Affiliation:
Ex-Professor of Physiology, Cochin Medical College, Kochi 683503, Kerala, India
Email: vallathr@gmail.com
ORCID: https://orcid.org/0000-0003-3428-2416
Explor Neurosci. 2024;3:434–477 DOI: https://doi.org/10.37349/en.2024.00059
Received: July 20, 2024 Accepted: August 23, 2024 Published: September 24, 2024
Academic Editor: Ameneh Rezayof, University of Tehran, Iran
The overt expression of circadian rhythms is a manifestation of the suprachiasmatic nucleus (SCN). This integrated complex function based on the transcriptional/translational feedback loops (TFFLs), neurotransmitters, genes, networking, and synchronization is essential for this molecular mechanism to operate effectively. Neurotransmitters by participating in the entrainment to the environmental light conditions and synchronization contribute to the robustness of the rhythm. Neurotransmitter signaling is the hallmark of circadian rhythm expression. Even during development, neuropeptides contribute to the dramatic cellular, genetic, and network circuit changes. Participating neurotransmitters are seen in afferent inputs, efferent output, and the SCN. There are numerous neurotransmitters involved in SCN function. Astrocytes co-exist with neurons in the SCN. Autonomous clocks seen in astrocytes can drive circadian behavior like neurons. Astrocytes and neurons are acting as two arms of the clock. Coupling through glutamate released from astrocytes gives additional evidence for the role of astrocytes. Glutaminergic signaling from astrocytes may also be responsible for timekeeping. The neurotransmitters can independently and in combination execute the functions making SCN a unique pacemaker for the overt expression of circadian rhythms. This reassessment also highlights its role in underlying molecular mechanisms, genetic linkage, and the recently known role of astrocytes.
Neurotransmitters, neuropeptides, and neuromodulators in a broader sense synthesized by the suprachiasmatic nucleus (SCN) neurons participate in the coordination of endogenous oscillators and entrainment of the SCN. Neuronal diversity along with diverse neurotransmitters and their abundance in the adult SCN makes the study of neurotransmitter function complex. Our timekeeping system focused on the SCN requires a multitude of peptide hormones and neurotransmitters. Hundreds of neuropeptides and small molecules like gamma-aminobutyric acid (GABA), nitric oxide (NO), and others are present in the SCN [1]. An estimate shows that among the heterogeneous population of neurons of about 20,000, they secrete more than 100 neurotransmitters, neuropeptides, cytokines, and growth factors [2]. Studies have also shown five different classes of neurons in the SCN based on the co-expression of various neuropeptides [3]. Another report [4] has identified eight major cell types with each group having a pattern of circadian gene expression. Based on connections, chemo architecture, anatomically and functionally distinct regions of the core that lie adjacent to optic chiasma and shell surrounding the core, are demarcated in the SCN, although the demarcation of core and shell is hardly clear-cut [5]. Studies have demonstrated [6] that core synthesizes GABA, calbindin (CalB), vasoactive intestinal peptide (VIP), calretinin (CALR), gastrin releasing peptide (GRP), neurotensin (NT) while arginine vasopressin (AVP) expressing neurons are seen in the shell. Others in the shell include GABA, CalB, angiotensin II (ANGII), met-enkephalin (m ENK), and galanin (Gal). The core receives dense visual and midbrain raphe afferents, and the shell has more non-visual cortical and subcortical inputs. It is seen that mouse SCN and its connections are observed in hamsters, rats, and humans [5, 6]. An estimate in rats has put 37% AVP, 24% VIP, CALR, and GRP each 14%, while others NT, ENK, somatostatin (SSA), and substance P (SP) constitute less than 5% each [7]. It is remarkable that a relatively small area of the brain, SCN, with a small number of neurons and one-third as many astroglia can synchronize circadian rhythms in the body and guide so many rhythms. A probable answer to this lies in the fact that genes within cells and cells in circuits work together coherently to carry out complex functions to enable normal body function. The intimate linkage of genes and neurotransmitters observed in the molecular basis of the working of the clock also multiplies the relevance of neurotransmitters in SCN function. The very fact that the Nobel Prize in Medicine and Physiology for the year 2017 was awarded to three pioneers working in the molecular mechanisms in the circadian field speaks volumes about the research going on in the field and the fertile nature of this ongoing research area.
The complex heterogenous structure with histological diversity underlies the functional specificity of the SCN. Electrically, chemically, and peptidergically, the heterogenous nature of the neurons is a fundamental feature of the SCN and these neurons individually generate circadian oscillations [8, 9]. An internal molecular oscillating clock based on transcriptional/translational feedback loops (TTFLs) of clock genes [10, 11] drives circadian rhythms in electrical activity and is more active during the day than at night [12]. This landscape nature regarding excitability within the SCN is being reinforced by external signals from the retina [13].
Genetic studies indicate autoregulatory feedback loops form the basis of circadian rhythms not only in the SCN but also in extra SCN oscillators [14]. TTFL generates noisy oscillations of gene expression and firing rates [15] of nearly all neurons because of the orchestrated coupled network of these neurons. Synchronization among the cells [16] with high precision [17] produced by the coupling mechanism is attributed to a variety of neuropeptides mainly VIP and AVP [18–21]. SCN generates rhythms of firing rate by adjusting light inputs through the retinohypothalamic tract (RHT) [22]. The connectome of the SCN along with afferent inputs and neural and humoral outputs are considered important aspects of the synchronization process of the SCN to signal time to the rest of the body [23].
Neurotransmitters are classified based on various parameters but no rigid classification is possible as parameters can be overlapping. An immunocytochemical analysis to identify and locate 25 neurotransmitters in the SCN and its projections has been reported.
Many reviews have been cited [24–29] in the literature highlighting various neurotransmitters and their function. The purpose of this review is to revisit the neurotransmitters since there are new additions to the list of neurotransmitters, neurotransmitter-based changes in the molecular mechanism of the clock working, and advancement in the knowledge regarding genetically linked neurotransmitter working. Not only neurons but astrocytes are also reported to have circadian clocks and molecular mechanisms like the one seen in neurons. Neurotransmitters are secreted by the glia as well and are important for the integrated clock function. Hence a discussion on the role of astrocytes is also in order.
The review is divided into two parts: part 1 is devoted to SCN, connections, input and output, receptors, and neurotransmitters present. Astrocytes in the SCN are also discussed. Part 2 of the discussion is about individual neurotransmitters their properties and their involvement.
The identification of the neurotransmitter dates to the identification of SCN as the seat of the biological clock. A list of the neurotransmitters with their probable location is given in Table 1. The molecular mechanism involved in the function of SCN is complex and by no means fully established at present. It is generally observed that a particular neurotransmitter of the SCN has more than one function. Neurotransmitters may influence the function by way of acting by itself and by way of modification of function. Colocalization of peptides is seen in the SCN. However, coexpression is not seen in all SCN neurons [30]. In some neurons, there is VIP and CALR, whereas in others it is GRP and CALR. VIP, peptide histidine isoleucine (PHI), and GRP are also reported in some neurons. Another report [31] states that 91% of SP cells, 42% of GRP cells, and 60% of VIP cells in the core of SCN coexpress CalB.
Neurotransmitters and their probable location
| Neurotransmitters | Afferent input | SCN | Efferent output |
|---|---|---|---|
| Arginine vasopressin (AVP) | + | + | |
| Vasoactive intestinal peptide (VIP) | + | + | + |
| gamma-aminobutyric acid (GABA) | + | + | + |
| Gastrin releasing peptide (GRP) | + | ||
| Pituitary adenylate cyclase-activating peptide (PACAP) | + | ||
| Dopamine | + | ||
| Glycine | + | + | |
| Glutamate | + | + | |
| Serotonin (5HT) | + | ||
| Neuropeptide Y | + | ||
| Acetylcholine | + | ||
| Cholecystokinin | + | ||
| Melatonin | + | ||
| Melanopsin | + | + | |
| Prokineticin 2 | + | + | |
| Orexin | + | ||
| Pigment dispersing factor (PDF) | + | ||
| Nitric oxide (NO) | + | ||
| Histamine | + | ||
| Neurotensin (NT) | + | ||
| Met-enkephalin (m ENK) | + | ||
| Substance P | + | + | |
| Neuromedin S (NMS) | + | ||
| Somatostatin (SSA) | + | + | |
| Calbindin (CalB) | + | + | |
| Calretinin (CALR) | + | + | |
| Angiotensin II (ANGII) | + | + | |
| Galanin (Gal) | + | + | |
| Oxytocin (OX) | + |
SCN: suprachiasmatic nucleus; +: presence of the neurotransmitter
It is reported [26] that a wide range of neuropeptides and neuropeptide receptors are present in the neurons of the SCN for the execution of its function. These neuropeptide receptors mediate input from extra SCN afferent pathways as well as intra-SCN networks. Various signaling pathways are identified within SCN. As regards the action of neurotransmitters and neuropeptides, it is known that they activate receptors and ion channels on the plasma membrane to produce intracellular signaling events. This includes the activation of guanine nucleotide (G) protein-coupled receptors (GPCR), G protein-gated ion channels, ligand-gated ion channels, voltage-gated ion channels, ionotropic receptors, and tyrosine kinase receptors (Trk) [32]. Trk receptor belongs to a family of tyrosine kinases that regulate synaptic strength and plasticity in the mammalian nervous system.
Neurotransmitters are seen in the afferent inputs to the SCN, efferent output, and in the clock itself. Neurotransmitters in the input to the SCN perform the light-dependent entrainment. These afferent projections with their neurotransmitters were described in the early 1980s in hamsters. Earlier reports suggested that about 40 brain regions have monosynaptic projections to the SCN [33]. However, recent research reports suggest at least three principal afferent inputs of which two are the photic inputs from retinal ganglion cells (RGCs) by way of the RHT and from intergeniculate leaflet (IGL) by way of geniculohypothalamic tract (GHT). RHT from the photosensitive ganglion cells in the retina mediates circadian rhythmicity by photic regulation and in this utilizes glutamate secreted into the core region of the SCN having VIP neurons. These inputs are mainly to the core part of the SCN and spread sparsely to the shell. Retinorecipient core region of SCN is delineated by VIP, GRP whereas the shell region has cells expressing AVP and receives input from the core containing VIP receptor 2 (VPAC2). RHT is the main afferent input to the SCN and terminates in the retinorecipient core area of the SCN and carries non-image forming visual input from intrinsically photosensitive RGCs (ipRGCs) sufficient to entrain SCN. Melanopsin, a photopigment expressed by the ipRGCs causes depolarization in response to light and releases glutamate as well as pituitary adenylate cyclase-activating peptide (PACAP) from RHT terminals in the SCN. Photo-sensitive ganglion cells by secreting glutamate into the core VIP region of SCN participate in the entrainment. RGCs also possess PACAP that helps to relay information about light. PACAP from RGCs relays light information for potentiation of glutamate’s action on the SCN. GHT has neurotransmitters like neuropeptide Y (NPY), GABA, and m ENK out of which NPY is the primary one acting on the pacemaker neurons of the SCN. GABA is reported to have a dual effect of excitatory during the day and inhibitory during the night and NPY acts pre-synaptically to inhibit GABA-mediated transmission to the SCN. A third input is from the median raphe nucleus and has serotonin (5HT) as a neurotransmitter. Neurotransmitters in the efferent projection mainly are AVP and VIP. GHT and RHT have a partial overlap in innervation within the SCN. 5HT from the median raphe nuclei is delivered to the retinorecipient core regions of the SCN rich in VIP neurons [34]. 5HT can regulate SCN neurons by both presynaptic and post-synaptic mechanisms. It modulates pacemaker responses to light within the SCN by potentiating glutamate input during daytime and inhibiting input at nighttime. Afferent projections other than these to the SCN carry cholinergic neurotransmitters [35]. Monosynaptic efferent projection from the SCN to the nearby hypothalamic and thalamic nuclei also terminate in them. These projections with major neurotransmitters present namely VIP and AVP coordinate peripheral circadian clocks. NPY, dopamine (DA), and 5HT are the principal mediators for non-photic entrainment. These inputs and outputs to the SCN [36] determine the working of the clock and coordinate with peripheral clocks (Figure 1).
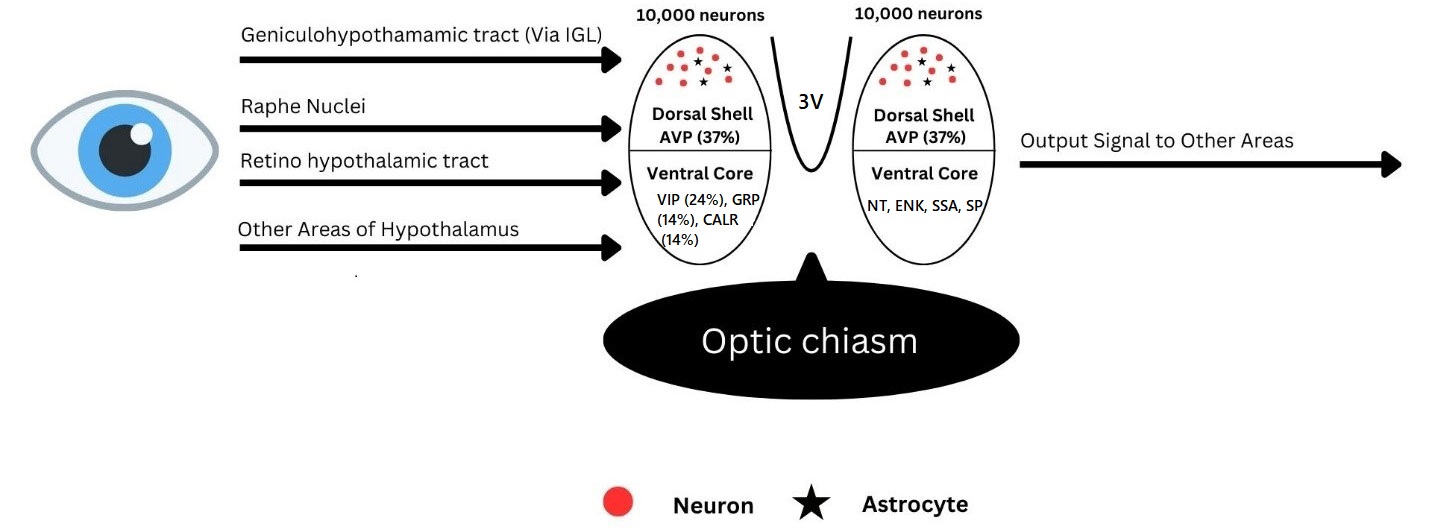
SCN with its afferent connection and output. 3V: third ventricle dorsal shell ventral core; IGL: intergeniculate leaflet; AVP: arginine vasopressin; VIP: vasoactive intestinal peptide; GRP: gastrin releasing peptide; CALR: calretinin; NT: neurotensin; ENK: enkephalin; SSA: somatostatin; SP: substance P
To begin with, before discussing the individual neurotransmitters, some of the neurotransmitters in the molecular mechanism leading to the integrated overt expression of circadian function, having a stable and plastic 24-hour rhythm function in the SCN are being addressed. Their role in network and coupling, circuity and signaling, gene expression, underlying electrical activity, and cation chloride co-transporters in the circadian system are described.
The robust pacemaker, SCN is adaptable to the changes imposed by the environmental cycles. The circadian clock relies on molecular mechanisms to generate rhythmicity in all cells. Circadian oscillations occur at the molecular and cellular levels as indicated by the molecular mechanism in the working of the clock. This efficient nature of the pacemaker for circadian timekeeping is possible with the help of multiple autonomous oscillator networks and their tight coupling to the long day (LD) cycle as well as to each other by intercellular coupling. Interaction between cell-autonomous oscillators determines the robust nature of the clock and its resilience to phase shifts [37]. Although each of the SCN neurons possesses an intrinsic circadian oscillator [15, 38] they display heterogeneity of circadian period and amplitude.
Connections among the different peptidergic cell types of the SCN are highly essential for the network organization. An analysis of the contacts among AVP and m ENK-containing neurons of the shell and VIP, GRP, and CALR-containing neurons of the core which holds the key for the circadian timing has shown [30] that there is less contact of AVP fibers with VIP neurons and the reverse is true more in the wild type (WT) of mice. Similarly, AVP contacts to GRP are more than in the reverse direction. Networking is dependent on coupling, coupling among the different oscillators, molecular coupling involving TFFL, cellular coupling, cell-to-cell communication, core, and shell region coupling, clock coupling with non-SCN tissues, and systemic coupling, all are involved.
SCN neurons which are the clock units are coupled using a system of signaling mechanisms like chemical synapses, gap junctions, and paracrine signaling [39]. Spontaneous oscillations in membrane excitability during the LD cycle are seen among SCN neurons [22, 40]. It has been demonstrated that SCN neurons have a diurnal electrical activity with higher activity during the circadian day and low during the night [22, 41] and this circadian pattern of electrical activity is seen even when isolated from the circuit present since the SCN neurons have stable sustained oscillators to generate circadian rhythms in electrical activity. Experiments with blockage of neural transmission with chemicals produce neuronal desynchronization and decreased or reduced gene expression [16]. Thus, the molecular loop depends on electrical activity and interneuronal signaling for which neurotransmitters and gene expression are essential. It is seen that whatever may be the coupling, all rely on the neuronal activity and the molecular mechanism of clockwork. This includes the TFFL, genes, and the neurotransmitters related to it [42].
It is generally agreed that neuropeptides have an essential and crucial role in the synchronization of circadian rhythms, although the precise mechanism leading to the synchronization of cellular rhythms is not known fully with clarity. Synchronization of cellular rhythms is mediated by neuropeptides and evidence strongly suggests VIP [18] and AVP [20, 43] are among them. Paracrine signals involving VIP, AVP, and GABA can restore cellular synchronization in weakly coupled rhythms of VPAC2 deficient SCN [19] and this suggests the possibility of diffusible signals for synchronization. GRP and GABA have been identified as other neurotransmitters taking part in the neural network contributing to synchronization. An interesting point to be mentioned here is a report [44] indicating a vascular pathway, probably a second one after the hypothalamic portal system, connecting the SCN with nearby areas of the brain for communication of diffusible signals. GABA an important neurotransmitter seen in almost all SCN neurons irrespective of core or shell region, takes part by way of GABA A and GABA B, two types of receptors, and has excitatory and inhibitory actions. Neurotransmitters responsible for network organization within the SCN are AVP, VIP, and GABA. VIP produced by the neurons of the ventrolateral (VL, core) region communicate with each other as well as with neurons of the dorsomedial (DM, shell) region. AVP of the DM region communicates between neurons. GABA has communication between neurons of the VL and DM [18, 45, 46]. It appears that the coupling strength between VL neurons to DM neurons is much larger than in the reverse direction [47]. The heterogeneity property of neurons of the SCN does affect the behavior concerning entrainment to the LD cycle as well as free-running rhythms [48–50]. Neuronal oscillators are heterogenous in their properties and have intrinsic amplitude, period, sensitivity to light information as well as cellular coupling strength. It is observed [51] that heterogeneities can alter free running under constant conditions of darkness and entrainment ability to external cycle. It is stated that this property probably explains the difference in free-running period and entrainment ability observed in different species studied. An emerging result from these studies is the heterogeneity effect on the collective behavior of the SCN. Further, it is observed [51] that disassortativity of the SCN network has a role in the synchronization in the SCN neurons with an increase in synchronization with an increase in disassortativity. The heterogeneity of the SCN neurons seems vital for rhythm generation among weakly coupled oscillators and is probably a method for strengthening the circadian rhythm [52].
This is another area of the SCN function where neurotransmitters have a major role. VIP, AVP, and GABA signaling are among the notable participants in this function of which VIP and GABA have major roles in synchronizing [49, 53–55]. A direct role for AVP in synchronization is now reported [39, 56]. In addition, others like GRP, SP, and neuromedin S (NMS) also have their role in coupling. Although AVP is considered traditionally an output signal, AVP projects within SCN neurons and exogenous AVP can regulate the activity of neurons of the SCN. The role of AVP in synchronization through vasopressin 1a (V1a) and V1b receptors [57] is evident as the loss of these receptors leads to loosening of coupling. Loss of AVP signaling does not preclude circadian oscillations, but in the absence of receptors, there can be a phase shift more rapidly to resetting ones which shows weaker coupling across the network [26]. A report [43] states that mice with a brain and muscle ARNT-like protein 1 (BMAL1) deletion, specific to AVP-producing neurons showed marked lengthening of the free running period as well as activity time of behavioral rhythms. It is known that mice with BMAL1 deletion are used as a tool for the study of the nature of coupling. Of late, it has been reported that glial cells also participate [58, 59] along with neurons in the SCN in the regulation of circadian rhythms. Glial cells and astrocytes possess persistent circadian gene expression to participate in daily rhythms in neurons [60]. Yet another study [61] based on the deletion of BMAL1 in the astrocytes had shown a role for them in circadian locomotor behavior for the first time.
Circadian rhythms present at the cellular level and the intercellular interactions among neurons not only synchronize but also amplify and finally stabilize them [17]. Spatiotemporal gradients in electrical activity at the network level are also an output of interaction among the SCN neurons which can be adjusted to environmental conditions [62, 63].
Circadian gene expression in the SCN depends on metabolism, protein biosynthesis, and degradation [64]. Secreted substances, AVP, VIP, and prokineticin 2 (PK2) are under circadian control in the SCN at the transcriptomic or proteomic level [65, 66]. The molecular mechanism operating in the circadian clock is based on and driven by TFFLs having circadian clock genes [67]. The genes in question in mammals are period (Per 1, Per 2, Per 3) cryptochrome (Cry 1 and Cry 2) clock, and BMAL1. This core TFFL loop operation involves activation of period (Per 1, Per 2, Per 3), and cryptochrome (Cry 1 and Cry 2) gene transcription by a BMAL1/clock heterodimer [68]. Light entrainment of the clock involves the induction of c-Fos [69] and clock genes Per 1 and Per 2 [70, 71]. Possible clock genes for glutamate and PACAP appear to be Per 1 and Per 2 which are induced by light, in the SCN at night. VIP also induces Per 1 and Per 2 expressions in a phase-dependent manner [72] indicating that VIP causes light-induced phase shift at night.
PACAP mediates synchronization of the SCN neurons through PAC1 receptor targeting signaling pathways that produce immediate early gene (IEG) FOS as well as Per 1 and Per 2 [73–75]. Melatonin retinal ganglion cells (mRGCs) along with PACAP and glutamate can transmit light information to the SCN neurons. Light signaling causes induction of the expression of IEG Fos, early growth response protein 1 (ERG1). In mice light entrainment and phase shift of circadian rhythms utilizes PAC1 receptor and PACAP signaling [74, 76–78]. Mice lacking receptors for PACAP show smaller phase delay in the early part of the night [76, 78]. In a recent study [79] it was observed that PACAP signaling through RHT is involved in light induction of EGR1 expression in SCN neurons.
Clock-related genes are one of the factors that modulate the working of the clock. Among the genes, the period circadian clock gene (Per 2) is important for modulation, expressed in the SCN [80], and associated with the possible generation of circadian rhythms [81, 82]. Circadian rhythms are found to modulate [83] the release of some of the neurotransmitters like DA, glutamate, and GABA. This indicates that Per 2 is capable of modulating at least some of the if not all neurotransmitters. A relation between the dopaminergic system and Per 2 [84–86] has been reported and DA release and DA gene expression also have been modulated by circadian rhythms [87–89]. Glutamate (Glu) has a circadian rhythm pattern and the level is regulated by Per 2 [90] and this indicates a positive relation between glutamate and Per 2. The release of GABA is also related to circadian rhythms [83]. Studies show that Per 2 regulates GABA levels and GABA regulates Per 2 expressions. Neurotransmitters like VIP, AVP, and GABA have key roles in intercellular coupling and the release of these [91–94] is regulated by clock genes the transcription of which is activated by the neurotransmitters [95]. Neurotransmitters and clock genes thus help to maintain intercellular coupling. It is also interesting to note that Per 2 can interact with a variety of other genes and proteins and thus the entire process of linkage of neurotransmitters and genes becomes an important factor not only for the functioning of SCN but also beneficial for understanding and treating neurodegenerative disease conditions of depression and sleep states if judiciously used with clinical studies.
Neurons of the SCN are reported to be electrically and chemically heterogeneous. TFFL present in the neurons drives circadian rhythms, electrical activity in the neurons is more active during the day as compared to night when the activity is low [96]. Studies from nocturnal rodent species by way of electrophysiological recordings have given us information regarding the electrophysiology of the SCN [13, 22, 97]. It is known that oscillations in gene expression, intrinsic membrane properties, and synaptic communication involving neurotransmitters determine the electrical features of the activity making them more active during the day than at night.
During the day increased membrane excitability is observed due to voltage-dependent and independent Na+ and Ca2+ currents as well as K+ currents and there is a higher firing frequency. At night there is membrane hyperpolarization and hence decreased firing. This membrane excitability is influenced by several neuromodulators and changes the action potential and rhythmicity of SCN neurons [97]. Electrical activity is less and hence SCN neurons are silent in terms of electrical activity at night. They start generating action potentials at the start of the day and continue all day long [22]. Rhythms in electrical activity are critical for the expression of clock genes and circadian timing system function. SCN neurons can be classified into three classes [98] with each displaying spontaneous firing rates. In other words, neurons of the SCN are heterogeneous in basic and active membrane properties and are classified into three clusters I, II, and III. While cluster I fires spontaneously in a regular pattern, cluster II fires in an irregular mode, and cluster III cells have low firing rates and change to a burst-like firing mode on receiving appropriate input.
It is also observed that the varying frequencies between groups is likely to influence the release of neuropeptides which regulate oscillations in circadian-relevant genes [99]. It is also important to note that different subgroups of clock neurons in the SCN perform a specific role in the network neuronal activity which fluctuates with a specific time of day, allowing the synchronized output to shape the circadian locomotor behavior [99].
VIP neurons of the SCN have circadian rhythms in firing activity and have demonstrated two types of instantaneous firing activity patterns [100]. It is also shown that the firing frequency of VIP neurons mediates circadian entrainment with higher frequencies causing an increase in VIP release, larger phase shifts, and faster circadian entrainment. VIP neuron stimulation with high frequencies shifted gene expression rhythms but not with low-frequency stimulation. Light input to the SCN is detected through the release of glutamate and PACAP from the retina [101–103]. Neurons of the SCN of the ventral hypothalamus are unique in the sense that they have self-sustained and spontaneously synchronizing circadian rhythms in firing activity and gene expression [104, 105]. Nearly 24-hour TTFL of core clock genes maintain the rhythms [10, 106, 107]. Electrical stimulation of SCN neurons is sufficient to phase shift and entrain circadian rhythms in gene expression [108].
Cation chloride cotransporters (CCCS) are associated with circadian rhythms largely as per recent studies. Two solute carriers 12 (SLC12) CCCS have been identified and they are Na+/K+/Cl– co-transporter 1 (NKCC1) and K+/Cl– co-transporter 2 (KCC2) which cause influx and efflux of Cl– respectively. Neuronal chloride is regulated by the above two cation chloride co-transporters NKCC1 and KCC2 seen in the SCN neurons [109]. Studies highlight the expression of NKCC1 and KCC2 in the SCN and the regulation of Cl– by these two cotransporters. NKCC1 is expressed across the SCN while KCC2 is seen only in the ventral region of the SCN [110]. Other studies [111, 112] have indicated NKCC1 and KCC2 in VIP-expressing neurons of the core region, while NKCC1 in the AVP neurons of the shell region. The important fact that emerged from these studies is the critical role of NKCC1 and KCC2 in the circadian system.
A growing number of studies have shown the importance [Cl–] in the SCN not only for GABA but also for AVP and VIP-expressing neurons. This is interesting since, among the heterogeneous population of neurons in the SCN, only 13% are AVP and 9 % are VIP.
It is reported [113] that an increased or elevated [Cl–]i is seen in immature neurons during development which changes to low [Cl–]i when activated. Mature neurons thus have low [Cl–]i leading to hyperpolarization from depolarization as seen in GABA-expressing neurons. KCC2 thus takes part in GABA-mediated hyperpolarization. NKCC1 expression which is high during development as compared to low levels of KCC2 during development changes in reverse pattern or order with NKCC1 decrease and KCC2 increase in maturing neurons [114]. This paves the way for a shift in polarity from excitatory to inhibitory which has been proved in the case of GABA using a pharmacological blocker of NKCC1 [113]. There are also studies [115, 116] to show that light has an important role in the expression and activity of NKCC1 and KCC2 in the SCN. As far as the effect of environmental lighting on the regulation of NKCC1 is concerned it was observed in experiments under constant light (LL) and LD conditions. A hypothesis based on this was put forward [115] indicating that the expression of NKCC1 protein is regulated by environmental lighting and circadian mechanisms within the SCN. In hamsters NKCC1-ir in the SCN was greater in LL as compared to LD or darkness duration (DD) conditions suggesting that light duration increases NKCC1 level in SCN. Both short and long exposure to light changes the expression.
Thus, intracellular chloride concentration (Cl–) is regulated across cell bodies dendrites, axons, and glial cells by the above two major categories of Cl– cotransporters. NKCCs have two isoforms NKCC1 and NKCC2 while KCCs have four isomers KCC1, KCC2, KCC3, and KCC4 [29]. VIP and GRP neurons of ventral SCN have KCC2 but not in AVP cells [117]. NKCC1 is seen in the VIP, GRP, and AVP neurons of the SCN [118]. NKCC1 expression is reported to be significantly higher [116, 119] during the night than during the day in the dorsal but not in the ventral SCN. Another study using a genetically coded Cl– sensor [120] reported that intracellular Cl– was high during the day as compared to night in both AVP and VIP neurons in the SCN. The Cl– co transporter-dependent regulation of SCN clock function appears to be complex but important and provides insight into the therapeutic application options with properly regulated clinical trials.
Astrocytes seen in the SCN of adult mice, rats, and hamsters [60, 121, 122] possess a cell-autonomous circadian clock. It is capable of driving mammalian SCN neuronal timekeeping [9]. They display daily rhythms in cellular physiology like neurons of the SCN. A crucial role for astrocytes has been re-emphasized in mammals based on recent experiments using newer techniques [60, 61]. The robust nature of the circadian rhythm arises from reciprocal network interactions among the neurons and astrocytes [123]. The circadian clock in astrocytes contributes to the circadian oscillations [124].
Contrary to the earlier observation that astrocytes have a supporting role for neurons [125], present-day information indicates that they also have a role in “gliotransmission” [126]. Astrocytes partner with the neurons to perform the functioning of the network for circadian timing of course with separate circadian properties. It is interesting that not only the neurons of the SCN but also the cell-autonomous clocks of astrocytes drive circadian behavior in mammals [127]. Astrocytes and neurons are acting as two arms of the central clock [122]. Based on the presence of an abundance of clock protein PER in glial cells and more so in astrocytes, it has been suggested that there is an existing functional circadian clock in astrocytes. Rhythmic expression of clock genes in astrocytes has also been reported [128–130].
The role of recently identified astrocytes of the SCN in controlling circadian behavior cannot be ignored [123]. Astrocytic cytoskeletal factor glial fibrillary acidic protein (GFAP) has a circadian variation in the SCN [131]. Circadian properties and functions of astrocytes have been studied in detail now with the use of genetic access to astrocytes. SCN astrocytes can control the circadian period by regulating GABA uptake as well as glutaminergic signaling [9, 61, 122].
Astrocytes of the SCN have an essential role in regulating activity and entrainment of the clock as they possess a molecular TTFL [60, 122, 129]. TTFLs form the basis for circadian rhythms in mammals. It is generally agreed that there is interaction between TTFL, neuronal and glial activity of the neurons, and astrocytes of the SCN for the generation of circadian time signals [132]. TTFLs are found in neurons and astrocytes alike in the SCN and the cell autonomous astrocytic TTFLs can alone drive molecular oscillations in the absence of other cellular clocks. TTFL in SCN astrocytes alone driving circadian timekeeping and behavior [133] has prompted the conclusion that astrocytes initiate SCN rhythms and control period but with lower potency than neurons. Molecular circadian clocks of the neurons and SCN astrocytes possess the same functional clock genes and perform as circadian oscillators. Timekeeping in most cells of the body uses TTFL in which period and cryptochrome proteins act on transcription factors CLOCK and BMAL1 to repress their transcription [134]. SCN acts as the central point for gene-to-cell as well as cell-to-circuit to the final behavior in mammals [9]. TTFL-based oscillations along with the strong network of the SCN enable robust timekeeping at the circuit level. This is displayed as high amplitude oscillation tightly ensemble period and phase and complex cellular synchrony [135, 136].
Astrocytes control circadian timekeeping through glutaminergic signaling [122]. Research has shown [129] that astrocytes if cocultured with SCN explants can sustain the circadian rhythm of astrocytes since the diffusible factors from the SCN can entrain circadian oscillations in astrocytes. Astrocytes also can regulate neuronal networks by way of the release and uptake of glutamate and other neurotransmitters [137, 138]. A new model for circadian timekeeping put forward [122] suggests glutamate released from astrocytes maintains higher calcium levels in the pre-synaptic terminals through the N-methyl-D-aspartate (NMDA) receptor subtype 2C (NR2C) activation which leads to neuronal GABA release. The peak phase of circadian calcium rhythms in astrocytes has been observed at night and GABA release from neurons via glutamate from astrocytes increases at night. This leads to a decrease in neuronal activity at night. In other words, this also states that SCN has “day active” clock neurons and “night active” astrocytes. Thus, the SCN circadian network has been reported to have two different cellular populations of day-active neurons and night-active astrocytes. It is also worth mentioning that neurons of the SCN clock are active during circadian daytime and SCN astrocytes are active during circadian nighttime. Anatomical and functional evidence also favors the suggestion of the role of SCN astrocytes in photic resetting. Glu release forms the signal transduction pathway for synchronization by light [139–141]. Neuronal and astrocytic clocks are coupled via glutamate released from astrocytes which increases presynaptic GABA release and suppresses postsynaptic neuron activity during night. Astrocytic clocks can drive molecular oscillations in the SCN by reinstating clock gene expression via glutaminergic signals.
Glu released from astrocytes assists in the coupling of the neuronal and astrocytic clocks by increasing GABA and suppressing the activity of postsynaptic neurons at night [127]. It is not only glutamate but other neurotransmitters are also likely to participate in the actions of astrocytes like it occurs in the SCN. It is reported that glutamate and glutamate receptors sensitive to NMDA have a dual role in SCN by coupling astrocytic and neuronal cell oscillators as well as resetting the phase to light [142].
The rhythmic output of the astrocytes is likely to modulate neuronal activity by way of pre- and post-synaptic interactions. It is also seen that [143] extracellular glutamate increases neuropeptide signaling from neurons. Astrocytes also can increase the circadian period and enhance neuronal synchronization as per the endogenous circadian period. Association of astrocytes with neurons occurs and VIP and AVP are involved in the process [144, 145]. Though not precisely known yet, experimental studies have shown that GABA, glutamate, and VIP contribute to the effect of astrocytes on circadian behavior. Astrocytes can encode circadian information by way of bidirectional synaptic interaction utilizing VIP released from neurons. Daily neuronal rhythms of the core clock are controlled by the release and uptake of GABA and glutamate [146].
A bona fide astrocytic circadian clock contribution for the generation of circadian and seasonal rhythmicity reported [124] gives a new understanding not only about the complex mammalian circadian clock, and neuron astrocyte interaction [147] but also the ability to determine at least some clinical features of diseases as well.
The following neurotransmitters of the SCN will be discussed in detail now. Both VIP and AVP function as synchronizers and are involved in entrainment. Study VIP and GRP of the core and AVP of the shell are the best-understood peptides of SCN.
AVP, one of the first neurotransmitters identified in the SCN has a unique nature and is involved in diverse functions. It has a role as a neuromodulator in many areas of the brain including SCN. Identification of AVP in the SCN was almost at the same time SCN was established as the seat of the biological clock. AVP-containing neurons are reported in almost all species including humans. AVP is also the first output signal based on evidence from Brattleboro rats and vasopressin-deficient mice showing attenuated circadian rhythms [20, 57, 148].
AVP-positive neurons form the second largest peptide population in the SCN and constitute about 20% as compared to 10% in the case of VIP [6]. Reviews are available [27, 29, 149, 150] giving an in-depth understanding of AVP. AVP neurons are essential for the SCN function and loss of AVP neurons is associated with increased activity fragmentation without loss of amplitude in humans. AVP signaling is found to modulate SCN period and phase, that too in a spatially specific manner [150]. Among the two prominent synchronizers of the neuronal networks in the SCN, VIP, and AVP, AVP is weaker than VIP in function.
Lesion experiments demonstrated the loss of drinking and locomotor behavior. Extirpation studies along with cell cultures of the SCN neurons have established the role of SCN in circadian rhythms. Other studies [25, 151] have also highlighted the role of AVP in circadian rhythms.
Application of AVP locally in the SCN does not affect the free running wheel running rhythm in hamsters or circadian food, or water intake in rats. The absence of AVP shows abnormalities of the rhythm expressed. A report [152] also indicates a free-running period of locomotor rhythm being lengthened in Brattleboro rats, V1a deficient mice [57] but not in V1a and V1b deficient mice [20]. New recent techniques of behavioral, electrophysiological, and pharmacological studies have further strengthened the role of AVP signaling. The role of AVP in the molecular working of the clock has been addressed using genetic tools and two state-of-the-art studies [43, 153]. BMAL1, a gene of the TTFL core loop, one of the specific and essential transcription factors of TTFL, specific to AVP neurons when deleted in mice, had shown sufficient for the extinction of the circadian behavioral rhythm in mice. Among the three encoded clock-controlled genes (CCGs) PK2, cardiotrophin-like-cytokine, and AVP, AVP is the critical output molecule to link SCN and its efferent targets and the gene for neuropeptide AVP is well-studied. In a study [93] bioluminescent circadian reporter mice, cAMP response element (Cre) dependent was used to study in detail to assess the functioning of AVP and VIP. The interesting observation reported was circadian functions in AVP neurons are required for autonomous network synchrony as well as stability of circadian rhythmicity and not that of VIP. This is to be considered along with the suggestion that AVP and VIP both are to function in a complementary way [19]. Evidence favoring the role of VIP and VPAC2 receptor signaling for the coherence of the oscillators within the SCN [18, 92, 94, 154, 155] has been reported. Surprisingly it is also shown [19, 93] that it is not VIP but AVP which is essential for autonomous network synchrony. Coupling and networking among SCN neurons is a basic requirement for a coherent output of rhythms [156] and this is mostly neuropeptide mediated.
AVP signaling is found to reset SCN molecular rhythms along with VIP signaling and this is being influenced by sex. Thus, the study of clock function in males and females and significant integration in the central clock is useful for the control of daily rhythms in behavior and physiology [157]. The above-mentioned experimental evidence points towards the ability of AVP in the SCN to integrate circadian time function. It is worth mentioning here in the report [158] that the vasopresinergic neurons of the SCN have not only the ability to encode the afferent input from osmoreceptors to generate homeostatic responses but also can participate in integrating circadian time.
During the developmental stage and adult life neuropeptide signaling has a crucial function in clock neurons of SCN. It is known that both AVP and VIP are expressed during the early part of SCN development [159] but when exactly transcriptional onset occurs is not clear. However, the developmental pattern of neuropeptide expression may contribute to regional cellular function in adult SCN.
AVP has a deep involvement in SCN function as evidenced by recent genetic studies and this has given importance to AVP in maintaining circadian homeostasis as well as a target for therapeutic intervention [160], maybe jet lag and others.
VIP is secreted sparsely, probably by about 10% of the SCN neurons [146]. Two among the 3 VIP receptors VPAC2 and PAC1 are found in the SCN with the majority of SCN neurons expressing VPAC2 in the mouse [161, 162]. VIP binds in the SCN with VPAC2 receptor for its action and high levels of VIP immunoreactivity are seen in the SCN. VIP and VPAC2 receptor agonists when applied locally to the SCN cause firing rate and GABA signaling [163]. Using phase resetting and VIP receptor agonists it has been reported [164, 165] that while the application of VIP during the subjective day did not shift the peak in firing rate rhythm, the same application of VIP during early or late subjective night resulted in a small phase delay or large phase advance. In terms of the use of VPAC2 receptor agonist and not PACAP agonist or VPAC1 receptor agonist, succeeded in producing VIP phase advancing effect and this shows that VIP-dependent phase shift is mediated through VPAC2 and suggests and supports the role for VIP and VPAC2 receptor present in the SCN for photic entrainment. It is also interesting to note that AVP neurons in the SCN participating in the rhythmicity and AVP signaling can coordinate circadian activity in the SCN especially in the absence of VIP [19, 43]. Loss of AVP receptors can weaken the clock and accelerate re-entrainment [20]. VIP signaling changes electrical and molecular rhythms in the SCN. It has been pointed out [166] that VIP is crucial for the maintenance and synchronization of cellular clocks of the neurons in the SCN. In slice preparations of mice with a lack of VIP receptors, there is a reduction in the number of neurons showing oscillations in electrical firing and clock gene expression. Rhythmic neuron synchrony was also drastically reduced [94, 167]. VIP-deficient neurons restored circadian rhythmicity on the application of VIP agonist or coculture with SCN grafts [19] thereby emphasizing the importance of VIP. Evidence of VIP as a principal synchronizer is also available [100, 108]. VIP cells in the core receive direct innervation from RHT and convey photic information to other parts of the SCN. The presence of VIP in SCN slices in ex vivo during late subjective night advances the phase of neuronal firing and stimulation of the expression of genes Per 1 and Per 2 like the effects of light [72, 168]. In the case of mice lacking VPAC2, the receptor for VIP, nocturnal light pulse failed to induce clock gene expression and showed impaired gating or photic responsiveness [92, 169]. In vitro, VIP application can cause an increase in cAMP level leading to a phase and dose-dependent decrease in intracellular Ca2+ in the SCN.
To understand how VIP release due to the firing activity of VIP neurons entrain circadian rhythms, studies [100] were carried out. They concluded that VIP neuron stimulation in high frequencies, but not low, shifted gene expression rhythms in vitro. In vivo, high-frequency VIP neuron activation resulted in the entrainment of circadian locomotor rhythms. In effect, it is reasonable to believe that VIP neural firing frequency releases VIP and leads to the entrainment of molecular and behavioral circadian rhythms. By using optogenetic manipulation and bioluminescence imaging interaction between molecular electrical and behavioral circadian rhythms was studied [108]. In yet another study [170] emphasizing the role of VIP, it has been suggested that VIP neurons of the SCN have a role in lengthening the circadian period. This was based on the ablation of VIP SCN neurons in vivo and in vitro. The results also showed a differential role for VIP neurons in adults and developing SCN. VIP neurons are important for synchronizing circadian cells during development but not so in adults. Core peptide VIP increases during the dark period and is important for light entrainment and internal synchronization. It has been pointed out [166] that VIP is crucial for the maintenance and synchronization of cellular clocks of the neurons in the SCN. The absence of VIP signaling as seen in VIP receptor-deficient mice leads to attenuated behavioral rhythms, maybe along with multiple period components [18, 92, 154]. In slice preparations of mice with a lack of VIP receptors there is a reduction in the number of neurons showing oscillations in electrical firing and clock gene expression. Rhythmic neuron synchrony was also drastically reduced. In VIP knockout (KO) mice and those lacking VPAC2, there is a reduction in the coherence of the circadian rhythms which is due to a lack of coupling among neurons [92, 171]. Variations within individual animals in the relation between behavior and rhythmic expression in SCN have also been reported. Mostly mice lacking VPAC2 receptors do not sustain behavioral rhythmicity and do not have detectable electrical rhythms in vitro. While adult mice with no production of VIP show less severe disruption of locomotor activity, VIP KO mice show a broad range in both period and phasing of electrical rhythms. These varied results as well as much of the present information do not clearly state how VIP and its receptor participate in interneuronal coupling in the SCN. However, it strongly indicates the important role of the normal period and phase of SCN neuronal rhythms.
Using bioluminescent circadian reporter mice now developed which are Cre dependent allows the genetically defined population of cells to be studied in real-time, it was observed [93] that among the two important peptidergic neurons studied, AVP and VIP, AVP neurons and not VIP neurons are required for an essential autonomous network synchrony of the SCN.
The role of VIP in SCN circuits, which is not yet fully known, was also studied using the DNA-editing enzyme Cre recombinase [172] and it was reported that VIP-IRES-Cre transgene interferes with VIP expression to affect clock function. However, it was also evident in their studies that molecular rhythms present in VIP-Cre-SCN are not due to residual VIP, but due to AVP signaling which helps to maintain SCN function at both intracellular and intercellular levels.
VIP-expressing cells of the SCN and its cognate receptor VPAC2 are separate in nature neurochemically and electrophysiologically. It is reported [136] that there is a VIP/VPAC2 cellular axis that acts as a neurochemically and topographically specific pacemaker hub to determine the properties of the SCN network. Neuropeptides AVP, PK2, PACAP, and VIP along with their cognate receptor [173] are capable of mediating not only paracrine but also autocrine signaling [20, 150]. It is possible that neurons contributing to VIP to the VPAC2 axis can define the network-level properties for the role of SCN as a pacemaker.
A study [174] had shown that VIP neurons of the SCN and the internal molecular clock present in it maintain locomotor activity rhythms, and circadian rhythm of body temperature in addition to sleep and wheel-running behavior. Recent studies are pointing to the participation of non-VIP SCN cell groups. An alternate but surprising viewpoint put forward is based on a combination of neurons expressing AVP, DA 1a receptor (Drd1a), or non-VIP NMS [43, 153, 175], present in the SCN, dominant to SCN VIP neurons controlling circadian pace making. However, this theory has not found much favor due to a lack of supporting evidence.
GABA, synthesized in the SCN from glutamate with the help of GABA synthesizing enzyme glutamate decarboxylase (GAD) is expressed by nearly all neurons. Multiple circadian oscillators are synchronized to each other through neurotransmitters and GABA is one among them for the oscillatory coupling mechanism. However, the role of GABA in the circadian clock function remains controversial [176]. Though initially recognized as an inhibitory neurotransmitter, GABA is now reported to be both inhibitory and excitatory on SCN neurons [5, 46, 177] with the effects varying with the region within the SCN, time of the day, photoperiod, and even development. GABA effects on cellular activity have been studied with one study reporting inhibitory effects on SCN neurons [178] while others pointing both excitatory and inhibitory effects [119, 179].
Two major classes of GABA receptors namely GABA A (gamma subunit) and extra synaptic GABA A (delta subunit) [180] and GABA B have been identified in the SCN [118]. GABA can function through signaling and the two types of receptor subunits referred to as GABA A appears to be a chloride ion channel and GABA B is a G protein-coupled receptor. Further GABA B receptors are mainly expressed in the dorsal area of SCN [118] also containing AVP-positive neurons. Individual SCN cells with heterogeneous oscillators present in the SCN must couple with each other to have a robust output and synchronized circadian rhythms are required for coordinating peripheral circadian oscillators as well [181]. Synchronization process in the SCN can be supported [182] or impaired [183] by GABA coupling.
The functional role of GABA in the clock has been elusive for some time now. Studies used to address the function of GABA in the SCN have mainly used a pharmacological approach since it is difficult to assess the role of GABA in the SCN by using a genetic study as mice with genetic GABA deficiencies like vesicular GABA transporters (VGAT) and GAD 65/67 deficient mice do not survive for long [184, 185] after birth. Two distinct forms of GAD namely GAD 65 and GAD 67 exist. VGAT is also present [118] in the GABAergic neurons of the SCN. Circadian oscillations occur at cellular, molecular, and systems level. A major reason for the dual excitatory/inhibitory effects of GABA in the SCN is the intracellular chloride (Cl–) concentration. GABA acts as an inhibitory neurotransmitter when intracellular Cl– concentration is low and excitatory when the Cl– concentration is high. GABA function also changes during development. GABA which has an excitatory effect initially during development because of the high intracellular Cl– concentration becomes inhibitory during development [186]. A study using genetically encoded radiometric probe Cl-Sensor has been reported [187] that [Cl–]i is under circadian regulation in AVP and VIP neurons and this has probably provided a basis for the reports of increased excitatory GABA transmission during day and early night.
GABA A, a heteropentameric ligand-gated ion channel permeable to chloride [188, 189] takes part in GABA signaling [5, 29, 190]. It is also suggested that the functional role of GABA may be for the refinement of circadian firing rhythm, rather than for rhythmic generation and population synchronization [190]. Genetic studies of recent times have revealed that GABA refines circadian output rhythms but does not affect circadian oscillations in the SCN [29]. A new computational insight into GABA signaling has been developed to give more information regarding mammalian circadian timekeeping [180]. This also explains the interplay between gamma and delta GABA A receptors in the SCN which is crucial for signaling. It also tells us to some extent seasonal variation in circadian timekeeping. This acts as a pointer towards GABA-based therapeutic regimes for shift work or jet lag. The possibility of GABA levels and GABA A receptor densities balancing to time electrophysiological output of the SCN for neuronal firing [190–192] is also reported.
Studies to understand the function of GABA in the SCN have been done by using both in vivo and ex vivo methods. SCN from neonatal animals [182, 193] and from adult animals [46] have been used in experiments for recording circadian rhythms. The results of these experiments have not given a complete picture of the developmental effects of GABA on SCN circadian oscillations. However, reports [21, 194] have indicated that Cry, a clock gene is involved in cellular coupling in the adult SCN and this may have a crucial role in the cellular coupling leading to circadian outputs.
GABA takes part in the transmission regulating synaptic input from RHT [195], mediates phase shifts regulates firing frequency [196], and contributes to circadian synchrony. GABA, a primary inhibitory neurotransmitter remains excitatory during embryonic and neonatal development. Chloride homeostasis conveyed by GABA A receptors and their co-transporters [116, 197, 198] which is modulated by photic changes [177] determines GABA intercellular signaling. It is also likely that some of the neurons within the SCN neural network that are excited by GABA may be involved in encoding day length [116, 199]. GABA A receptor is primarily permeable to Cl– ions and intracellular Cl– concentration determines depolarization or hyperpolarization. Based on the finding that the application of GABA decreased firing frequency at night and increased firing frequency during the day, it was suggested that the effect of GABA on SCN neurons is related to the circadian phase. This is contradictory to other reports [178] stating that there is no difference in the excitatory/inhibitory effects of GABA during subjective day as compared to night. GABA A agonists like muscimol and baclofen and GABA A antagonists like biculline produce excitatory and inhibitory effects across circadian day or night irrespective of the circadian time.
GABA is colocalized sometimes with other neuropeptides like VIP and GRP and studies [200] have tried to explain the interaction of multiple signals in the SCN. GABA is likely released synaptically and non-synaptically in the SCN. However, it is not known about the relative contribution of the two types of signaling for the coupling of the clock cells for the robust functioning of the SCN. Based on the evidence it may be thought that neuropeptides of AVP, VIP, and GABA by participating in network communication allow oscillators to work and bring out the interplay of molecular, electrical, and behavioral components of the clock.
SCN neural network utilizes GABAergic neurotransmission for synchronization and all SCN neurons communicate using GABA [5, 176]. Light-induced phase shifts, synchronization between the dorsal and ventral SCN, and the sensitivity of the circadian clock to light-entraining signals are among the many functions of GABA in the SCN. Fast “phasic” signaling between the SCN neurons is achieved by the action of GABA on synaptic GABA A receptors whereas extra synaptic GABA A receptors activation provides “tonic” GABA A current [201].
GRP and its receptor Gq-coupled Bombesin-2 (BB2) are synthesized in the SCN neurons of rodents, mice, and hamsters. Glu, identified as the main neurotransmitter for photic entrainment along with GRP and SP participates in photic response. It is observed [202] in rats and hamsters that GRP application causes phase delay in SCN neuron firing during early subjective night, and phase advance during late subjective night with no response to application during subjective day. Phase shift blocking by BB2 receptor antagonist confirmed GRP and its receptor participation in photic entrainment. The presence of GRP within CalB cells may indicate GRP for intra-SCN communication [203].
BB2 receptor is densely expressed in the shell [204]. GRP applied can mimic resetting actions of light and produce the response in both early and late subjective night. From a developmental point of view, it is interesting to note that GRP expression occurs at a later age as compared to some other neurotransmitters like VIP and SP in not only hamster SCN [146] but also in mice [205–207]. The difference in late timing in the expression of GRP seen at an early age is absent in adult mature SCN and are retinorecipient [206, 208]. GRP neurons in adults display light-induced increase in firing rate, IEG expression, and neuropeptide release [209, 210]. A subset of SCN neurons is found to co-express GRP and VIP and there is a synergistic response to combined GRP and VIP administration [200]. Yet another interesting observation is that BB2 signaling can synchronize SCN neurons when VIP signaling is not available [19, 211]. However, there is no evidence to show that GRP is required for SCN timekeeping since BB2 deficient mice though exhibit a lack of photic responses show normal rhythms in behavior [204]. Probably this aspect may make one believe that late expression of GRP is related to a more specific role or function of GRP in the mature adult SCN. Further, it is also seen that GRP neurons of the retinorecipient ventral area of the SCN can convey photic signals from the ventral part to the dorsal part of the SCN by way of induction of mPer gene.
PACAP belongs to the secretin family and has a role in mammalian clock resetting. It is a neurotransmitter with many functions and is co-localized with glutamate in RHT terminals. It is associated with melanopsin required [212, 213] for entrainment of the mammalian clock. It is suggested [214] that PACAP action is to augment the PACAP signaling pathway at times when glutamate stimulation alone is not sufficient.
Light entrainment of the circadian clock is through mRGCs as well as glutamate and PACAP. Light signaling involves the expression of the IEGs Fos and Egr1 and clock genes of Per 1 and Per 2. Using PACAP deficient mice to study the role in light-induced gene expression of EGR1 in neurons of the SCN, it was reported [79] that light-mediated EGR1 induction at early night at low light intensities depends on PACAP signaling. Although this role of PACAP in light NMDA/glutamate-mediated neurotransmission is suggested there is a need for further investigation about the role of PACAP.
The PAC1 receptor responsible for PACAP signaling is involved in light entrainment and phase shift of the circadian rhythms in mice [76, 77, 78, 215]. In the absence of PACAP or its receptor PAC1 smaller phase delay is seen at early night, probably due to altered light sensitivity, and light-mediated phase advances are less compromised at late night.
Of late DA is recognized as an important modulator of circadian rhythms and hence regulation of biological rhythms by DA appears to be an emerging area of interest. Experimental evidences show that DA signaling is functional in adult SCN and both in vitro and in vivo elevated DA signaling lengthens the circadian period [216]. This effect of DA on circadian rhythms is opposed by valproic acid, a mood stabilizer to highlight the role of DA signaling. The reciprocal, bidirectional relationship between DA and the circadian clock is interesting as DA signaling is an important modulator of circadian rhythms and DA is also under the control of the circadian clock. It is also suggested that this bidirectional process is seen at the level of genes and neural structures as evidenced by the experiments of depleting DA release using 6-OHDA to lesion DA neurons projecting to dorsal striatum leading to loss of rhythmicity of the Per 2 gene in striatum [84]. From a clinical viewpoint, this relationship may be critical for the development of schizophrenia as well [217]. The circadian clock modulates the synthesis and breakdown of DA. DA imbalance produces many neurological conditions and circadian disruptions can be seen in these conditions [218]. DA signaling thus becomes important for consideration under the circumstances. DA, a member of the family of catecholamine neuromodulators once known for regulating movement, reward, and learning has entered the circadian field with its involvement in central and peripheral clocks as a neuromodulator. It is not known clearly whether DA has a causal role in regulating circadian rhythms or is acting as a motor relay for other known bona fide circadian pacemakers [219]. There is a direct connection between dopaminergic neurons of the ventral tegmental area (VTA) and SCN. DA signaling in the embryonic SCN to synchronize with maternal fetal circadian rhythms was reported many years ago. Studies at that stage gave the impression that sensitivity to DA was a transient one and was lost after the development of RHT. Present studies indicate a direct modulation of DA signaling in the circadian clock and studies including those involving genetic ones have reported DA participation directly in the modulation of the circadian clock in adulthood. DA circadian rhythmicity is observed with the highest peak of synthesis during the daytime in the retina which is reduced during night.
Light is responsible for the photic entrainment of the circadian clock. However, non-photic cues like social interaction physical exercise, and many others also influence the circadian molecular clock in the SCN to complete the generation of circadian rhythms. DA appears to be a neurotransmitter responsible for the non-photic entrainment of the above-mentioned behaviors. It is also interesting to note that perturbations of circadian rhythms are seen [220] in patients with psychiatric disorders, Parkinson’s disease, Schizophrenia, and drug addiction.
Evidence is available to show that DA has a role in the regulation of circadian rhythms and this is through modulation of SCN entrainment and behavioral oscillators [217]. The function of two important components namely the retina and the SCN of the circadian system is influenced by dopaminergic signaling [221, 222]. Light adaptation and visual acuity are two functions for which DA is required in the retina [221]. DA can control the amplitude of rhythmic gene expression in the clock [223]. Evidence is available to show that DA participates [224] in the rhythmic expression of melanopsin in the iPRGCs via D2 receptors (D2R). In this way, DA becomes a main modulator of the non-visual light detector for the photic entrainment of the clock. Many dopaminergic neurons having Drd1 expressing neurons are reported approximately 60% in rodents [175]. As stated, earlier DA is required for the entrainment of the developing SCN [225]. Recent investigations [108, 175, 216, 226] have revealed that DA modulation of the central clock exists in adulthood. Drd1 positive cells overlap with AVP and VIP neurons in the SCN and hence DA involvement in circadian function cannot be fully ascertained [226]. It is also demonstrated that Drd1 absence can reduce circadian entrainment and the same can be restored by the restoration process or presence of Drd1. Midbrain DA neurons innervating SCN if stimulated can also accelerate circadian entrainment.
DA neurons can also release GABA, the inhibitory neurotransmitter that can modulate clock protein expression in the retina. GABA may likely influence the actions of DA neurons in the brain and SCN may have a direct bearing on circadian rhythms [227].
Two classes of DA receptors are seen, one D1-like receptor including D1 and D5, and D2-like receptors D2 D3, and D4 [228, 229]. The presence of D1 and D5 receptors is reported in the SCN. SCN clock communicates timing information with other clocks in the brain to regulate DA activity as well as DA appears to have feedback effects on the SCN [225]. DA seems necessary to entrain the developing fetal SCN.
Among 5 different types of receptors mediating actions of DA, Gs-coupled D1 and D5 and Gi-coupled D2 D3 D4 are the receptors found in the anatomically distinct regions of the brain and body [230]. D1 type receptor subtypes D1 D5 coupled to G protein Gs and activate adenyl cyclase. Another receptor subtype belongs to D2 and subtypes of D2 D3 and D4, GPCR inhibit adenyl cyclase and activates K+ channels.
Glycine and glycine receptor chloride ion channels (GlyRs) are seen in the central nervous system (CNS) [231] where it can function as an inhibitory neurotransmitter. Glycine-containing nerve terminals are seen in close relation to the VIP neurons in the SCN. Glycine produces both inhibitory and excitatory effects on SCN firing rates [232] and the effect is dependent on circadian time. It is also known that glycine from SCN slice cultures shows time-of-day-dependent fluctuations like the rhythmic release of AVP. Also, a subset of neurons of the SCN showed biphasic responses to glycine, a rapid transient increase in firing rate, and then a suppression. There is a suggestion [233] that glycine regulates some SCN functions through NMDA receptors.
Based on electrophysiological studies, it is reported that strychnine-sensitive GlyRs are present which act as an inhibitory neurotransmitter and excitatory neuromodulator like GABA. High concentrations of glycine when present can reset the clock in brain slice preparations [234]. A circadian release of glycine is also reported in slice culture preparations. Some studies using patch-clamp recordings and long-term recordings in brain slices have shown [232] that glycine can modulate circadian rhythm activity on specific receptors GlyRs in the SCN neurons. Glycine can act in the SCN to synchronize SCN neurons as well as to potentiate NMDA responses indirectly for synchronization thereby enhancing transmission of photic information.
While studying the effect of glycine on the sleep-promoting mechanism it was observed that glycine produces the effect through the activation of NMDA receptors in the SCN shell by causing peripheral vasodilatation in the case of rats [233]. It was also observed that when bilateral microinjection of glycine was made into the SCN, there was an increased cutaneous blood flow in a dose-dependent manner and ablation of SCN could abolish the sleep-promoting effect of glycine [233]. In effect, glycine promotes sleep by modulating thermoregulation and circadian rhythms through NMDA receptors of the SCN.
In yet another study [235] it has been reported that glycinergic transmission has a role in the synchronization of the circadian network in the SCN which leads to the organization of circadian behavior.
Glu is required for the molecular timekeeping mechanism in the SCN, especially the extracellular glutamate levels are important. These extracellular levels and their rhythmic oscillation are controlled by astrocytes [236]. Glu release from astrocytes is mediated by the ultimate Glu metabotropic glutamate receptors (mGluRs) which when activated causes an increase in astrocytic [Ca2+]i with ultimate Glu release from astrocytes. Glu seems to be involved in photic signaling and this is now well established. SCN neurons express all the 3 subtypes of glutamate receptors Glu addition to SCN explants induces neuronal firing whereas optic nerve stimulation causes the release of glutamate. These effects are mostly due to NMDA and non-NMDA-dependent glutamate receptors as seen in experiments using receptor antagonists in vitro and in vivo [237]. SCN astrocytes release glutamate [122] and thereby raise levels of extracellular glutamate during the night. During subjective day astrocytes release less glutamate and will lead to reduced spontaneous activity of SCN neurons. RHT mainly uses glutamate as a neurotransmitter but PACAP and SP also act as other two peptide co-transmitters in RHT transmission. Surprisingly these co-transmitters regulate glutamate transmission but the mechanism of such an action is not known [238]. Glu receptors present in the SCN belong to metabotropic and ionotropic types. However, the distribution and abundance of Glu receptor subunits varies and this leads to different effects of Glu on SCN neurons. Melanopsin, a photopigment expressed by the iPRGCS causes depolarization in response to light and releases glutamate as well as PACAP from RHT terminals in the SCN.
A dense serotonergic projection mainly from raphe nuclei to the retinorecipient region of the SCN and that too specifically to the VIP-containing core region of the SCN [239] has been reported. There is a reciprocal projection from SCN to raphe nuclei also. IGL also has a serotonergic projection from the dorsal raphe. The presence of many 5HT receptor subtypes has been reported in the SCN [240, 241]. 5HT receptor-mediated signaling within SCN appears to be complex. The function of serotonergic projection to the SCN is mainly to modulate the pacemaker responses to light and in this raphe-retina projection may function as an indirect photic input to the clock. 5HT can regulate SCN neurons by both pre-synaptic and post-synaptic mechanisms [34]. However, 5HT modulates glutamate release from RHT terminals as well as participates in GABAergic signaling through 5HT1B receptors present in SCN clock neurons [242, 243]. In vitro and in vivo 5HT receptor agonists cause phase shifts of the SCN if administered at times of the circadian cycle during which light does not cause phase shifts. Raphe nuclei lesions are found to reduce amplitudes of circadian activity rhythm in rats.
5HT agonists inhibit optic nerve-induced field potentials in SCN brain slice preparation, light-induced Fos expression, and phase shifts of the circadian rhythm of wheel running activity. 5HT antagonists produced an increase in light-induced firing rates of SCN neurons as well as light-induced phase shifts. 5HT modulates pacemaker response to light within SCN by potentiating glutamate input during daytime and inhibiting input at night time.
In addition to modulation of photic input serotonergic system is also involved in non-photic effects of phase shifting of neuronal firing, locomotor activity rhythm, melatonin secretion, and body temperature rhythm as well as gene expression [244]. NPY, DA, and 5HT neurotransmitters are the principal mediators of non-photic entrainment. There is some difference of opinion as regards the role of 5HT signaling. The possibility of different subtypes of receptors mediating these reactions cannot be forgotten. Despite all these serotonergic projection remains an important signaling mechanism for the SCN function.
Of the four afferent inputs to the SCN, GHT from the IGL mostly has immunoreactivity for NPY and is the neurotransmitter in the photic input [33]. Though not required for entrainment or normal expression of circadian rhythmicity, it does exert an influence on entrainment. Neurotransmitters involved in GHT which is an indirect photic input mediated by both photic and non-photic stimuli are NPY, GABA, and ENK. NPY is the primary neurotransmitter acting directly on pacemaker neurons of the SCN [25, 245].
NPY is a 36-amino acid peptide involved in various physiological processes. In the SCN, NPY has multiple functions of modulating the phase and amplitude of circadian rhythms, altering the timing and strength of SCN output signals depending on the time of day and environmental conditions. NPY also influences the response of SCN to light stimuli and is responsible for SCN activity and sensitivity to various stressors and metabolic signals like glucocorticoids, leptin, and Ghrelin. NPY modulates neurotransmitter action in the SCN, particularly the inhibition of GABA release. NPY exerts its effects on the SCN through the receptors Y1-Y6 coupled to different intracellular signaling pathways. It is seen that the application of NPY to SCN slices produces reversible inhibition of neuronal firing in a concentration-dependent manner which subsequently leads to phase advance in circadian rhythms. Of the receptors, NPY acts both on Y1 and Y2 which involves reducing the frequency of spontaneous miniature inhibitory postsynaptic currents thereby suggesting a presynaptic mechanism of NPY action. The exact mechanism and interaction of NPY and its receptors are yet to be known completely and require further investigation. The distribution of NPY and receptors in the SCN varies across species and circadian phases. In addition, NPY acts pre-synaptically to inhibit GABA-mediated transmission to the SCN. A recent review article [36] goes into more detail about NPY along with other neurotransmitters in the afferent inputs to SCN.
Evidence both in favor and against acetylcholine (Ach) as a neurotransmitter for the regulation of circadian rhythms in SCN is available. A role for Ach in the light input pathway has been suggested. Electrophysiological studies showing the excitation of some neurons of the SCN by cholinergic agents have also been reported. Cholinergic agonist, carbachol when injected into the lateral ventricles produced delays in the onset of activity like those seen in response to light. Light pulses also increased Ach levels at the SCN, in experimental animals, hemicholinium used to deplete Ach failed to block the ability to respond to light [246]. Ach application directly to the SCN did not produce a light-like pattern of circadian resetting. These experimental data instead of establishing the role of Ach in clock resetting raise doubt about the role of Ach as such. SCN is rich in cholinergic neurons and has nicotinic cholinergic receptors. Ach levels peak during the early subjective day and decline during the mid-subjective night. The action of Ach through nicotinic and muscarinic receptors on cholinoceptive neurons may suggest cholinergic signaling to modulate photic input to the SCN. It is also reported [247] that in rats, cholinergic projection to the SCN, if damaged can reduce and increase light-induced phase advances and delays respectively which suggests cholinergic modulation of photic resetting. Another study [248] in hamsters shows that cholinergic cells in the basal forebrain are required for arousal-induced phase shifting. However, this has not been confirmed yet. Carbachol when applied directly to the SCN produced a non-photic-like phase advance, whereas through cerebroventricular system produced a biphasic response comparable to the effect of light. SCN is densely innervated by cholinergic neurons [249]. Carbachol-induced phase shifts are likely to be mediated through muscarinic receptors. However, it appears [250] that structures other than SCN are responsible for locomotor rhythm regulation involving the cholinergic system and this is supported by the fact that carbachol induces hyperpolarization via K+ currents which was blocked by M4 antagonists and to a lesser degree by M1 antagonists.
Activation of the neurons of the SCN by cholinergic agents in general, clearly shows the relation between circadian rhythms and cholinergic mechanism. Results obtained using carbachol, Ach receptor agonist, like the ones of the influence of light and its indirect relation between light and SCN can be interpreted as a modulation function of Ach. A consideration of all this information points towards a close relation between Ach and circadian rhythm and sleep [251].
Cholecystokinin (CCK) is both a hormone and neurotransmitter. Though originally discovered in the gastrointestinal tract, is seen in the mammalian brain also, that too in high concentration. It meets the criteria of a neurotransmitter and is active via receptors CCK1 and CCK2. CCK is colocalized with other neurotransmitters like DA GABA and 5HT. CCK neurons are seen in the SCN and receive many afferent inputs from other areas of the brain especially the ventromedial hypothalamus which may explain how feeding behavior can entrain circadian rhythms through CCK neurons of SCN [252].
The exact role of CCK, seen as a distinct population of neurons in the shell part and a few neurons in the core part of SCN in circadian timekeeping is not known completely. Though CCK neurons do not express either AVP or VIP, have synaptic contact. CCK neurons in the SCN do interact with VIP neurons in the light entrainment pathway. CCK neurons have no direct input from the major pathways to the SCN and are not light-responsive [151, 252, 253]. Also, CCK-deficient mice have normal entrainment and show light-induced phase shift. CCK neurons may likely act in non-photic regulation within the clock rather than photic regulation [253].
It is also reported [254] that the activities of CCK neurons in the SCN of mice preceded the onset of behavioral activities that too under different photoperiods. In addition to shortened free-running periods, CCK-deficient mice failed to compress the activities under a long photoperiod. CCK neurons are not directly light sensitive, but their activation can produce phase advance and counter light-induced phase delay mediated by VIP neurons. Further low slow-responding CCK neurons can control the rate of recovery during jet lag thereby suggesting that these neurons are crucial for the robust nature and plasticity of the clock.
Melatonin also referred to as “darkness hormone” is of importance in the function of SCN and is synthesized in the pineal gland. The highest number of melatonin receptors are seen in the SCN and hence it is likely to be the most important target for melatonin action in humans. Two high affinity G-protein coupled receptors namely MT1 and MT2 are seen in the SCN with MT1 receptor high density in the core area [255]. MT2 is expressed at a lower level. It is reported [256] that SCN directs melatonin synthesis and receives melatonin feedback and this feedback alters gene expression and electrical activity in SCN. Another report states that MT1 receptor activation inhibits SCN neuronal activity as well as PACAP-mediated signal transduction. MT2 receptors on the other hand mediate receptor-induced phase-shifting effect on SCN electrical activity which is abolished by MT2-specific antagonists [257]. This is seen in mice genetically deficient for MT1 receptors also. All these indicate the differential response of the receptors. Melatonin has a key role in the integration of circadian rhythms [258] based on some of the experimental evidence. Melatonin can suppress neuronal SCN activity towards nighttime levels [259] as an immediate action and phase shift and amplify circadian rhythmicity as a long-term effect. Another action is to lower the AVP secretion from SCN neurons in rats. Melatonin has also been recognized as a chrono biotic and used to overcome some changes seen in jet lag and shift work. A temporal melatonin-controlled expression of the clock gene is also reported [260]. Melatonin release from the pineal gland occurs by a direct excitatory glutaminergic input from the SCN.
Melatonin, initially known for regulating the sleep-wake cycle by controlling the circadian rhythm has additional functions as well, as revealed by recent research. In rodents and humans, melatonin administration can entrain activity rhythms by binding and altering through acute and clock resetting mechanisms. The role of melatonin is thus beyond sleep regulation and extends to our overall circadian health.
Melanopsin belongs to the opsin family of light-sensitive retinal proteins and plays crucial roles in various biological processes including circadian function. ipRGCs are specialized photoreceptor cells containing melanopsin and are sensitive to light. The advent of melanopsin has provided a new meaning to circadian entrainment [261]. It is observed [262] that melanopsin-expressing ipRGCs are depolarized by light with subsequent firing of action potentials at a higher sustained frequency having brighter illumination. This also makes them capable of acting independently of the image-forming circuits. These cells do not contribute to image formation and directly communicate with SCN. ipRGCs when absorbing light relay this information to the SCN and lead to photoentrainment [263]. Melatonin being part of the cells virtually regulates circadian functions. mRGCs form only a very small percentage (0.3–0.8) of the ganglion cells of the retina but still act as a third set of photoreceptors of the retina expressing melanopsin [261, 264]. A required number of mRGCs and their maintenance is essential for circadian rhythms. With the aging process and certain neurodegenerative diseases, circadian disorders are observed with reduction as well as change in the structure of melanopsin ganglion cells which indicates a correlation between circadian alterations and pathological states. It is known that conventional photoreceptors, rods, and cones, are not required for circadian photoreception, instead, it is RGCs with expression of melanopsin that are required indicating the inner retinal layer might contribute to circadian photoreception [265].
PK2 is a cystine-rich secreted protein and is identified as an output signaling molecule for the SCN. PK2 oscillates in a circadian phase with peaks in subjective daytime. PK2 is a CCG and has a crucial role in the functioning of SCN. SCN, the epicenter of daily rhythms is entrained by light and PK2 expression responds to light-dark cycles. The molecular rhythm of PK2 in the SCN seems to be a delicate interplay between the endogenous circadian clock and light and this clock governs PK2 while light has a modulatory role. Vasopressin and PK2 are two SCN output molecules derived from two different CCGs [266, 267]. The receptor for PK2 namely PKR2 is expressed in the SCN target sites abundantly [268] which emphasizes that it is an output molecule. PK2 is involved within the SCN to synchronize the output as well as in the transmission of behavioral circadian rhythm.
An earlier study [269] reported that PK2 is not necessary for SCN timekeeping or entrainment, but is required for coordination of circadian behavior and physiology. Many lines of evidence involving experiments have shown and confirmed PK2 signaling in input and output pathways. Further confirmation of PK2 as an output signal for SCN has been reported [270]. Reduced circadian rhythms in mice deficient in PK2 or its receptor and reduced oscillation of PK2 expression in transgenic mice are observed. One report [271] also observed that PK2 from ipRGCs to the SCN neurons has a signaling role. Overexpression of PK2 caused a significant reduction of BMAL1, Per 1, Per 2, and Cry 2 genes in the SCN. Thus, it becomes evident that PK2 signaling has a role in both the input and output pathways of the SCN. There is a possibility that more information about the role of PK2 as a neurotransmitter in the SCN is equally useful when considering and interpreting its therapeutic possibilities.
Orexin or hypocretin neurons originating from lateral and posterior hypothalamic areas send excitatory fibers to many areas of the brain. Orexin-containing fibers and orexin receptors in the SCN have suggested that orexin may have a role in the SCN function [272]. The interaction of orexin-containing neurons of the tuberal hypothalamus and SCN helps to regulate the daily rhythm of sleep and wakefulness [273]. The orexin-A/hypocretin-1 and orexin-B/hypocretin-2 are two neuropeptides synthesized by neurons in the lateral hypothalamus and perifornical area [274]. Orexin A has been reported to have a dose-dependent enhanced neuronal activity on many neurons and inhibitory activity on other neurons. Orexin A can influence the neuronal activity of the SCN in brain slices as well as in dispersed cell cultures [272]. A variety of inputs received by Orexin neurons project to parts of the CNS which makes them perform “multi-tasking” and regulate many vital body functions like sleep/wake states, energy homeostasis, cognition mood, and others. Dysfunction of the orexinergic system also leads to different pathological conditions.
It is found that SCN regulates orexin neurons and is much more active during the circadian night than the circadian day. However, it is not clear whether the orexin neurons reciprocally regulate the SCN. Orexin suppresses mouse SCN Per 1-EGFP (enhanced green fluorescent protein) expressing clock cells and augments the SCN clock-resetting effects of NPY. It also potentiates NPY’s inhibition of SCN Per 1-EGFP cells [275]. This report modifies the present view that orexin signaling is exclusively excitatory.
Orexin produces an increase in inhibitory postsynaptic currents with low doses while high doses cause a decrease in inhibitory postsynaptic currents and orexin 1 receptor antagonist can prevent the same. The ability of orexin A to induce phase shifts on long-term recordings, and the discharge rate of SCN neurons suggest modulation of neuronal activity in rodent SCN.
The involvement of orexin in a multitude of functions from energy homeostasis to cognition and dysfunction of the orexinergic system leading to pathological states has prompted to describe its role as necessary for a healthy life. It is equally noteworthy that the role of orexin is not limited to brain areas as it finds its way in metabolic regulation too.
The pigment-dispersing factor (PDF) is an 18-aminoacid peptide that acts as a neuromodulator and is produced by circadian clock circuit neurons [276]. It is a neuromodulator signaling peptide produced only by circadian clock circuit neurons. Two important functions of PDF appear to be the regulation of locomotor activity timing and the maintenance of clock circuit intracellular cycling. Both functions are mediated through the activation of PDF receptor (PDFR) in neurons [276]. PDF has an important but complex role in the phasing of Drosophila activity rhythm [277]. Another observation is that loss of PDF produces desynchrony among clock neurons under DD conditions but not under LD 12:12 and retains the neurons synchronized. This may indicate PDF is not very essential to maintain synchrony under entrained conditions [277]. PDF-present pea aphids are likely to mediate photoperiodic signaling to insulin-producing cells [278]. Thus, PDF has a pivotal role in the circadian clocks of many insects, fruit flies, and cockroaches. It is considered an output molecule of specific circadian clock neurons. Some of the findings suggest that PDF conveys day-length information to the photoperiodic system. In some insects, PDF may signal LDs, while in others it signals short days (SDs).
NO of the SCN plays a pivotal role in regulating the body’s circadian rhythms. It helps to synchronize the body’s internal clocks with the external environment. Neurons release NO and it acts as a neurotransmitter to communicate with neighboring cells and this helps to maintain proper phase relationship between internal rhythms and the night cycle. NO also affects the expression of clock genes like Per 1, Per 2, and Cry 1 of the SCN neurons which regulate the mechanism responsible for circadian rhythms. Exposure to light during the daytime causes the release of NO and this reinforces the synchronization of the body’s clocks to the external day-night cycle. SCN neurons nitric oxide synthase (nNOS) immunoreactivity are seen in dwarf hamsters and rats which indicates the participation of NO in SCN [279]. Although presently NO has been reported to be an extracellular modulator of photic transduction in the SCN it can also perform both intracellular and transcellular signaling in the SCN as per some experimental evidence [280–282].
Production of NO in the SCN is linked to NMDA induced cyclic guanosine monophosphate (cGMP) production and administration of cGMP produces phase shifts of circadian rhythms in vitro. NOS inhibitors prevent NMDA-induced phase shifts of circadian rhythms in vitro and in vivo. Light transmission to the SCN is disrupted by blocking NO production suggesting a role in the light-input pathway. Glu release from retinal terminals can also be interrupted by blockade of NO action in intact animals. Other effects of NO blockade include interruption of expression of IEGs, intracellular increase in calcium activation of nNOS, augmented production of cGMP, and others, all indicating the requirement of NO in the SCN. NO appears to be an important neurotransmitter in generating the phase shifts of circadian rhythms. Aging-induced alteration of NO levels seen was significant in the liver. Melatonin, a known antiaging agent decreases with aging and on exogenous administration could produce a differential restoration of age-induced alterations NO daily rhythms [283]. The role of extracellular NO communication within SCN for synchronization to LD cycles has been demonstrated [281]. This occurs by single light pulses activating NO intracellular signaling which leads to phase shifts and synchronization. Since the knowledge about the 3 different forms of NOS is limited at present, the exact role of NO is not established yet. However, NO is now known as a crucial mediator for aligning our internal rhythms with the outside world, for healthy functioning of rhythms.
Histamine is a neurotransmitter/neuromodulator and has a role in mammalian timekeeping. Although Histamine was first identified in the brain about 50 years ago, its role as a neurotransmitter in physiological functions was identified only later. Despite substantial evidence suggesting a role for histamine as a neurotransmitter in circadian entrainment, its role has not been identified with authenticity. Histamine can induce phase shifts in circadian rhythms in a manner like that of light pulses. Histamine may be acting as a final neurotransmitter on which photic and non-photic entrainment converge [284]. Intracerebroventricular injection of histamine is also found to alter circadian function in hamster and mouse SCN slices, exogenous application of histamine exerts a phase-shifting effect on circadian neural activity rhythms [285]. Four subtypes of GPCR, H1, H2, H3, and H4 have been identified for the action of histamine [286] and the synthesis and release of histamine are regulated by feedback inhibition. Among histamine receptors H1R, H2R, H3R, and H4R, H1R mediates the excitatory action of histamine in the SCN. Histamine by acting on the H1R receptor can increase Ca2+ concentration [Ca2+] in the mouse SCN neurons [287] and it is likely that the molecular mechanism responsible for histamine-induced-phase delay is based on this. Histamine signaling in histamine-induced resetting of the clock is also seen. However, in this case, the receptors mediating the effect may not be H1R but may be H3R also. Despite accumulating evidence for the role of histamine in circadian function, it is not yet possible to give a specific role in circadian activity currently.
NT and its receptors are present in rats, monkeys, and humans with a larger number of NT neurons in humans [288, 289]. NT is a 13 amino acid neuropeptide having NT1, NT2, and NT3 receptors for mediating its function. First evidence of NT and its receptors NT1 and NT2 having a role in circadian pacemaker regulation has been provided [290]. As per the studies NT can phase shift the firing rate in SCN neurons and play a role in regulating pacemaker function. Studies using receptor agonists and antagonists have also provided valuable information. Application NT produces an increase in the firing rate of SCN neurons [291]. NT-positive cells projecting to SCN can be seen in hamster IGL and It is reported that NPY can regulate SCN neuronal activity when applied with NT producing dampening of the inhibitory action of NPY [291]. These results point towards a possibility of NT modulating circadian function in the89 SCN involving NT1 and NT2 receptors. Despite the presence of NT and its receptors in the SCN suggesting its probable role in SCN activity based on some studies, a specific role is still awaited.
m ENK also referred to as metenkephalin, is an endogenous opioid pentapeptide found in the CNS. ENK neurons of the IGL also contribute to GHT and project to the VL part of the SCN [25, 292] in several species. Enkephalinergic cells in the dorsal SCN can release this neuropeptide to contribute to the circadian action. This may indicate its role in control of the circadian phase. The opioid agonist fentanyl is a homolog of morphine and is a µ receptor agonist that induces phase shifts of circadian rhythms in hamsters when administered during the mid-to-late night period [292]. Phase shifts are blocked by naloxone, which leads to a conclusion that there is opioid receptor involvement in circadian timing and opioid receptors mediate the actions of fentanyl. Morphine when used also produces a similar effect [293, 294]. It is interesting to note that Fentanyl can affect light input, per gene expression, and electrical activity in the SCN of hamsters. Fentanyl and light can block each other’s phase shifts of behavioral activity rhythms and this is due to a strong interaction between opioids and light input. More studies about opioids in the SCN are likely to tell us about the role of ENKs in circadian timekeeping which may lead to benefits in dealing with clinical conditions.
SP, a member of the tachykinin peptide family of neuropeptides can act both as a neurotransmitter and neuromodulator. SP is an undecapeptide having a chain of 11 amino acid residues, found within the SCN, and plays a pivotal role in regulating our circadian rhythm. SP immunoreactive nerve Fibers are seen throughout SCN with the largest amount seen in the ventral part. SP found in the RHT and within a group of SCN cell bodies can participate in the generation and entrainment of circadian rhythmicity. It is reported that Ionophoretically applied SP activates hamster SCN neurons. GABA A receptor antagonist bicuculline blocked NMDA and SP-induced phase shifts of SCN neuronal activity rhythms [295]. SP and GRP extensively colocalize with CalB D28K and are strongly light-responsive. SP by itself did not enhance GRP-induced phase shifts in the early-subjective night and significantly attenuated GRP-induced phase shifts during the late-subjective night. Based on SP-induced Fos expression only in the late subjective night, it was suggested [296] that SP may not be a part of the photic phase shifting during the early-subjective night, but may participate in modulating phase shifts during the late-subjective night. Based on this reliable evidence, it is considered that SP is useful in orchestrating our daily rhythms and thereby essential for internal timekeeping.
The presence of NMS, a 36 amino acid neuropeptide in the SCN was reported in 2004 [297]. NMS, seen in the core part of the SCN is a neurotransmitter of the circadian oscillator system and has a diurnal peak under the LD cycle [298]. NMS-containing neurons contribute about 40% among the SCN neurons and these neurons have an essential role in the generation of daily rhythms in behavior [153]. NMS neurons appear to be sufficient to lengthen the period of the SCN and behavioral circadian rhythms. Interestingly, it is closely related to another neuropeptide called neuromedin U which is involved in the mammalian circadian oscillator system.
NMS neurons appear critical and it is reported [299] that deletion of the gene that decodes for the VGAT from NMS neurons can lead to arrhythmia of locomotor activity and sleep as well as impairment of intrinsic clock gene rhythms. This suggests that the removal of GABA release from the adult SCN does not affect the pacemaker’s ability to sustain overt circadian rhythms, but its removal from NMS neurons within the SCN throughout development removes the SCN’s ability to sustain circadian rhythms. It is also known that NMS can regulate circadian rhythms through autocrine and/or paracrine actions [298].
NMS, thus appears as one among the ever-growing list of neurotransmitters of the SCN having multiple functions. It operates like a conductor orchestrating the timing symphony of SCN to maintain the integrity of the clock for the expression of robust circadian rhythms and participates in the integration and coordination of the thousands of clocks of the SCN.
SSA synthesized in both the core and shell of the SCN has been assigned a significant role in SCN function. Length of the day influences the expression of SSA and studies have shown [300] the involvement of SCN responses to light. SSA produced by a group of SCN neurons is an inhibitory neuropeptide that acts as a neuromodulator to regulate SCN function. SSA acts through 5 receptors [6] to perform its action. Some of the SSA and SP peptides are found colocalized in the SCN. This is mostly due to the synapse of SSA on VIP and AVP neurons and the presence of SSA receptors in the SCN. SSA likely has a regulatory role on the other peptidergic neurons of the SCN. A study [301] reported an increase in SSA and SP immunoreactivity in aged Wistar rats, while another study [302] in Syrian hamsters reported no effect of age of SSA m RNA. Though this can be explained based on the species-specific effect seen in the SCN, an increase in SSA immunoreactivity may be related to the VIP decrease with aging, especially given the inhibitory modulatory role of SSA on VIP rhythmicity in the SCN. SSA influences retinorecipient neurons of the SCN core which can reset the molecular clock and this clearly shows a direct role of SSA in the entrainment of circadian activity to the external LD cycle. Studies also suggest SSA signaling [49, 116, 303] can influence the circadian mechanism responsible for the waveform of daily rhythms more than period or phase. Based on these reports one can suggest that SSA may not be necessary for intrinsic circadian timekeeping or photoentrainment, but can contribute to the robustness of circadian activity with change in lighting conditions.
Two calcium-binding proteins, CalB and CALR, have a role in SCN function. These peptide-containing neurons of the SCN have a complex organization and this is similar among all species [304]. Both proteins are involved in buffering intracellular calcium levels, crucial for the regulation of neuronal excitability and neurotransmitter release. Both the core and shell parts of the SCN have CalB neurons with more density in the caudal part and receive direct retinal input [305]. CalB neurons are essential for circadian locomotor activity rhythm. Lesion and transplantation studies in animals have shown that lesions that destroyed CalB neurons but spared other neurons of the SCN lost rhythmicity of locomotor activity and transplants with CalB neurons promptly restored the same. There is colocalization of CalB with peptides like GRP, VIP, and SP. CalB also has interconnections with AVP, GRP, CCK, NPY, and VIP and some interconnections are bilateral while others are not [31]. In addition, CalB cells also have intra-SCN as well as a projection to extra SCN region [31]. CalB cells are non-rhythmic and receive input from RHT and GHT and the light information to CalB facilitates circadian output [306]. Various pieces of evidence suggest that CalB cells in the SCN may function as gates to relay photic signals.
CALR, another calcium-binding protein seen in the SCN is small to medium-sized and is found in the core ventral part of dorsal and lateral SCN It is co-localized with VIP neurons. Optic tracts show immunoreactivity for CALR and there is a reduction in CalB-D28k expression parallel to RHT formation There is also an increase in CALR expression with development [307]. Although the precise roles of CalB and CALR are not yet known, it is expected that they are important for the fine tuning of circadian rhythms.
ANGII, is an octapeptide found in the shell part of the SCN [6] whose presence has been confirmed by light microscopy and electron microscopy in normotensive rats [308]. SCN-derived ANGII has been reported to act as a neurotransmitter and neuromodulator and their effects are mediated through angiotensin 1 receptor (AT1). SCN has ANGII and AT1 [309]. Its influence on circadian rhythms is likely to be indirect as there is no evidence of a direct effect. Its effect on blood pressure and electrolyte balance may have indirect effects on the sleep-wake cycle and other circadian functions. ANGII stimulates the synthesis, and release of AVP and increases its production. AVP also enhances the coupling of SCN with the LD cycle and helps in the synchronization of circadian rhythms in the SCN. Modulating neuronal activity, and altering the firing rate and phase of SCN neurons are listed as some other functions. Expression of clock genes like Per 1, Per 2, BMAL1, and neuropeptide VIP are also affected by ANGII. However, these effects depend on time of day, type of receptor as well as interaction with other neurotransmitters. ANGII receptors and signaling pathways within the SCN can tell us more about the interplay among the neurotransmitters and the changes they make in the clock function. The molecular mechanism operating in the clock depends on the TTFL of clock genes and is present in the SCN as well as in the clocks present in peripheral organs. There is synchronization between central and peripheral clocks and this helps to anticipate external stimuli and maintain homeostasis. Damage to this synchronization can lead to cardiovascular diseases and other abnormalities. One study reported [310] that infusion of ANGII caused changes in blood pressure and other cardiovascular parameters. In addition, there was a distinct daily rhythm in Per2 expression in the SCN with 28 days infusion causing significant phase advance. This was considered as ANGII modulating the functioning of a central and peripheral circadian system based on Per2 expression and locomotor activity.
ANGII produces dose-dependent stimulation and inhibition of many SCN neurons in recordings [309] and AT1 receptor antagonist was found to block both these actions. AT1 receptor activation by ANGII depolarizes SCN neurons and causes increased GABA release in the mouse SCN.
ANGII is not a clock gene. It shows diurnal variation in its activity and could be influenced by the clock. The precise mechanism of ANGII interaction should provide some insights into the development of a chronotherapeutic approach to combat cardiovascular diseases and information on the regulation of the biological clock.
Gal is yet another 29/30 amino acid neuropeptide, present in the SCN [311] and has a significant role in the SCN function. In human SCN Gal and AVP-containing neurons and Gal receptor subtype R2 (Gal-R2) are seen [311]. Neurons of the shell area of the SCN having GABA, CALB, AVP, and ANGII m ENK receive input from Gal and VIP [6] Gal acts through GPCR and exerts inhibitory effects on neurotransmitter and hormone secretion. The inhibitory actions of Gal remind us of those of GABA and NPY. It also has modulation of neuronal activity function. Gal influences various physiological processes and may form a part of the complex interplay of neuropeptides in the SCN for controlling the clock. In animal models, a suppressive effect is reported on insulin secretion [312] which points to Its influence on metabolic processes related to circadian rhythms. However, this effect is not seen in humans. Like some other neurotransmitters not much is known about its specific role in SCN. With the availability of specific high affinity Gal antagonists, it is expected that new dimensions will be given to its function.
Unlike AVP, it is not possible to assign specific neurotransmitter-related functions for oxytocin (OX) in the SCN. However, the involvement of OX in various neural functions and its regulation has been reported [313]. OX, a nonapeptide is produced in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) and can affect SCN. It has a crucial role in social bonding, childbirth, and post-childbirth. It is seen that OX action in the brain can prevent or attenuate the disturbance of the circadian feeding rhythm [314] due to the irregular light-dark cycle seen in shift work or jet lag. In the context of the SCN, While the exact role of OX is debatable, it influences circadian rhythms and may modulate neural activities related to circadian timing. Regulation of the sleep-wake cycle, and social behaviors are among them. SCN coordinates autonomic, endocrine, and behavioral functions in response to light and the role of OX may be as part of a broader network of hormonal signaling for synchronization of circadian activities in SCN.
OX functions in the body are abundant and many neural connections can produce controversial effects. Interaction with other hormones also makes the situation different. Clinical trials or usage of OX and OX-related compounds will contribute to therapeutic actions for the benefit of human health.
Neurotransmitters in the SCN, afferent inputs, and efferent projections are crucial for the generation and maintenance of robust circadian rhythms which is a fundamental mechanism for regulating most of the physiological functions of our body. The presence of more than 100 neurotransmitters makes their role in the working of the clock important and complex. More information about the link between neurotransmitters and genes has improved our understanding of the role in molecular mechanisms. The co-localization of some of the neurotransmitters, and the influence of some of them on others makes it more intricate to explain the role. Neurotransmitter signaling mechanisms, at least some, described possess potential therapeutic properties for various disease conditions including neuropsychiatric disorders for which there is still no definite answer yet. This review offers a complete understanding of the old and new neurotransmitters identified. It also discusses their linkages and probable approaches to therapeutic interventions for human benefit. Many conditions like sleep disorders, insomnia, jet lag, and shift work have started getting benefits in terms of improvement.
5HT: serotonin
Ach: acetylcholine
ANGII: angiotensin II
AT1: angiotensin 1 receptor
AVP: arginine vasopressin
BB2: Gq-coupled Bombesin-2
BMAL1: brain and muscle ARNT-like protein 1
CalB: calbindin
CALR: calretinin
CCGs: clock-controlled genes
CCK: cholecystokinin
cGMP: cyclic guanosine monophosphate
CNS: central nervous system
Cre: cAMP response element
DA: dopamine
DM: dorsomedial
Drd1a: dopamine 1a receptor
G: guanine nucleotide
GABA: gamma-aminobutyric acid
GAD: glutamate decarboxylase
Gal: galanin
GHT: geniculohypothalamic tract
Glu: glutamate
GlyRs: glycine receptor chloride ion channels
GPCR: guanine nucleotide protein-coupled receptors
GRP: gastrin releasing peptide
IEG: immediate early gene
IGL: intergeniculate leaflet
ipRGCs: intrinsically photosensitive retinal ganglion cells
KCC2: K+/Cl– co-transporter 2
LDs: long days
m ENK: met-enkephalin
mRGCs: melatonin retinal ganglion cells
NKCC1: Na+/K+/Cl– co-transporter 1
NMDA: N-methyl-D-aspartate
NMS: neuromedin S
NO: nitric oxide
NPY: neuropeptide Y
NT: neurotensin
OX: oxytocin
PACAP: pituitary adenylate cyclase-activating peptide
PDF: pigment-dispersing factor
PK2: prokineticin 2
RGCs: retinal ganglion cells
RHT: retinohypothalamic tract
SCN: suprachiasmatic nucleus
SP: substance P
TTFLs: transcriptional/translational feedback loops
V1a: vasopressin 1a
VGAT: vesicular gamma-aminobutyric acid transporter
VIP: vasoactive intestinal peptide
VL: ventrolateral
VPAC2: vasoactive intestinal peptide receptor 2
The author acknowledges Saanvi Sharma for her help during the preparation of the manuscript and profusely thanks her for the same.
VR: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
The author declares that there is no conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
