Abstract
Intracranial artery dolichoectasia (IADE) is a vascular anomaly characterized by dilation and/or tortuosity of one or more intracranial arteries. While many cases are incidental findings on imaging, the associated ischemic neurological complications of IADE can be severe and must be promptly recognized. This study aims to increase awareness among general practitioners and neurologists regarding this rare and potentially life-threatening condition. We utilized reputable databases for this scoping review, including PubMed, Scopus, and Google Scholar, from database inception through September 2024, using a combination of terms such as “Dolichoectasia of Basilar Artery”, “Intracranial Dolichoectasia”, and “Ischemic Stroke”. Our scoping review revealed that IADE is a challenging and often underdiagnosed condition, with an estimated prevalence of less than 1% in the general population. Hypertension, atherosclerosis, and advanced age are well-documented risk factors. To minimize the risk of misdiagnosis, we briefly elucidated the pathophysiology of IADE, correlating it with clinical and radiological features. We discuss the diagnostic criteria for IADE based on radiological imaging, addressing the advantages and limitations of different techniques. Finally, we highlight the unmet clinical needs related to IADE management, which may involve pharmacological and surgical therapies tailored to individual cases, with careful consideration of safety and efficacy.
Keywords
Dolichoectasia, intracranial aneurysm, basilar artery, cerebrovascular disorders, vascular malformations, strokeIntroduction
Intracranial artery dolichoectasia (IADE) is a medical condition defined by the enlargement of one or more intracranial arteries, which presents in the form of a long and tortuous shape and/or increased diameter. The majority of patients affected by IADE are asymptomatic, and the diagnosis is often made incidentally. However, some patients may experience ischemic neurological complications, as well as compression of adjacent structures such as the brainstem and cranial nerves, cerebral hemorrhages, and obstruction of cerebrospinal fluid flow, leading to the development of hydrocephalus [1–6].
IADE may clinically manifest as a stroke, although its true prevalence is still uncertain in the scientific literature. According to the 2013 National Health Survey, an estimated 2,231,000 individuals suffered a stroke that year, and approximately 25% of these individuals presented some degree of severe disability as a sequela. In addition, a 2021 publication in The Lancet reported a national stroke incidence rate of 141 to 158 individuals per 100,000 inhabitants, with the majority of these cases being ischemic. It is possible that IADE underlies a significant number of these ischemic stroke cases, although this has not yet been fully elucidated [7, 8].
It is estimated that IADE affects less than 1% of the general population. IADE is frequently associated with stroke and approximately 12% of stroke patients present with this type of intracranial dilatation. The majority of IADE cases are found in the posterior cerebral circulation, especially in the basilar artery. These events seem to occur more often in men in their sixth to seventh decades of life [1]. IADE is associated with medical conditions such as hypertension, atherosclerosis, and renal disease, increasing the risk of its occurrence. Age is also a risk factor, with the majority of cases occurring in individuals over 50 years of age [9, 10].
The first population-based estimate of dolichoectasia frequency in stroke patients, published in Neurology by Ince et al. [3] (1998), demonstrated the presence of dolichoectasia in 3.1% of all patients with a first episode of ischemic stroke. The study utilized computed tomography (CT) or magnetic resonance imaging (MRI) of the brain to detect IADE, highlighting that the use of more sensitive diagnostic modalities, such as MRI or cerebral angiography, may increase the detection frequency of IADE [3].
As a rare disease, IADE is frequently underdiagnosed by general neurologists, resulting in several unmet needs for the coming decades. This article will briefly delve into IADE pathophysiology, risk factors, classification diagnosis criteria, and therapeutic approaches to increase awareness of this vascular complication.
Objectives
The aim of this scoping review is to raise awareness about IADE as an underdiagnosed condition potentially associated with cerebrovascular events, particularly ischemic strokes, among general practitioners and neurologists. This scoping review discusses the prevalence of IADE in the population, identifies its risk factors, evaluates diagnostic criteria on the basis of radiological imaging by highlighting the advantages and limitations of various techniques, and explores pharmacological and surgical therapeutic strategies.
Methods
To achieve the proposed objectives, we conducted a scoping review, utilizing reputable databases such as PubMed, Scopus, and Google Scholar from database inception through September 2024 to gather data from 80 articles. We employed a combination of search terms, including “Dolichoectasia of Basilar Artery”, “Intracranial Dolichoectasia”, and “Ischemic Stroke”. Epidemiological, imaging, and clinical studies addressing the prevalence of IADE, its association with ischemic stroke, and its risk factors were included. Article selection was rigorous and limited to studies published in English or Portuguese.
Results
Our scoping review revealed that IADE is a rare but potentially underdiagnosed condition, with an estimated prevalence of less than 1% in the general population. Although the true prevalence remains uncertain, studies suggest that IADE may underlie a significant proportion of ischemic stroke cases, especially in the posterior cerebral circulation, such as the basilar artery. The associations of IADE with risk factors such as hypertension, atherosclerosis, and advanced age are well documented in the literature. Additionally, the sensitivity of imaging methods, such as MRI and cerebral angiography, significantly influences the detection of IADE. However, further epidemiological studies are needed to better understand the prevalence of IADE in the population and its contribution to the burden of ischemic stroke.
Pathophysiology
The pathogenesis of IADE is multifactorial and involves both anatomical factors, such as variations in the circle of Willis, and hemodynamic factors. The basilar artery is frequently affected by IADE because of its peculiar anatomical features. The open angulation and apical bifurcation of the basilar artery predispose patients to the development of turbulent blood flow, leading to ectasia. Furthermore, the basilar artery has a reflection wave that contributes to increased vascular resistance and consequent shear stress on the arterial walls [2].
Endothelial cells are essential for maintaining vascular integrity and function and perform various biochemical and mechanical functions that contribute to the regulation of vascular homeostasis. One of these functions is the production of a glycocalyx layer that coats the luminal surface of arteries and acts as a protective barrier against damage caused by blood flow and interaction with blood components. However, in pathological conditions such as hypertension and atherosclerotic disease, endothelial function may be compromised [11–14].
Endothelial dysfunction and pathophysiological changes in the endothelium may be considered possible triggering events in the formation of intracranial aneurysms [15, 16]. Under shear stress on the vascular wall, endothelial cells may undergo stretching, change their alignment with respect to the direction of blood flow, and alter cell density. In addition to structural changes, functional modifications may occur, including increased activity of the prostaglandin E2 pathway, a mediator of the inflammatory response, and amplification of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [17].
Furthermore, vascular smooth muscle cells (VSMCs), which are responsible for maintaining the structural integrity and contractility of the vascular wall, are capable of undergoing phenotypic modulation from differentiated cells to cells with a pro-inflammatory and pro-remodeling matrix phenotype, with increased expression of inflammatory factors and matrix metalloproteinases (MMPs). VSMCs, which are arranged in parallel, migrate to the vascular intima layer where they proliferate, causing myointimal hyperplasia [14, 18–20].
Similar to the endothelium, changes in the expression of transcription factors such as protein C-Ets-1 (Ets-1), p47phox, and MMPs have been reported to promote inflammation, reactive oxygen species (ROS) generation, and vascular matrix remodeling [21, 22]. These inflammatory insults and loss of the protective structure of the vascular wall allow for differential biosynthesis and processing of collagen. Unlike healthy vessels, where type I collagen expression is restricted to the adventitia and where fibronectin is found in the media, in aneurysms, type I collagen and fibronectin are dispersed throughout the vascular wall, whereas type III and IV collagen expression is reduced compared with that in normal vessels. These changes in the vascular media layer increase apoptosis, loss, and altered proliferation of VSMCs, as well as thinning of the aneurysmal wall [23, 24].
Patients with coronary artery disease have increased levels of MMPs, especially MMPs 2, 3, and 9, which degrade extracellular proteins in the medial layer of blood vessels, such as collagen, proteoglycans, and elastin, resulting in rarefaction of the elastic tissue and fragmentation of the internal elastic lamina [1, 2, 25–29]. As described by Jin et al. [30] (2007), MMPs 2 and 9 were found at higher levels in ruptured aneurysms than in nonruptured aneurysms in a series of 30 patients, suggesting that excessive breakdown of the extracellular matrix eventually led to rupture.
The inflammatory response to vascular injury involves the participation of leukocytes; cytokines such as interleukin-1β, interleukin-6, tumor necrosis factor-α (TNF-α), adhesion molecules, immunoglobulins (IgM and IgG), and complement; and the production of ROS, which damage endothelial cells [11, 12].
Macrophages play a pivotal role in vascular remodeling resulting from blood flow. Several components, such as monocyte chemoattractant protein-1 (MCP-1), Ets-1, and NF-κB, appear to be crucial in attracting macrophages to the aneurysm wall, initiating the inflammatory response, and contributing to aneurysm formation and progression. T lymphocytes actively participate with macrophages in this inflammatory milieu [31]. According to Frösen et al. [32] (2004), infiltration of the vascular wall by macrophages and T lymphocytes is associated with aneurysm rupture.
A study conducted by Ishibashi et al. [33] (2010) demonstrated an increase in the number of mast cells during the formation of intracranial aneurysms, as well as an increase in MMP expression in VSMCs after mast cell degranulation. Moreover, the administration of a mast cell degranulation inhibitor interrupted aneurysm progression.
Inflammatory cytokines, such as interleukin-1β and TNF-α, play crucial roles in the apoptosis of VSMCs and can activate MMPs. This cascade of events contributes to vascular remodeling and may result in aneurysm formation [34–41].
In 2006 and 2010, Tulamo et al. [42, 43] reported an increase in complement expression in patients with ruptured aneurysms. Complement activation occurs through the classical pathway and was confirmed in 2010 by the presence of classical pathway activators, such as IgG, IgM, C-reactive protein, and oxidized low-density lipoprotein, as described by the same author.
Endothelial dysfunction leads to a decrease in the production of nitric oxide, a crucial factor in regulating arterial vasodilation and maintaining vascular integrity. This reduction causes decreased arterial vasodilation and increased vascular resistance, which may contribute to the progressive dilation and elongation of intracranial arteries [11].
In the medial layer of the arterial wall, there is an accumulation of extracellular tissue, resulting in thickening of the layer and arteriosclerosis in smaller arteries, known as medial calcinosis. These findings are supported by Jia et al. [44] (2018). According to the authors, the vessel wall of segmental dolichoectasia is prone to atherosclerosis and calcification due to premature aging of the vascular tissue. In other words, the abnormal histological segment of dolichoectasia is vulnerable and prone to decompensation because of insufficient adaptation, vascular remodeling, and fragility [4, 18].
In patients with IADE, there is an increase in the expression of MMPs, notably MMPs 2, 3, and 9, which have the ability to degrade extracellular proteins in the medial layer of blood vessels, such as collagen, proteoglycans, and elastin. This can lead to rarefaction of elastic tissue and fragmentation of the internal elastic lamina [1, 21, 25–29]. Figure 1 presents a succinct overview of the pathophysiology of IADE.
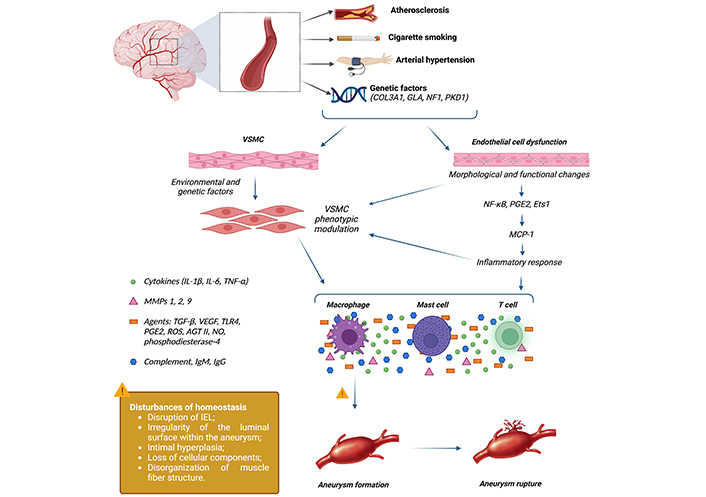
Inflammatory response associated with the formation and rupture of intracranial aneurysms. Aneurysm formation begins with endothelial dysfunction, triggering an inflammatory response involving various cells and inflammatory mediators. This inflammatory response results in rupture of the vessel intima layer, extracellular matrix remodeling, and eventually aneurysm formation. Additionally, apoptosis and degeneration of the vessel wall can lead to definitive aneurysm rupture. AGT II: angiotensin 2; COL3A1: collagen type III α 1 chain; Ets1: protein C-Ets-1; GLA: α-galactosidase; IEL: internal elastic lamina; Ig: immunoglobulin; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; MMPs: matrix metalloproteinases; NF1: neurofibromin 1; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NO: nitric oxide; PGE2: prostaglandin E2; PKD1: polycystin 1, transient receptor potential channel interacting; ROS: reactive oxygen species; TGF-β: transforming growth factor-β; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor-α; VEGF: vascular endothelial growth factor; VSMC: vascular smooth muscle cell. Created in BioRender. Nascimento, R. (2025) Biorender.com/m29t855
In summary, the pathophysiology of IADE involves structural and functional changes in the arterial wall. Endothelial dysfunction and inflammation play crucial roles in this process, mediated by inflammatory cytokines and reduced nitric oxide production. Understanding these mechanisms is essential for the development of new therapeutic approaches.
Risk factors for IADE
The etiological causes of IADE are still under investigation, and further research is needed to comprehensively understand this relationship. However, any conditions that alter the vascular structure may be triggering factors. The main etiology is atherosclerosis; however, several risk factors, such as advanced age, smoking, hypertension, Fabry disease, and Ehlers-Danlos syndrome, are also considered possible causes. In some cases, IADE can occur as an isolated condition, without an apparent cause [2, 5, 9, 45].
Some congenital etiologies of IADE include Marfan syndrome and neurofibromatosis type 1. In Marfan syndrome, a mutation occurs in the gene that encodes fibrillin, resulting in arterial wall fragility and thus predisposing patients to aneurysmal formation and dilation of arteries. In neurofibromatosis type 1, the presence of benign tumors in the nerves can compress arteries and lead to the development of dolichoectasia [45–47].
The association between IADE and abdominal aortic aneurysm (AAA) is highly relevant. Studies, such as the one conducted by Flemming et al. [47] (2005), which analyzed 159 cases of intracranial fusiform aneurysm (IfA) or dolichoectasia, demonstrated a significant 18% rate of association with AAA. Therefore, investigating the presence of AAA in IADE patients is crucial since these individuals have a 7.6-fold greater risk of developing this condition [46].
However, there is evidence indicating possible protective factors against IADE. Studies suggest that a healthy lifestyle, including a balanced diet, control of arterial hypertension, and regular physical activity, may reduce the risks of cardiovascular and cerebrovascular diseases, which are known risk factors for IADE. These data emphasize the importance of adopting preventive measures to reduce the risk of developing this condition, especially in individuals with a family history or other predisposing risk factors [48, 49]. A recent study published in the European Journal of Epidemiology in 2019 indicated that adherence to the Mediterranean diet, which is considered a healthy diet, may reduce the risk of stroke in patients with high cardiovascular risk [48]. Another 2022 work published in the Frontiers in Aging Neuroscience suggested that regular physical exercise may confer significant beneficial effects for the prevention and treatment of cerebrovascular diseases [49].
Importantly, there is no uniformity in the relevance of risk and protective factors in all cases of IADE. Each individual exhibits particularities regarding these factors, which may influence their risk of developing the condition.
Clinical and radiological features of IADE
The clinical symptoms of IADE are diverse and nonspecific, which can make accurate diagnosis challenging. These symptoms include persistent and/or atypical headaches, balance disorders, dysphagia, dysarthria, visual disturbances, motor deficits, and cognitive disorders. However, these symptoms can also be present in other neurological conditions, which can lead to diagnostic difficulties [6, 50, 51]. Furthermore, IADE is a rare pathology, which often results in the omission of this condition as an initial diagnostic hypothesis. This can lead to delays in patient identification and treatment. Therefore, it is essential for physicians to remain aware of the possibility of IADE and to include it in the differential diagnosis for patients with nonspecific or atypical neurological symptoms.
Owing to its prevalence and clinical importance, many studies have been conducted with the aim of establishing consensual diagnostic criteria for IADE. For its diagnosis, CT angiography and MRI are the most widely used exams (Figure 2) [1, 52–54].
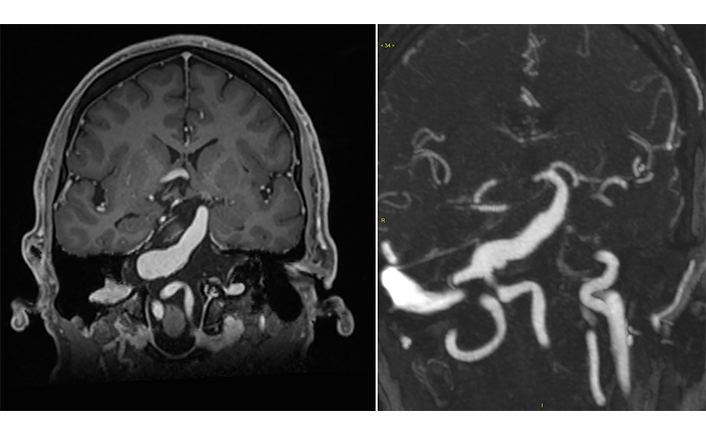
Radiographic features of dolichoectasia. Contrast-enhanced T1-weighted coronal magnetic resonance imaging demonstrating a fusiform aneurysm, from the proximal third to the middle third of the basilar artery, with a diameter of 1.8 cm, that compresses the pons and medulla oblongata without signs of ischemia or bleeding
CT angiography is considered the gold standard method for the diagnosis of basilar artery dolichoectasia because of its high resolution and ability to detect changes in artery structure and size. Through this examination, it is possible to evaluate several aspects of the basilar artery, including basilar artery diameter, laterality, and bifurcation height (Table 1) [1, 4, 51].
Diagnostic criteria for IADE based on AngioCT
| Items | Parameters |
|---|---|
| Basilar artery diameter* | 0 1.9–4.5 mm1 > 4.5 mm (ectasia) |
| Laterality▲ | 0 Midline1 Mid-lateral margin of the clivus or dorsum sellae2 Lateral-lateral margin of the clivus or dorsum sellae3 Cerebellopontine angle |
| Bifurcation height▲ | 0 Dorsum sellae or below1 Suprasellar cistern2 At the level of the third ventricle3 Recession and elevation of the floor of the third ventricle |
AngioCT: computed tomography angiography; IADE: intracranial arterial dolichoectasia; mm: millimeters; * value of 1 is deemed abnormal; ▲ value of 2 or more suggests abnormality
Additionally, three-dimensional (3D) imaging models of aneurysmal blood vessels were constructed using digital subtraction angiography, an advanced imaging technique. These 3D imaging models were then utilized to simulate fluid dynamics and calculate hemodynamic parameters, including the wall shear stress (WSS) and the mean oscillatory shear index. This analysis enabled the hemodynamic characterization of aneurysms, identifying areas of high and low flow, WSS distribution, and distinctive features between stable and unstable aneurysms. This technique plays a fundamental role in estimating aneurysm rupture risk and provides valuable information for planning and executing therapeutic procedures in patients with cerebral aneurysms [52, 53]. Furthermore, most large and giant aneurysms are not filled with thrombi but instead contain turbulent blood flow, which can be observed on MRI [54].
The assessment of IADE can be performed using high-resolution vessel wall imaging (VWI) or the black blood sequence protocol of MRI, which employs T1- and T2-weighted sequences to obtain sharper images. This technique allows for the evaluation of arterial wall layers and identification of areas of thickening or narrowing in the basilar artery and is capable of detecting focal aneurysmal abnormalities and regions of high shear stress. Abnormalities in blood flow, such as low flow velocity and high shear stress, can also indicate the presence of unstable aneurysms [4, 53].
Patients with an aneurysm that shows increased wall enhancement detected through VWI have been associated with higher inflammatory activity, as evidenced by more suggestive myeloperoxidase staining via histopathology. This inflammation is an indicator of greater instability and may increase the risk of bleeding [55, 56].
However, it is important to note that the VWI protocol of MRI is a more elaborate and time-consuming technique than CT angiography and may be less accessible in some medical establishments. Therefore, the selection of the imaging method for the diagnosis of IADE should consider availability, radiologist experience, and the specific demands of the patient.
In patients with IADE without known risk factors, it is important to consider the possibility of genetic conditions related to the condition, such as Fabry disease, Ehlers-Danlos syndrome, neurofibromatosis type 1, polycystic kidney disease, or autoimmune disease such as IgG4-related disease (IgG4-RD).
Fabry disease is a lysosomal storage disorder characterized by several neurovascular manifestations, including ischemic strokes and transient ischemic attack (TIA). Additionally, Fabry disease is recognized as a risk factor for IADE, contributing to elongated and dilated brain arteries. The disease is driven by the accumulation of globotriaosylceramide (Gb3) in vascular endothelial cells due to deficient α-galactosidase A (α-Gal A) enzyme activity [57]. This pathological buildup leads to vascular occlusion, ischemia, and subsequent damage to vital organs, including the kidneys, heart, peripheral nervous system, and central nervous system. Biomarkers such as plasma or urinary Gb3 levels are essential for diagnosis and disease monitoring, particularly in males, where α-Gal A activity in plasma or leukocytes is diagnostic. In females, genetic testing is necessary because of variable enzyme levels. More than 300 different mutations in the α-galactosidase (GLA) gene have been identified, including missense, nonsense, and splicing mutations. In some cases, kidney and heart tissue biopsies can be performed and may reveal zebra bodies under electron microscopy, indicating the presence of Gb3 deposits [58–60].
IgG4-RD is an autoimmune condition characterized by chronic inflammation and fibrosis in several organs, including neurovascular structures. Neurovascular manifestations are often associated with ischemia, hemorrhage, and vascular compression of nearby brain structures [61]. Histopathologically, IgG4-RD is defined by dense infiltration of IgG4-positive plasma cells in affected tissues, often exceeding 50% of total IgG-positive plasma cells, as confirmed by immunohistochemical staining. Tissue biopsy of fibroinflammatory lesions typically reveals storiform fibrosis, obliterative phlebitis, and a lymphoplasmacytic infiltrate, sometimes accompanied by eosinophils. Serum IgG4 levels are typically elevated in two-thirds of cases, and the IgG4/IgG ratio in affected tissues serves as an important diagnostic marker. In neurovascular IgG4-RD, such histopathological findings combined with the presence of obliterative phlebitis and storiform fibrosis are frequently observed in lesions such as pachymeningitis and intracranial pseudotumors. The obliterative nature of phlebitis, coupled with the infiltration of IgG4-positive plasma cells, reflects the autoimmune etiology of the disease. Clinical diagnosis often involves imaging studies to identify vascular abnormalities and targeted biopsies to confirm the fibroinflammatory process, guided by immunohistochemistry [62]. According to the classification criteria, a biopsy is not mandatory but should be performed if other differential diagnoses cannot be ruled out. As IgG4-RD may lead to significant neurovascular damage, early recognition and treatment with immunosuppressive therapy are crucial to prevent irreversible damage [63].
These conditions can cause abnormal dilation of intracranial arteries and other neurological and nonneurological symptoms. Therefore, genetic sequencing through Sanger, next-generation sequencing (NGS), or whole exome sequencing (WES) may be useful in determining the underlying cause of IADE and guiding appropriate treatment [59, 64, 65].
Despite the above investigations, many complex cases exist that go beyond initial evaluation. Therefore, it is necessary to interpret the results on the basis of clinical and radiological phenotypes. In the case of doubt or the presence of known alterations, it is essential to refer the patient to a specialist in the field or to referral centers for the treatment of IADE.
Treatment
The approach to IADE is complex and may involve outpatient and/or surgical interventions, depending on the severity of the condition and the symptoms presented by the patient [4]. Treatment with IADE involves controlling blood pressure and prescribing anticoagulant or antiplatelet medications to reduce the risk of ischemic or hemorrhagic stroke. Regular follow-up of patients with IADE is recommended to monitor the progression of the condition and assess the need for additional therapies [4].
The prevalence of clopidogrel resistance may reach 60% in the Asian population and 20% in Caucasian patients. Johnston et al. [66] (2020) conducted a randomized study comparing secondary prevention of stroke/TIA using combined ticagrelor and aspirin versus aspirin alone. Both groups showed a reduction in recurrent ischemic stroke; however, hemorrhagic events were more frequent in patients who received dual antiplatelet therapy.
Furthermore, the possibility of using cilostazol, which has antiplatelet, anti-inflammatory, and vasodilatory activities, was evaluated. This drug is widely used for the secondary prevention of ischemic stroke in Japan. A randomized study by the CSPS (2019) revealed that adding cilostazol to antiplatelet therapy significantly reduced the recurrence rate of stroke/TIA by approximately 30% [67].
In 2010, the CSPS2 study, a randomized study comparing monotherapy with aspirin and cilostazol, with patient follow-up for up to 5 years, was conducted. The study suggested the superiority of cilostazol over aspirin for the secondary prevention of ischemic stroke, with a lower incidence of hemorrhagic events [68].
Various clinical and radiological predictors can help in selecting the appropriate treatment for patients, thus improving survival. The PHASES score (population, hypertension, age, size of aneurysm, earlier subarachnoid hemorrhage from another aneurysm, site of aneurysm) (Table 2) helps evaluate the risk of intracranial saccular aneurysm rupture over a period of five years and can be applied to fusiform aneurysms in the absence of a specific score for this subtype. However, the probability of bleeding in fusiform aneurysms is slightly lower. Most of these patients develop stroke and compression of adjacent structures, which is the leading cause of death in this population [46, 69–71].
PHASES score
| Items | Points |
|---|---|
| (P) Population | |
| North American, European (except Finnish) | 0 |
| Japanese | 3 |
| Finnish | 5 |
| (H) Hypertension | |
| No | 0 |
| Yes | 1 |
| (A) Age | |
| < 70 years | 0 |
| ≥ 70 years | 1 |
| (S) Size of aneurysm | |
| < 7.0 mm | 0 |
| 7.0–9.9 mm | 3 |
| 10.0–19.9 mm | 6 |
| ≥ 20 mm | 10 |
| (E) Earlier SAH from another aneurysm | |
| No | 0 |
| Yes | 1 |
| (S) Site of aneurysm | |
| Internal carotid artery | 0 |
| Middle cerebral artery | 2 |
| Anterior cerebral artery/posterior communicating artery/posterior circulation | 4 |
| Risk of rupture of intracranial aneurysms in 5 years | |
| 0.4% | ≤ 2 |
| 0.7% | 3 |
| 0.9% | 4 |
| 1.3% | 5 |
| 1.7% | 6 |
| 2.4% | 7 |
| 3.2% | 8 |
| 4.3% | 9 |
| 5.3% | 10 |
| 7.2% | 11 |
| 17.8% | ≥ 12 |
SAH: subarachnoid hemorrhage; mm: millimeters; PHASES: population, hypertension, age, size of aneurysm, earlier SAH from another aneurysm, site of aneurysm
An additional option is the assessment of the diameter of the posterior communicating artery. Values above 1 mm may indicate a more favorable outcome after surgical intervention. However, it is important to note that approximately 8% of patients may develop posterior circulation ischemia [72].
A giant vertebrobasilar fusiform aneurysm is a concentric dilation with a length of 2.5 cm or more that affects the internal carotid artery (ICA) and/or the anterior cerebral artery (ACA), middle cerebral artery (MCA), or posterior cerebral artery (PCA) and is considered to have one of the worst prognoses. The reported mortality rate is approximately 30% [73, 74].
Further meta-analyses have shown that combined therapy with cilostazol significantly reduces the incidence of stroke by 42% and that of stroke recurrence by 28%, surpassing cilostazol monotherapy [75].
With respect to the treatment and support of patients with IADE and associated genetic diseases, such as Fabry disease, Ehlers-Danlos syndrome, neurofibromatosis type 1, and polycystic kidney disease, controlling symptoms and complications, as well as genetic counseling, are fundamental. For Fabry patients, enzyme replacement therapy with α-Gal A infusion is indicated, which can help reduce glycosphingolipid accumulation in tissues and organs of the body. Control of neurological symptoms, such as pain, dysautonomia, and renal complications, is also essential in the treatment of Fabry and other genetic diseases associated with IADE [58, 60, 76–78].
Applicable therapeutic strategies consist of surgical or endovascular interventions for blood flow reversal through proximal vessel occlusion. There is currently insufficient evidence to accurately define the benefits of using flow reversal devices in patients with giant vertebrobasilar fusiform aneurysms. A review of seven patients treated with this approach revealed four deaths in the perioperative period, and three of the patients had a severe functional disability with a Medical Research Council (MRC) score of 5 [73, 74]. Nevertheless, indirect approaches for managing unruptured giant intracavernous aneurysms include ICA occlusion, achieved through either direct ligation or gradual closure using a Selverstone clamp; superficial temporal artery-to-middle cerebral artery bypass combined with ICA occlusion; trapping; or high-flow bypass with ICA occlusion or trapping. Additionally, flow diversion has become a well-established method for treating cerebral aneurysms. A meta-analysis encompassing 29 studies involving 1,524 patients and 1,732 aneurysms reported complete or near-complete occlusion rates of 84.4% following treatment with flow diverters [79].
Conclusions
In conclusion, IADE is a rare vascular condition that presents a highly heterogeneous clinical course, with the possibility of developing severe ischemic neurological manifestations. Given the challenges posed by IADE, the next years demand early identification of the disease, medical education about the corresponding pathology, investment in infrastructure, and optimization of financial resources for specialized centers in the approach to the disease. In summary, the management of IADE may involve drug and surgical therapies, which are selected according to the severity of the condition and the symptoms presented by the patient. The choice of surgical intervention is based on an individualized assessment of each case, accounting for safety and efficacy criteria [80].
Abbreviations
| 3D: | three-dimensional |
| AAA: | abdominal aortic aneurysm |
| CT: | computed tomography |
| Ets-1: | protein C-Ets-1 |
| Gb3: | globotriaosylceramide |
| IADE: | intracranial artery dolichoectasia |
| ICA: | internal carotid artery |
| IgG4-RD: | IgG4-related disease |
| MMPs: | matrix metalloproteinases |
| MRI: | magnetic resonance imaging |
| NF-κB: | nuclear factor kappa-light-chain-enhancer of activated B cells |
| ROS: | reactive oxygen species |
| TIA: | transient ischemic attack |
| TNF-α: | tumor necrosis factor-α |
| VSMCs: | vascular smooth muscle cells |
| VWI: | vessel wall imaging |
| WSS: | wall shear stress |
| α-Gal A: | α-galactosidase A |
Declarations
Author contributions
LDD, RRNRdN, and LSV: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
According to the International Committee of Medical Journal Editors (ICMJE) and Committee on Publication Ethics (COPE) guidelines, this review does not require ethical approval.
Consent to participate
The informed consent to participate from each participant has been obtained.
Consent to publication
The informed consent to publication was obtained from relevant participants.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.