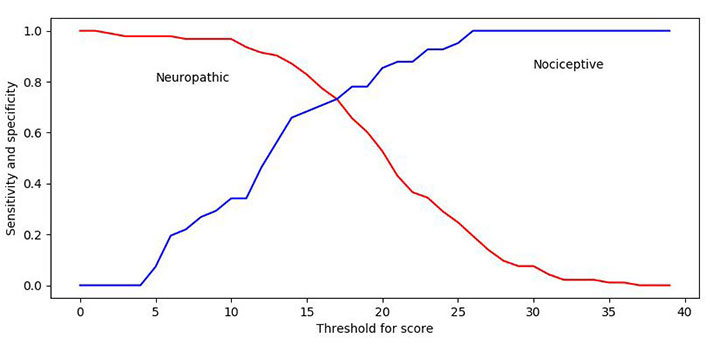
ROC curves of sensitivity and specificity for the upper and lower boundaries. The cut-off point of > 18 has 80% sensitivity and 60% specificity. The cut-off point of < 13 has 90% sensitivity and 50% specificity. (ROC, receiver-operating characteristics)
Overall, the results of this study confirmed the validity of the PD-Q for the assessment of NeP in a Hong Kong Chinese population. For the upper limit to differentiate NeP, the authors elected to use > 18 as the cut-off point for higher sensitivity on the basis of the statistical results derived from the study, rather than an a-priori numerical pre-set value. The authors considered this to be clinically relevant after convening a meeting among the expert members of the MPNP. For the lower limit to define non-NeP (PD-Q negative), the authors selected < 13 as the cut-off point based on the statistical results derived from the study, rather than an a-priori numerical pre-set value; indeed, the sensitivity and specificity were slightly better than for the NeP cut-off of > 18. The determined cut-off points for the neuropathic (PD-Q positive) and non-neuropathic (PD-Q negative) diagnosis was found to be identical with the original PD-Q study (≤ 12, and ≥ 19) [14] and other validation studies [17–22]. This results in the same mixed/unclear range from 13 to 18, which may have advantages in clinical practice (i.e. more certainty for doctors who would like to help with diagnosing NeP using our PD-Q).
The sensitivity and specificity for the upper and lower limits were similar to the original German version [14], and the results were generally comparable with those seen in the validation study of the Japanese version (PD-Q-J) [18] of the PD-Q. It must be pointed out that most validation studies of the German version were showing lower sensitivity and specificity. In our study the sensitivity and specificity for the upper limit of > 18 were 80% and 60% respectively, and for the lower limit of < 13 were 90% and 50%. While there is some variation, overall the sensitivity and specificity were broadly comparable with validation studies of the Korean version (KPD-Q, ≥ 19: 95.4% sensitivity, 73.8% specificity; ≤ 13: 95.4% sensitivity, 73.8% specificity) [20], Hindi version (Hi-PDQ, > 19: 78.7% sensitivity, 92.5% specificity; ≤ 12: 90% sensitivity, 66.2% specificity) [22], Arabic version (Arabic PD-Q, ≥ 19: 67.3% sensitivity, 81.1% specificity) [23], Turkish version (Turkish PD-Q, ≥ 19: 77.5% sensitivity, 82.5% specificity; ≤ 12: 90% sensitivity, 67.5% specificity) [21], and Spanish version (Spanish painDETECT, ≥ 19: 75% sensitivity, 84% specificity; ≤ 12: 93% sensitivity, 68% specificity) [17]. In the study from the Philippines, the sensitivity and specificity of Tagalog PD-Q were both 80% for an upper limit cut-off value of ≥ 17, while for the Cebuano PD-Q, the sensitivity and specificity were 62.5% and 80% respectively, for an upper limit cut-off value ≥ 18.0 [24]. Interestingly, both the PD-Q and PQ-J were translated from the original German version of the PD-Q, while the KPD-Q, Hi-PDQ, Arabic PD-Q, Turkish PD-Q, Spanish painDETECT, and Filipino PD-Q were all translated from the English version of the PD-Q. Although there is insufficient clinical evidence to assess whether translation from a different language version of the PD-Q could potentially impact sensitivity or specificity at cut-off points to a significant extent, this may be worth bearing in mind for future validation studies of translated versions of the PD-Q.
The mean PD-Q score for patients diagnosed with NeP in this study was 19.9, just above the cut-off point of > 18. This may be because patients did not wish to exaggerate their pain, or because the Chinese translation for severe pain does not have the same connotations as that of the original German. There could be a difference in the perception of severe pain in the Chinese and German cultures. In addition, this study included many subjects with trigeminal neuralgia, for which the worst of the pain occurs infrequently; thus, patients on a routine clinic follow-up visit may not have been experiencing such severe pain as they do during an acute episode of trigeminal neuralgia. A follow-up version of our study may need to address these issues.
Sensitivity and Specificity of this version of the painDETECT were found to be lower than in previous translations. This may be due to random scattering, or may hint towards real lower separation capacity of the Chinese version of the painDETECT. Reasons are unknown, but would likely indicate an imperfect translation of concepts from previous versions into Chinese. Long-term monitoring of specificity and sensitivity need to be employed, and reviewed on a larger data basis in the future, to ascertain causes. However, the limited specificity for distinguishing NeP from other types of moderate-severe chronic pain is important to recognize and we are well aware. The sensitivity and specificity of a quantitative test are dependent on the cut-off point above or below which the test is positive. In general, the higher the sensitivity, the lower the specificity, and vice versa. In the same way, the Youden Index gives equal weight to sensitivity and specificity. Sensitivity and specificity are inversely proportional, meaning that as the sensitivity increases, the specificity decreases and vice versa.
The factors affecting our sensitivity and specificity are as follows. First of all, they are dependent on the prevalence of the disease in the population of interest. The differences in sensitivity and specificity results may be due to the specific conditions in different countries such as the type of disease entity on which the scale was validated, or the intensity of pain in screened patients (e.g., moderate vs. more severe). There are validation studies published which showed that specific discriminants alone included in a tool, namely, numbness, hypoesthesia to touch and burning pain, had an influence on sensitivity and specificity [28]. Also inclusion or exclusion of patients with mixed-pain, demographic data related to race and ethnicity, different proportion of patients across the studies or even (statistically) not adjusting for measurement error could be mentioned as a substantial bias of sensitivity and specificity of a validated scale [29–32].
A brand new publication from the UK (2023 in press) dealing with the validation of the Self-Report LANSS (S-LANSS) in adolescents showed a comparable problem. Sensitivity was highest with inclusion of examination findings and lowest with self-completion (LANSS examination vs. S-LANSS interview vs. S-LANSS self-completed: 86.3% vs. 80.8% vs. 74.7%), but specificity was relatively low (37.8% vs. 36.7% vs. 48%). They stated that “contributory factors included classification of CRPS as non-neuropathic rather than NeP, female sex, and high levels of pain catastrophizing”. “The low specificity of S-LANSS and associations with impaired psychosocial function in adolescents with moderate-severe non-NeP, highlight the need for interdisciplinary assessment to more fully inform the classification and management of chronic pain” [33].
However, our data are within an acceptable range and indicate a good sensitivity with a relatively low specificity. We agree that a test with low specificity can be thought of as being too eager to find a positive result, even when it is not present, and with this, one may give a higher number of false positives. But in the case of pain, it might lead to a specialist interdisciplinary assessment and may be even useful to improve not only recognition of NeP or non-NeP but also lead to appropriate treatment. Moreover, the concordance between screening tool outcomes and clinical diagnosis makes the questionnaire easy and practical and can be considered useful to improve recognition and with this as the first step in identifying potential NeP cases. Especially for Hong Kong colleagues, we feel the urge of developing such easy-to-use screening tools in Chinese language.
Utilization of the PD-Q in Hong Kong may effectively identify such patients with a NeP component to their pain and improve clinical management of such patients. However, when mean current pain scores assessed using simple linear tools such as the VAS or NRS are compared with mean PD-Q scores seen in this study (NeP: VAS/NRS 4.75 ± 2.961; PD-Q: 19.9 ± 6.4 and NoP: VAS/NRS 4.54 ± 2.712; PD-Q 12.5 ± 6.2), and then taken into context with validation studies in other Asian populations, such as the Japanese (NeP: NRS 6.7 ± 2.0; PD-Q-J: 18.6 ± 6.3, NoP: NRS 4.2 ± 2.3; PD-Q-J: 11.8 ± 6.3) [16], Korean (NeP: NRS 6.7 ± 1.8; KPD-Q: 22.1 ± 5.5, NoP: NRS 4.8 ± 1.7; KPD-Q: 10.8 ± 4.4) [18], and Hindi (NeP: NRS 7.3 ± 1.0; Hi-PDQ: 20.7 ± 5.9, NoP: NRS 6.7 ± 0.8; Hi-PDQ: 9.9 ± 5.9) [20]. PD-Q validation studies, it appears that the mean PD-Q scores observed for NeP and NoP patients in this study are not particularly low.
There are a few limitations to the current study. Firstly, as performed in the original PD-Q validation study [14], a follow-up study to assess the consistency of evaluating PD-Q scores in the same patient population was not conducted. This was because between visits for the initial test and re-test, study subjects would not have been able to receive treatment for pain. Secondly, although this study included multiple centers and different disciplines such as orthopedics, traumatology, neurosurgery, neurology, geriatrics, anesthesiology, surgery, and rheumatology, the PD-Q may need to be tested extensively in other fields, such as oncology, to further broaden its use. Thirdly we acknowledge that a simple backward-forward translation may not be sufficient in a study of this nature, and reference has been made to other guidelines such as that of Wild et al. [31] and Beaton et al. [32]. Cognitive debriefing with patients may be a better choice as compared with that within the expert panel. Fourthly, exclusion of the mixed pain group will benefit the validity of PD-Q, but it can lessen its utility in clinical practice and therefore the mixed pain group is analyzed qualitatively. Finally, concurrent validity with other validated assessment tools for NeP, such as the Chinese ID pain questionnaire [26], were not conducted in this study, and such comparisons will need to be conducted in future. Last but not least, PD-Q is only a screening tool and it does not replace a thorough medical history and examination.
In conclusion, this study demonstrated the validity of a Chinese version of the PD-Q for the assessment of NeP in a Hong Kong Chinese population, and may have important implications for the assessment of NeP in Chinese-speaking populations around the world. NeP can be challenging to manage and often negatively impact quality of life, so the validation of the PD-Q provides a useful and easy tool to facilitate the detection and management of NeP in patients suffering from pain. Moreover, patients with lower scores on the PD-Q are more likely to have a NoP condition. It is hoped that inclusion of the PD-Q in routine screening of patients with pain will help to ensure timely treatment, appropriate management, and overall better outcomes. A screening tool cannot replace a thorough medical examination.
AUC: area under the receiver operating characteristic curve
IASP: International Association for the Study of Pain
ID: identification
LANSS: Leeds Assessment of Neuropathic Symptoms and Signs
NeP: neuropathic pain
NoP: nociceptive pain
NRS: numeric rating scale
PD-Q: painDETECT questionnaire
ROC: receiver operating characteristic
SD: standard deviation
S-LANSS: Self-Report Leeds Assessment of Neuropathic Symptoms and Signs
VAS: visual analogue scale
The authors would like to thank Dr. Moritz Kurtz for translating the PD-Q from German to Chinese. The authors would also like to thank all subjects and study personnel for participating in this study.
HL, JWYI, JMKL, GKWL, CCFL, RL, VM, THT, CPW, SHSW, CMC, and RF: Conceptualization, Investigation, Data curation, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
RF has received consultancy and speaker fees within the past 2 years from AOP Orphan, Grünenthal GmbH, Eli Lilly and Company, Merck Consumer Health, Mitsubishi Tanabe Pharma, Pfizer, Roche Pharma, and Scilex Pharmaceuticals.
This study’s protocol was approved by the respective Hong Kong Hospital Authority cluster or institutional review boards (IRB) of the six sites participating in the study.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This study was funded by the Multidisciplinary Panel on Neuropathic Pain of Hong Kong and Pfizer Hong Kong.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Hannah G. Matejowsky ... Alan D. Kaye
Sergei Kozlovskiy ... Dmitry Aminin
Bushra Yasin ... Alex Bekker
Tom Berfelo ... Jan R. Buitenweg
Youyi Peng ... Shan Chen
Laura Demartini, Cesare Bonezzi
Giustino Varrassi ... Matteo L.G. Leoni
