Affiliation:
Department of Neurosurgery, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, 08097, Barcelona, Spain
Email: mariaciscar@bellvitgehospital.cat
ORCID: https://orcid.org/0009-0005-8039-6272
Affiliation:
Department of Neurosurgery, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, 08097, Barcelona, Spain
Affiliation:
Department of Neurosurgery, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, 08097, Barcelona, Spain
Affiliation:
Department of Neurosurgery, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, 08097, Barcelona, Spain
Affiliation:
Department of Neurosurgery, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, 08097, Barcelona, Spain
Affiliation:
Department of Neurosurgery, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, 08097, Barcelona, Spain
Explor Neurosci. 2024;3:520–526 DOI: https://doi.org/10.37349/en.2024.00063
Received: August 01, 2024 Accepted: September 20, 2024 Published: October 16, 2024
Academic Editor: Katrin Sak, NGO Praeventio, Estonia
The article belongs to the special issue Current Approaches to Malignant Tumors of the Nervous System
Glioblastoma multiforme (GBM) is the most common malignant primary central nervous system (CNS) tumor. It presents an aggressive pattern, with a tendency for intracranial progression despite optimal treatment. On the other hand, metastases and manifestations outside the CNS are exceptional, and very few cases have been described. The authors submitted a case of a 48-year-old male diagnosed with a left parietooccipital GBM isocitrate dehydrogenase (IDH) wild-type, non-methylated O6-methylguanine-DNA methyltransferase (MGMT). Six months after surgical treatment, followed by a chemotherapy (CT) and radiotherapy scheme, he developed a few subcutaneous erythematous nodules within the surgical scar. A punch biopsy confirmed the histopathology of GBM. The CT scan showed concomitant intracranial progression. We ruled out systemic metastases. As the performance status was good [Karnofsky Performance Status 90 (KPS 90)] and the subcutaneous implants were growing rapidly, limiting the quality of life, we decided to perform palliative surgery to remove the implants. The result was good. Unfortunately, the patient worsened during the following week, after ruling out medical complications, we attributed the worsening to cerebral tumor swelling, revealed in the CT scan, as unresponsive to steroids. He passed away a few days later. Based on the analysis of our case, we intend to provide useful information for the approach to this peculiar manifestation of GBM.
Gliomas represent almost 30% of all primary brain tumors and 80% of all malignant ones and are responsible for the majority of deaths from primary brain tumors. Glioblastoma multiforme (GBM) is the most frequent and most malignant glioma, predominantly manifesting in patients over 50 years of age. However, GBM can also occur in children, adolescents, and young adults, with increasing evidence that tumors in these age groups are genetically distinct from those in elderly patients [1]. Extracranial metastases from GBM or other gliomas are extremely rare. The most common sites of extracranial metastases from GBM are lungs and pleura, liver, mediastinal and cervical lymph nodes, and bone, or bone marrow [2]. The occurrence of skin and subcutaneous GBM metastases is usually associated with prior interventions, including craniotomy, biopsy, and ventriculoperitoneal shunting. The localization of metastases along the surgical incision site makes direct tumor seeding the most likely mechanism [3]. Nonetheless, metastases to the cranial bones prior to any surgical intervention has also been documented [4]. And more distant metastases may also result from the invasion of lymphatics and blood vessels by dural or scalp extension through the surgical defect [3].
Here, we submitted a case of GBM skin and subcutaneous tissue recurrence within the surgical scar, along with intracranial progression, after performing surgical treatment, followed by chemotherapy (CT) and radiotherapy (RT) scheme. In this case, direct tumor seeding seems to be the most likely mechanism.
A 48-year-old male presented with a constitutional syndrome. Henceforth, he was diagnosed with a left parietooccipital tumor. No neurological deficits were found, nor seizures nor other symptoms. He was treated surgically, achieving gross total resection of the dominant nodule, leaving only two unresectable millimetric satellite lesions within the corticospinal tract (Figure 1). The patient was positioned with ipsilateral shoulder elevation and Mayfield head fixation, turned 90° to the right. Neuronavigation was used during the intervention. The surgical steps are described next: a skin incision in a U-shaped form over the left parietal eminence, pericranium flap preservation, and partial sectioning of the temporalis muscle. A left parietal craniotomy from four burr holes. Subsequently, significant tumor infiltration of the dura mater was found; the affected dura was excised, and the remainder was opened in a U-shaped form. The dominant nodule was completely removed, and the satellite millimetric lesions were left, given their location within the pyramidal tract. Lastly, hemostasis, dural closure with the pericranium flap, bone repositioning, and soft-tissue suturing. Histological and immunohistochemical analysis results indicated GBM-isocitrate dehydrogenase (IDH) wild-type non-methylated O6-methylguanine-DNA methyltransferase (MGMT). The following CT + RT scheme was completed.
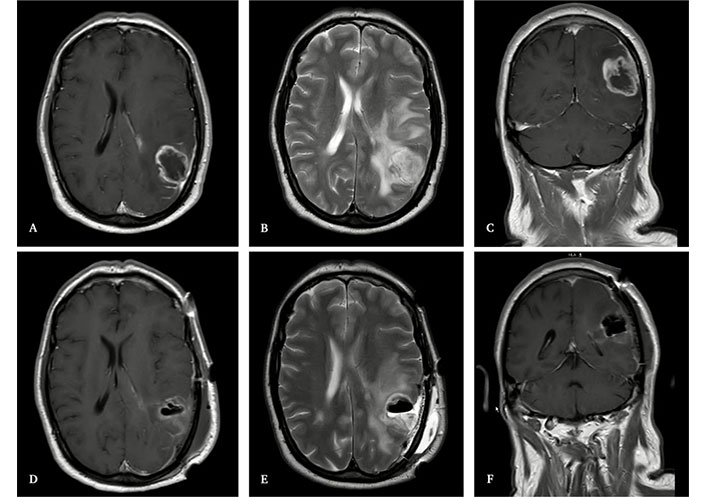
Magnetic resonance imaging (MRI) of the brain. Upper images: MRI before surgery, showing a left parietooccipital glioblastoma multiforme (GBM). A: T1-weighted imaging with contrast, axial. B: T2-weighted imaging, axial. C: T1-weighted imaging with contrast, coronal. Lower images: MRI during the prior 72 hours after surgery, showing complete resection of the dominant nodule and two millimetric satellite lesions located within the corticospinal tract. D: T1-weighted imaging with contrast, axial. E: T2-weighted imaging, axial. F: T1-weighted imaging with contrast, coronal
A month later, growth of the satellite lesions was seen in the control magnetic resonance imaging (MRI). Clinically, a sporadic language deficit was the only finding. A new RT regimen was carried out. Two months afterward (six months after the first surgery), the patient developed various erythematous subcutaneous nodules on the surgical scar. A punch biopsy confirmed tumor recurrence, and MRI showed intracranial progression (Figure 2). At that moment, the performance status of the patient was good [Karnofsky Performance Status 90 (KPS 90)], and for the first four weeks, we adopted a wait-and-see approach. However, the scalp lesions grew and evolved by forming a necrotic center, disrupting the skin, suppurating, and generating a bad smell. Additionally, the CT scan showed a significant increase in the size of the four soft-tissue nodules previously identified, as well as the cerebral edema. Different therapeutic alternatives were debated in the clinical session. We performed a thoraco-abdominal CT scan that ruled out systemic metastases.
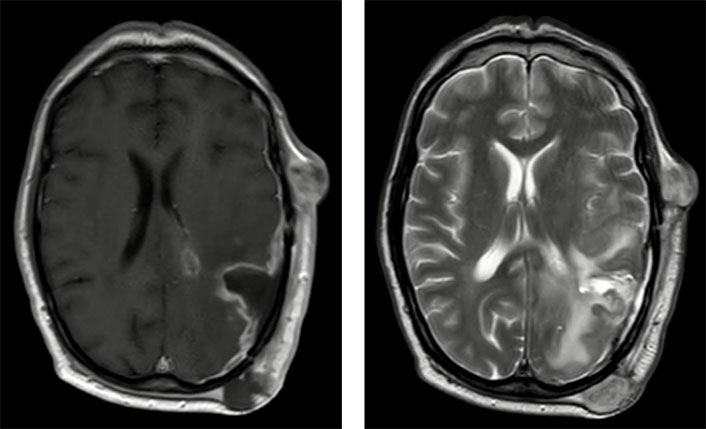
MRI, showing subcutaneous nodules and progression of the CNS disease, two months after the RT course and six months after the first surgery. Left: T1-weighted imaging with contrast, axial; right: T2-weighted imaging, axial. CNS: central nervous system; MRI: magnetic resonance imaging; RT: radiotherapy
So, since several months of survival were expected and the skin lesions were growing rapidly further limiting normal daily life, we agreed to operate on the affected soft tissue as a palliative measure in order to avoid complications such as bleeding or infections. The intervention was performed in cooperation with the Department of Plastic Surgery at our center. Firstly, we made a complete excision of the involved scalp, including all the nodules. We didn’t remove the bone, as it was macroscopically intact. After that, the plastic surgeons created an omental free flap that developed immediate thrombosis between the right gastroepiploic artery and the superficial temporal artery. The arterial anastomosis was cut, and the superficial temporal artery was washed with heparin. The saphenous vein graft was also cut and washed with abundant physiological saline solution. Then, it was replaced by a thoracodorsal artery perforator (TDAP) flap that turned out well. The skin defects were covered with split-thickness skin grafts, resulting in a satisfactory global appearance (Figure 3). Unfortunately, a week later, the patient started with a sudden and significant functional decline. The flap appearance was good, without any signs of wound failure. He remained afebrile, non-septic, and hemodynamically stable. Nevertheless, his arousal level dropped, being only responsive to intense stimuli, and his previously known language deficit worsened. A new control CT revealed greater cerebral swelling, with increased peritumoral vasogenic edema attributable to tumor progression, without showing response to steroids. Since the latter surgery didn’t involve the intracranial compartment we didn’t consider this worsening a postsurgical complication. A complication of CT or RT did not seem likely either, as the patient was not undergoing active treatment at that time. Finally, palliative care was implemented, and the patient passed away a few days later.
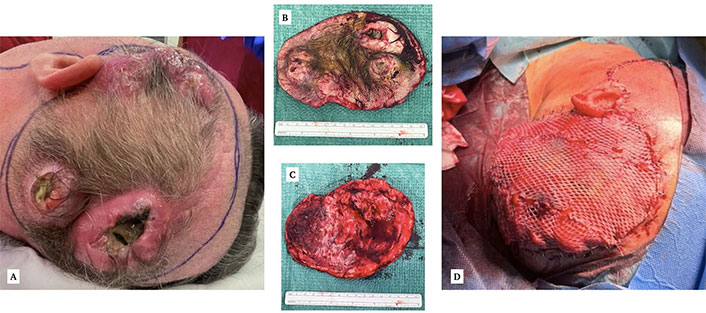
Surgical image. A: Macroscopic appearance of the scalp nodules, two of them with visible necrosis and suppuration. B: Surgical piece, external. C: Surgical piece, internal. D: Final result after covering the defect with a thoracodorsal artery perforator (TDAP) flap and split-thickness skin grafts
Based on our experience, we made a series of observations that we believe could be useful in guiding the management of future cases.
Firstly, even though GBM rarely metastasizes outside the central nervous system (CNS), patients previously operated on a high-grade glial tumor who present with subcutaneous erythematous nodules within the surgical scar or nearby should be biopsied, since histopathological examination is always necessary to obtain the diagnostic confirmation. In addition, as it is possible and seems to be related to intracranial progression, we consider it to perform at least a brain CT scan. On the other hand, given that instances of GBM dissemination to organs such as the lungs, liver, and bones have been described, we also believe it appropriate to perform an extension study to rule out systemic involvement, especially in those patients who present with unexplained systemic symptoms (elevated liver enzymes, thrombocytopenia, or bone pain, among others). In this sense, a thoracoabdominal CT scan may be sufficient to rule out such systemic dissemination, and other more specific tests should be considered based on clinical suspicion.
After studying each patient’s situation individually, the next step is to decide on the most suitable management. Is it an isolated skin or subcutaneous metastases? Is there intracranial progression? Are there systemic metastases? In any event, the best strategy seems to be the employment of multimodal therapies, since the complexity of GBM resistance mechanisms, with vast intratumoral and intertumoral heterogeneity, reduces the treatment efficacy.
The stem cell-like features of GBM promote resistance to CT, by upregulation of efflux transporters (between other metabolic mechanisms); radiation, by promotion of GBM stem cell proliferation in neurogenic zones; and immunotherapy, by immune suppression. Nonetheless, a personalized strategy through molecular techniques could be the answer, and future treatments may have more efficacy [5]. In this sense, molecular tests for clinically relevant biomarkers have become a standard procedure and some of them represent a prognostic marker [6]. The MGMT promoter methylation status has the highest predictive value for the success of temozolamide (TMZ) therapy in patients with GBM. Furthermore, a quantitative method (methylation quantification of endonuclease-resistant DNA, MethyQESD) has shown a correlation to survival, and patients who had a methylated MGMT promoter region of ≥ 10% had improved overall survival compared with patients with a methylated promoter region of < 10% (p = 0.002) [7].
From the neurosurgeon’s perspective, it is well known the correlation between maximal extent of resection and prognosis in newly diagnosed GBM. There is an indisputable association between maximal resection of contrast-enhanced tumor and overall survival, for all molecular subgroups. Additionally, maximal resection of non-contrast-enhanced tumor was associated with longer overall survival in younger patients, regardless of IDH status, and among patients with IDH-wild-type glioblastoma regardless of the methylation status of the promoter region of MGMT [8]. Surgery in GBM recurrence has also shown a significant, prognostic overall survival advantage independent of other prognostic factors in select patients [9].
There are no studies or scientific evidence on the best therapeutic option for GBM subcutaneous or skin metastases given the exceptional nature of this condition. In the case we presented, we believe it was appropriate to perform palliative surgery on subcutaneous lesions, considering that the implants were progressing and deteriorating the patient’s quality of life. Since fewer cases are reported and there aren’t studies comparing the efficacy of the different strategies that can be implemented or their impact on overall survival, the therapeutic decision should be based on the individual patient characteristics and bringing together the opinions of the different specialists involved, where we should always keep in mind the importance of palliative care, given the lethality and morbidity associated with this tumor. In fact, some studies highlight the relevance of working together with the palliative care team from the onset of the disease, due to the progressively disabling neurological course of GBM, as long as patients can communicate their preferences, goals, and values [10].
In conclusion, skin and subcutaneous metastasis of GBM are rare findings in the disease course, with very few cases reported and no evidence regarding the best therapeutic options. Nonetheless, from the present case, we learned the importance of ruling out systemic metastasis and intracranial recurrence to adapt treatment appropriately, as well as to prioritize patient well-being, even considering surgery if lesions are limiting in terms of quality of life. The impact on prognosis is unknown, but we hope to stimulate the development of more studies and that future research will contribute to improving our work and, most importantly, the lives of patients.
CNS: central nervous system
CT: chemotherapy
GBM: glioblastoma multiforme
IDH: isocitrate dehydrogenase
MGMT: O6-methylguanine-DNA methyltransferase
RT: radiotherapy
MCF: Writing—original draft, Writing—review & editing, Formal analysis, Investigation, Methodology. ADVB: Conceptualization, Data curation, Project administration, Supervision, Validation, Writing—original draft, Writing—review & editing. GBP: Investigation, Methodology, Visualization. MRQ: Project administration, Visualization. GPA and AGC: Supervision, Validation, Visualization.
The author declares that there are no conflicts of interest.
The material in the manuscript has been acquired according to modern ethical standards. An ethical committee approval is not needed for this study, according to the requirements of Bellvitge Universitary Hospital committee.
Informed consent to participate in the study was obtained.
Informed consent to publication was obtained.
All the data of this study was included in the manuscript.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Zohreh Khosravi Dehaghi
Maria Ciscar-Fabuel ... Andreu Gabarros-Canals
