Abstract
Neurodegenerative disorders, including Alzheimer’s, Parkinson’s, Huntington’s, and amyotrophic lateral sclerosis, are among the most significant health concerns worldwide, characterized by neuronal dysfunction, oxidative stress, neuroinflammation, and protein misfolding. Epigallocatechin gallate, a green tea polyphenol, has been reported to possess multifaceted neuroprotective properties. It reduces oxidative stress through free radical scavenging, activation of antioxidant enzymes, and stabilization of mitochondrial function. It also inhibits neuroinflammation through modulation of key signaling pathways. It suppresses amyloid-beta aggregation in Alzheimer’s and alpha-synuclein fibrillation in Parkinson’s, thus attenuating toxic protein accumulation. Its activity in the induction of autophagy and promotion of synaptic plasticity supports neuronal survival and function. However, low bioavailability and metabolic instability hinder its translation into the clinic. Strategies including nanoparticle encapsulation, structural modifications, and combination therapies are being explored to overcome these challenges. Future research could establish epigallocatechin gallate as a viable candidate for managing neurodegenerative disorders.
Keywords
Epigallocatechin gallate, neurodegenerative disorders, anti-inflammatory, neuroprotection, oxidative stress, mitochondrial function, therapeutic strategiesIntroduction
Neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, pose a significant global health challenge due to their progressive nature. These disorders are characterized by the degeneration of neurons and subsequent cognitive decline. Since early, many natural products, including polyphenols like epigallocatechin gallate (EGCG), have been explored for their therapeutic potential [1]. EGCG, the primary polyphenol in green tea, exhibits a wide range of neuroprotective properties, including antioxidant, anti-inflammatory, metal-chelating, neurotrophic, and amyloid-beta (Aβ) aggregation inhibitory activities. For example, EGCG scavenges harmful free radicals, reduces oxidative stress, and modulates inflammatory responses, thereby mitigating neuroinflammation associated with neurodegenerative diseases [2, 3]. However, further research is needed to fully understand its mechanisms of action and optimize its therapeutic potential. Ongoing clinical trials and formulation development efforts aim to translate the preclinical promise of EGCG into effective clinical applications [4]. EGCG, a potent polyphenolic compound derived from green tea, represents a fascinating molecular intervention in neurodegenerative disease management through intricate neuroprotective mechanisms [5]. Its molecular structure enables direct interaction with key neurological processes, specifically targeting protein misfolding and aggregation characteristic of disorders like Alzheimer’s and Parkinson’s. The compound’s unique mechanism involves inhibiting beta-amyloid and tau protein aggregation, which are hallmark pathological features of neurodegeneration. By neutralizing reactive oxygen species (ROS) and reducing oxidative stress, EGCG helps mitigate neuronal damage and supports cellular resilience [6]. The polyphenol’s ability to cross the blood-brain barrier facilitates direct neural tissue intervention, promoting neurogenesis, enhancing synaptic plasticity, and potentially modulating neuroinflammatory responses.
These multifaceted neuroprotective strategies position EGCG as a promising natural therapeutic agent with comprehensive potential in addressing complex neurodegenerative pathologies [7]. Green tea polyphenols emerge as a sophisticated molecular arsenal against neurodegenerative disorders, presenting a complex landscape of therapeutic potential through six distinct structural groups. The six primary structural classifications like flavonoids, phenolic acids, stilbenes, lignans, and tannins each contribute unique neurological intervention strategies that collectively target multiple pathogenic mechanisms in neurodegenerative conditions [8]. These compounds effectively cross the blood-brain barrier, directly interacting with neurological pathways associated with Alzheimer’s and Parkinson’s diseases. Phenolic Acids, including gallic and caffeic acids, complement this approach by mitigating oxidative stress and modulating critical cellular signaling mechanisms [9]. Molecular neurobiology reveals these polyphenols potential to interfere with pathological protein misfolding and potentially reverse early-stage neurodegeneration. Neuropharmacology further elaborated on the compounds multitargeted approaches in neural protection, highlighting their ability to modulate critical neurological signaling pathways [10]. Green tea polyphenols have shown potential as therapeutic agents in neurodegenerative disorder research, providing comprehensive cellular protection through antioxidants, anti-inflammatory, and anti-apoptotic activities.
They neutralize oxidative stress, mitigate neuronal damage, and intervene in neurological pathways [11]. In Alzheimer’s disease (AD) models, these compounds have shown significant potential in inhibiting beta-amyloid protein aggregation and preventing neuronal cell death. Experimental investigations in Parkinson’s disease (PD) paradigms reveal their ability to protect dopaminergic neurons by reducing mitochondrial dysfunction and supporting cellular resilience. Neuroinflammation studies using advanced transgenic mouse models have highlighted the polyphenols’ remarkable ability to downregulate pro-inflammatory cytokines and activate neuroprotective signaling mechanisms [11]. Specific investigations demonstrate their potential to enhance neurogenesis, improve synaptic plasticity, and potentially reverse early-stage neurodegeneration. Cutting-edge research by neuroscientists has uncovered the molecular complexity of these compounds, revealing their capacity to cross the blood-brain barrier and directly interact with critical neurological pathways. The multi-targeted approach of green tea polyphenols presents a promising avenue for developing comprehensive neurotherapeutic strategies [12]. EGCG is a complex polyhydroxylated compound found in green tea, a subgroup of catechins. EGCG’s interaction with various cellular components makes it a subject of intense research in nutrition, pharmacology, and medical science. Its potential therapeutic applications range from cancer prevention to metabolic health regulation, and its molecular architecture allows it to interact with various cellular components. This review explores into the neuroprotective mechanisms of EGCG, offering an innovative exploration of its potential in combating neurodegenerative disorders. By moving beyond traditional antioxidant assessments, the manuscript provides a deep molecular analysis of EGCG’s interactions with neuronal damage processes. The research synthesizes cutting-edge experimental findings to unravel EGCG’s unique therapeutic strategies, specifically examining its role in modulating protein misfolding, neuroinflammatory responses, and oxidative stress pathways. Through a critical lens, the review presents a nuanced perspective on EGCG’s therapeutic potential, revealing sophisticated neuroprotective mechanisms that transcend conventional antioxidant interventions.
EGCG as a polyphenol from green tea
EGCG is a potent polyphenolic compound found in green tea with significant health-promoting potential. Its complex chemical structure, including multiple hydroxyl groups, contributes to its antioxidant capabilities. EGCG can neutralize free radicals, chelate metal ions, and modulate cellular signaling pathways with unprecedented efficiency, making it a subject of multidisciplinary research across molecular biology, nutritional science, and therapeutic intervention strategies [13]. Advanced spectroscopic and computational studies reveal EGCG’s molecular interactions, cellular protection mechanisms, and potential therapeutic intervention. Current research focuses on bioavailability and metabolism, with innovative formulation strategies and delivery mechanisms improving EGCG’s bioavailability. Techniques include nanoencapsulation, lipid-based delivery systems, and chemical modifications [14]. EGCG’s multifaceted biological activities, documented in high-impact journals like Nature, Cell, and Molecular Nutrition & Food Research, include mitigating oxidative stress, modulating inflammatory responses, regulating cellular proliferation, and supporting metabolic homeostasis [15]. Research shows that EGCG has significant epigenetic modulation capabilities, influencing DNA methylation patterns, histone modifications, and microRNA expression. This has implications for disease progression and developing therapeutic strategies. EGCG also has immunomodulatory properties, regulating immune cell function and supporting immune system homeostasis. Computational biology and advanced molecular modelling techniques are revolutionizing the understanding of EGCG’s molecular interactions, allowing researchers to explore complex protein-ligand interactions and design targeted interventional strategies. These advancements are expanding EGCG research beyond traditional experimental boundaries [16]. Researchers are integrating genetic profiling, metabolomic analysis, and individual health assessments into interdisciplinary collaborations to explore EGCG’s potential applications in personalized medicine, recognizing it as a complex molecular entity with nuanced interactions across biological systems [17]. Recent systematic reviews emphasize context-specific research and cautious interpretation of EGCG’s potential benefits. Researchers advocate for rigorous, long-term clinical trials to definitively establish its therapeutic efficacy and potential limitations. EGCG, at the intersection of traditional nutritional wisdom and molecular research, offers insights into transformative health management and disease prevention approaches [18].
Chemical and biological properties of EGCG
EGCG is a complex polyphenolic compound with unique chemical structures and exceptional biological properties. Its unique polyphenolic framework with multiple hydroxyl groups allows for complex interactions across biological systems. Its tetracyclic backbone, containing interconnected rings and multiple hydroxyl substituents, significantly enhances its antioxidant capabilities [19]. EGCG, a compound with hydroxyl groups, is known for its ability to scavenge free radicals, chelate metal ions, and interact with proteins. Recent crystallographic studies reveal its intricate chemical behavior. EGCG’s versatility in cellular interactions, including modulating signaling pathways, allows for comprehensive biological interventions. Advanced proteomics research shows it can affect nuclear factor-κB and other regulatory proteins [20]. Oxidative stress mitigation represents one of EGCG’s most significant biological properties. The compound’s molecular structure enables direct neutralization of ROS, preventing oxidative damage and supporting cellular homeostasis. Sophisticated redox biochemistry studies have elucidated EGCG’s ability to activate nuclear factor erythroid 2-related factor 2 (Nrf2), a critical transcription factor governing antioxidant defense mechanisms, thereby enhancing cellular resilience against environmental and metabolic challenges [21]. Research shows that EGCG has significant epigenetic modulation capabilities, influencing DNA methylation patterns, histone modifications, and microRNA expression. This could help understand disease progression and develop therapeutic strategies. Computational molecular modelling provides insights into EGCG’s interaction with chromatin structures and gene expression. EGCG also has immunomodulatory properties, regulating immune cell function and inflammatory cytokine production [22]. EGCG, a compound with significant biological potential, is being explored for its therapeutic applications in managing chronic inflammatory conditions and supporting immune resilience. However, challenges in bioavailability and metabolism remain, and researchers are exploring innovative strategies like nanoencapsulation and lipid-based delivery systems. Advanced technology, including machine learning algorithms and molecular simulation techniques, is also being used to further understand EGCG’s molecular mechanisms [22, 23]. Computational approaches are enhancing scientific understanding of EGCG, a compound with complex properties. Researchers from biochemistry, pharmacology, computational biology, and nutritional science are collaborating to develop nuanced understandings. EGCG’s complex chemical properties and emerging technological capabilities make it a leading scientific exploration, offering insights into innovative health management and disease prevention approaches [24]. Neuroprotection is mediated by a multiplicity of biochemical targets and pathways including inflammation control, apoptosis control, and the regulation of oxidative stress. Such mechanisms are supported by pro-apoptotic factor inhibitors, anti-inflammatory cytokine inducers, and antioxidants. The signaling pathways include PI3K/Akt, Nrf2, and the mitochondrial regulators that contribute to such neuroprotective effects like enhanced performance of the brain, reduced cell death, and increased neuronal survival. Therefore, most of the focus will be directed toward these pathways for improving long-term neural health and developing treatments for neurodegenerative diseases Table 1.
Neuroprotective mechanism: pathways and molecular targets for neural cell preservation
| Mechanism category | Specific action | Molecular targets/effects | Neuroprotective outcome | References |
|---|---|---|---|---|
| Antioxidant defense | Direct ROS/RNS scavenging | O2, OH, ONOO−, H2O2 | Prevents oxidative damage to neurons and glial cells | [25] |
| Antioxidant signaling | Nrf2/ARE pathway activation | HO-1, NQO1, γ-GCS upregulation | Enhanced cellular antioxidant capacity | [26] |
| Mitochondrial protection | Complex I-IV activity enhancement | Electron transport chain efficiency | Improved neuronal energy metabolism | [27] |
| Membrane stabilization | Phospholipid organization | Membrane fluidity and integrity | Enhanced synaptic function and plasticity | [28] |
| Anti-inflammatory | Microglial activation suppression | TNF-α, IL-1β, IL-6 reduction | Decreased neuroinflammation | [29] |
| Protein aggregation | Amyloid-β fibril prevention | Direct binding to Aβ monomers | Reduced amyloid plaque formation | [30] |
| Protein homeostasis | α-Synuclein aggregation inhibition | α-Syn oligomer disruption | Prevention of Lewy body formation | [31] |
| Apoptosis control | Bcl-2/Bax ratio modulation | Caspase cascade regulation | Reduced neuronal death | [32] |
| Autophagy regulation | mTOR pathway modulation | LC3-II, p62 regulation | Enhanced cellular waste clearance | [33] |
| Metal ion homeostasis | Iron/Copper chelation | Metal-induced oxidation prevention | Protected neural tissue integrity | [34] |
| Synaptic function | BDNF expression enhancement | TrkB signaling activation | Improved neural plasticity | [35] |
| Protein quality control | Heat shock response activation | HSP70, HSP90 induction | Enhanced protein folding capacity | [36] |
| Blood-brain barrier | Tight junction protection | Occludin/claudin expression | Maintained BBB integrity | [37] |
| Glial support | Astrocyte function modulation | GFAP regulation | Enhanced neuronal support | [38] |
| Axonal transport | Microtubule stabilization | Tau phosphorylation reduction | Preserved axonal function | [39] |
| DNA protection | PARP-1 modulation | DNA repair enhancement | Maintained genomic stability | [40] |
| Calcium homeostasis | Ca2+ channel regulation | Intracellular calcium balance | Protected synaptic transmission | [41] |
| Mitochondrial biogenesis | PGC-1α activation | Enhanced mitochondrial function | Improved cellular energy status | [42] |
| Neurotransmitter balance | MAO inhibition | Dopamine/serotonin regulation | Enhanced neurotransmission | [43] |
ARE: antioxidant response elements; Aβ: amyloid-beta; BBB: blood-brain barrier; BCL-2: B-cell lymphoma 2; BDNF: brain-derived neurotrophic factor; γ-GCS: gamma-glutamyl cysteine synthetase; GFAP: glial fibrillary acidic protein; HO-1: heme oxygenase 1; HSP: heat shock proteins; IL-1β: interleukin-1β; LC3-II: LC3-phosphatidylethanolamine conjugate; MAO: monoamine oxidase; mTOR: mammalian target of rapamycin; Nrf2: nuclear factor erythroid 2-related factor 2; NQO1: quinone oxidoreductase 1; ONOO−: peroxynitrite; PARP-1: poly (ADP-ribose) polymerase-1; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1 alpha; RNS: reactive nitrogen species; ROS: reactive oxygen species; TNF-α: tumor necrosis factor-alpha; TrkB: tropomyosin receptor kinase B
Mechanisms of action free radical scavenging, anti-inflammatory effects
EGCG is a compound with a complex molecular structure that plays a crucial role in free radical scavenging and anti-inflammatory processes. Its intricate structure allows it to neutralize ROS and modulate complex inflammatory responses with precision. EGCG’s ability to interact with free radicals at multiple sites prevents oxidative cascade reactions and supports cellular homeostasis. Its anti-inflammatory mechanisms involve modulating cellular signaling pathways, including suppressing NF-κB activation, a critical inflammatory transcription factor [5]. EGCG inhibits pro-inflammatory cytokine production, interrupting inflammatory cascade processes and promoting resolution of responses. It activates Nrf2, a regulator of antioxidant defense, triggering comprehensive cellular protection strategies. This unique approach to managing oxidative and inflammatory stress involves suppressing pro-inflammatory pathways and activating protective cellular mechanisms, supporting mitochondrial function and promoting resolution of inflammatory responses [20]. Advanced proteomics research shows that EGCG can modulate protein interactions involved in inflammatory processes, inhibiting enzyme activities like cyclooxygenase and lipoxygenase, and providing multilayered protection against chronic inflammatory conditions. Its epigenetic modulation capabilities also suggest it can influence DNA methylation patterns and histone modifications, potentially providing long-term regulatory mechanisms for inflammatory processes [44]. Immunomodulatory properties represent another critical dimension of EGCG’s action mechanisms. The compound demonstrates a remarkable capacity to regulate immune cell function, modulating macrophage and neutrophil activities. By supporting balanced immune responses and preventing excessive inflammatory reactions, EGCG offers a nuanced approach to managing complex immunological challenges. Computational biology techniques have provided unprecedented insights into EGCG’s molecular interactions [45]. Machine learning algorithms and simulation platforms are enabling researchers to study protein-ligand interactions, predicting potential inflammatory pathway modulations with accuracy. This technology is expanding scientific understanding beyond traditional experimental methods. Interdisciplinary research is integrating molecular biology, immunology, computational modeling, and biochemistry to understand EGCG’s free radical scavenging and anti-inflammatory capabilities. While evidence shows potential, rigorous investigations are needed to definitively establish its mechanisms and potential therapeutic applications Table 2 [46].
Therapeutic mechanisms and associated molecular pathways in disease treatment
| Mechanism | Description | Molecular target/pathway | References |
|---|---|---|---|
| Nrf2 pathway activation | Upregulates antioxidant response elements (ARE) leading to increased expression of detoxifying enzymes | Nrf2/ARE pathway | [5] |
| Direct ROS neutralization | EGCG’s hydroxyl groups directly donate electrons to neutralize reactive oxygen species like superoxide and hydroxyl radicals | O2, OH, H2O2 | [47] |
| COX-2 suppression | Directly inhibits cyclooxygenase-2 enzyme activity and expression | COX-2 enzyme | [48] |
| iNOS downregulation | Reduces inducible nitric oxide synthase expression, decreasing nitric oxide production | Enzyme | [5] |
| Leukotriene reduction | Inhibits 5-lipoxygenase activity, decreasing pro-inflammatory leukotrienes | 5-LOX enzyme | [49] |
| AP-1 modulation | Suppresses activator protein 1 transcription factor activity | AP-1 complex | [50] |
| Adhesion molecule suppression | Decreases expression of VCAM-1, ICAM-1, reducing inflammatory cell recruitment | VCAM-1, ICAM-1 | [51] |
| Metal ion chelation | Forms complexes with transition metals (Fe2+, Cu2+) preventing their participation in Fenton reactions that generate free radicals | Fe2+, Cu2+ ions | [52] |
| SOD enhancement | Increases expression and activity of superoxide dismutase, improving cellular antioxidant defense | SOD1, SOD2 | [53] |
| Glutathione system support | Enhances glutathione synthesis and recycling, maintaining cellular redox balance | GSH/GSSG ratio | [54] |
| NF-κB inhibition | Suppresses nuclear factor kappa B activation, reducing pro-inflammatory gene expression | NF-κB signaling pathway | [55] |
| Pro-inflammatory cytokine reduction | Decreases production of TNF-α, IL-1β, and IL-6 through multiple pathways | TNF-α, IL-1β, IL-6 | [56] |
| Mitochondrial protection | Preserves mitochondrial function and reduces oxidative damage | Electron transport chain | [57] |
| MAPK pathway modulation | Inhibits stress-activated protein kinases involved in inflammatory signaling | p38 MAPK, JNK, ERK | [58] |
| Lipid peroxidation prevention | Protects cellular membranes from oxidative damage through radical scavenging | Membrane lipids | [59] |
| NADPH oxidase inhibition | Reduces cellular superoxide production by inhibiting NOX enzymes | NOX family enzymes | [60] |
| Myeloperoxidase suppression | Decreases production of hypochlorous acid and other oxidizing species | MPO enzyme | [61] |
| Prostaglandin synthesis inhibition | Reduces inflammatory mediator production through multiple mechanisms | PGE2, PGD2 | [62] |
| Heat shock response | Induces heat shock proteins that protect against oxidative stress | HSP70, HSP90 | [63] |
| STAT3 pathway inhibition | Reduces inflammatory signaling through STAT3 suppression | STAT3 pathway | [64] |
5-LOX: 5-lipoxygenase; AP-1: activator protein 1; COX-2: cyclooxygenase-2; EGCG: epigallocatechin gallate; ERK: extracellular signal-regulated kinase; GSH: glutathione; GSSG: glutathione disulfide; HSP: heat shock proteins; ICAM-1: intercellular adhesion molecule-1; IL-1β: interleukin-1β; iNOS: inducible nitric oxide synthase; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; MPO: myeloperoxidase; NADPH: nicotinamide adenine dinucleotide phosphate; NOX: NADPH oxidase; Nrf2: nuclear factor erythroid 2-related factor 2; PGD2: prostaglandin D2; PGE2: prostaglandin E2; ROS: reactive oxygen species; SOD: superoxide dismutase; STAT3: transducer and activator of transcription 3; TNF-α: tumor necrosis factor-alpha; VCAM-1: vascular cell adhesion molecule-1
Mechanisms of EGCG in neuroprotection
EGCG, a polyphenol found in green tea, is a promising compound in neuroprotective research due to its multifaceted protective mechanisms against neurological disorders. Its complex interactions within neural systems help mitigate oxidative stress, neuroinflammation, and neurodegenerative processes. EGCG’s molecular mechanisms involve intricate signaling pathways that modulate neuroinflammatory responses and promote neuronal survival. Research indicates that EGCG can effectively suppress pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), which are typically elevated during neurological disorders. Moreover, it activates critical neuroprotective transcription factors such as Nrf2, which triggers the expression of antioxidant response elements, enhancing the cell’s endogenous defense mechanisms against oxidative stress and neuronal degradation [65]. Recent neurobiological studies suggest that EGCG is crucial in preventing neurodegenerative diseases like Alzheimer’s and Parkinson’s. It inhibits protein aggregation, particularly in the misfolding and accumulation of beta-amyloid and tau proteins, potentially interrupting neuronal damage. EGCG also enhances neuroplasticity and cognitive function by stimulating neurogenesis, promoting synaptic plasticity, and supporting brain-derived neurotrophic factor (BDNF) signaling. These mechanisms contribute to improved neural connectivity, potentially mitigating age- EGCG, a natural compound, has shown promising potential in neurological disease prevention and management. It can cross the blood-brain barrier, allowing direct interactions with neural tissues. Research also indicates EGCG’s ability to modulate neuronal calcium homeostasis and protect against excitotoxicity. However, further clinical investigations are needed to fully understand its neuroprotective mechanisms and establish standardized therapeutic protocols [66].
The communication between microglia and the gut microbiota forms a critical aspect of neuro-immune interactions, influencing brain health and disease. Microglia, the primary immune cells of the central nervous system, are deeply affected by signals originating from the gut microbiota, including microbial metabolites like short-chain fatty acids (SCFAs) and endotoxins such as lipopolysaccharides (LPS). These signals can regulate microglial activation states, shaping their roles in neuroinflammation, neuroprotection, and overall brain function. Conversely, microglial responses influence gut physiology and immune responses through bidirectional pathways, including the vagus nerve and immune mediators. This intricate crosstalk highlights the interconnectedness of the gut-brain axis, where disruptions in microbial balance (dysbiosis) or microglial dysfunction can contribute to neurodegenerative and neuroinflammatory disorders Figure 1.
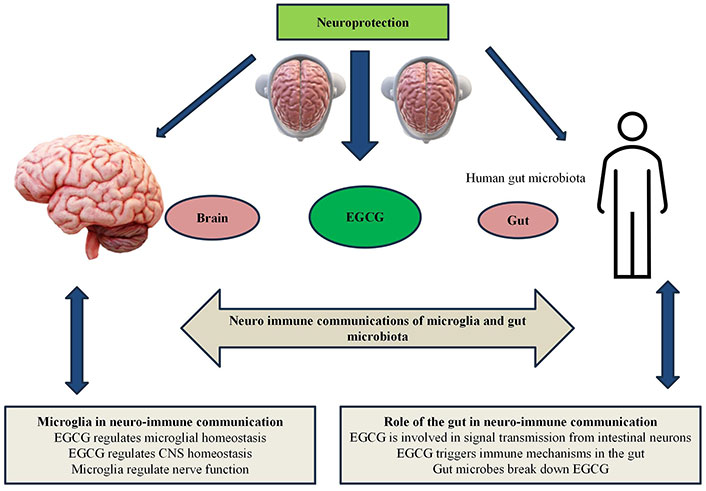
Mechanisms of EGCG in neuroprotective disorder of microglia and gut microbiota in neuro-immune communication. EGCG: epigallocatechin gallate; CNS: central nervous system
Antioxidant and anti-inflammatory pathways
EGCG is a potent bioactive compound that significantly impacts antioxidant and anti-inflammatory biological pathways. It neutralizes ROS and mitigates oxidative stress. EGCG activates the Nrf2 signaling pathway, triggering the expression of antioxidant enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase, enhancing cellular resilience against oxidative damage [67]. EGCG has impressive anti-inflammatory capabilities, suppressing inflammatory responses through intricate molecular interactions. It effectively inhibits pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 through complex mechanisms that interrupt inflammatory cascades. EGCG’s molecular structure allows it to scavenge free radicals and modulate cellular signaling pathways, providing a comprehensive approach to cellular defense. This dual-action mechanism intercepts potential damage at multiple levels and supports overall cellular homeostasis [68]. EGCG’s potential in managing oxidative and inflammatory processes is being explored through its ability to influence epigenetic modifications. This suggests a long-term protective strategy beyond immediate inflammatory suppression. EGCG’s intricate interactions with cellular signaling pathways reveal a sophisticated mechanism of action beyond traditional antioxidants [69]. EGCG, a natural compound, offers a comprehensive approach to cellular protection by targeting multiple molecular targets simultaneously. Its ability to cross cellular membranes and interact with various molecular targets makes it potent in managing oxidative stress and inflammatory responses. However, more clinical studies are needed to fully understand its mechanisms and develop targeted therapeutic interventions. EGCG’s complex role in cellular protection makes it a critical compound for understanding and potentially mitigating oxidative and inflammatory challenges in human health Figure 2 [69].
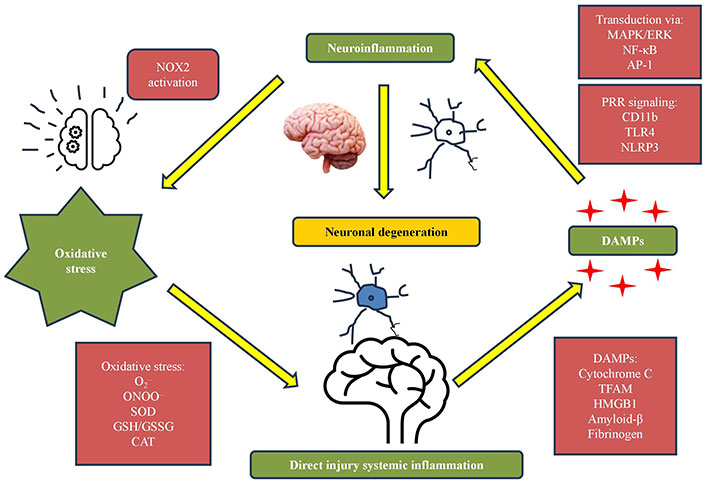
Anti-inflammatory pathways. NOX2: NADPH oxidases 2; MAPK: mitogen-activated protein kinase; ERK: extracellular signal-regulated kinase; AP-1: activator protein 1; PRR: pattern recognition receptor; CD11b: cluster of differentiation molecule 11B; TLR4: toll-like receptor 4; NLRP3: nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; DAMPs: damage-associated molecular patterns; ONOO−: peroxynitrite; SOD: superoxide dismutase; GSH: glutathione; GSSG: glutathione disulfide; CAT: catalase; TFAM: mitochondrial transcription factor A; HMGB1: high mobility group box 1
Modulation of mitochondrial function and oxidative stress
Recent research on EGCG, the primary catechin found in green tea, has revealed significant insights into its effects on mitochondrial function and oxidative stress regulation. Studies have demonstrated that EGCG can directly influence mitochondrial bioenergetics by enhancing the efficiency of the electron transport chain and promoting mitochondrial biogenesis through activation of key signaling pathways, particularly peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α). The compound has been shown to upregulate antioxidant defense mechanisms by activating Nrf2, a transcription factor that controls the expression of various antioxidant enzymes. EGCG’s ability to scavenge ROS while simultaneously boosting cellular antioxidant capacity makes it particularly effective in protecting mitochondria from oxidative damage [70]. Recent investigations have highlighted EGCG’s role in maintaining mitochondrial membrane potential and reducing mitochondrial fragmentation under stress conditions. The polyphenol has also been found to modulate mitochondrial dynamics through regulation of fusion and fission proteins, contributing to improved mitochondrial network stability. Notably, research has uncovered EGCG’s capacity to enhance mitochondrial ATP production while reducing excessive ROS generation, suggesting a balanced approach to energy metabolism regulation. These effects have been particularly relevant in neurodegenerative conditions and metabolic disorders, where mitochondrial dysfunction plays a central role. The compound’s ability to cross the blood-brain barrier and accumulate in mitochondria-rich tissues has made it an interesting candidate for therapeutic applications targeting mitochondrial health Figure 3 [71].
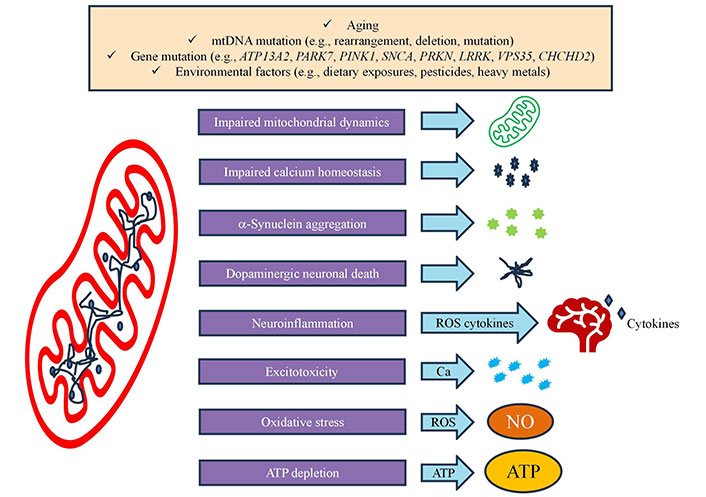
Oxidative stress of mitochondria and modulation in neurological disorders. ATP13A2: ATPase cation transporting 13A2; PARK7: Parkinson’s disease 7; PINK1: PTEN-induced kinase 1; SNCA: alpha-synuclein; PRKN: Parkin; LRRK: leucine-rich repeat kinase 2; VPS35: vascular protein sorting ortholog 35; CHCHD2: coiled-coil-helix-coiled-coil-helix domain containing 2
Role in reducing neurotoxic aggregates (e.g., amyloid-beta, alpha-synuclein)
EGCG is a promising drug for neurodegenerative diseases due to its ability to prevent and mitigate protein aggregation through various mechanisms. Its polyphenolic structure allows it to form non-covalent interactions with unfolded or partially folded proteins, targeting hydrophobic regions prone to aggregation. EGCG also interferes with the early stages of oligomerization in Aβ peptides, binding to monomeric and low molecular weight oligomeric species, and converting mature Aβ fibrils into non-toxic, amorphous aggregates through remodeling. It also prevents toxic oligomers and fibrils by stabilizing the protein’s native conformation [72]. EGCG, a compound with anti-aggregation properties, also plays a role in protein homeostasis by enhancing the unfolded protein response (UPR) and activating heat shock proteins. It also upregulates autophagy, facilitating the clearance of protein aggregates from neurons. Its ability to cross the blood-brain barrier can lead to significant accumulation in brain tissues [73]. EGCG, a compound with neuroprotective effects, has been found to modulate microglial activation, reduce neuroinflammation, suppress pro-inflammatory cytokines, and decrease oxidative stress. It also promotes the non-amyloidogenic processing of amyloid precursor protein, reducing Aβ peptide production. In synucleinopathies, EGCG enhances alpha-synuclein clearance and protects mitochondrial function. These findings are particularly relevant for neurodegenerative disorders, with studies showing improvements in cognitive function in Alzheimer’s and motor function in PD models. EGCG’s multiple mechanisms of action make it a promising candidate for therapeutic development [74].
Regulation of apoptosis and autophagy in neural cells
EGCG regulates cell death and survival in neural cells, modulating apoptosis and autophagy mechanisms. Its influence on apoptotic pathways is context-dependent, promoting survival in healthy neurons and preventing unnecessary cell death in stressed or damaged ones. EGCG also modulates key apoptotic regulators, such as Bcl-2 family proteins, increasing anti-apoptotic expression while regulating pro-apoptotic proteins under normal physiological conditions [71]. EGCG influences survival signaling cascades, including PI3K/Akt and extracellular signal-regulated kinases 1 and 2 (ERK1/2), for neuroprotection against stressors. It regulates autophagy through cellular recycling mechanisms, enhancing autophagic flux through adenosine monophosphate-activated protein kinase (AMPK) activation and mammalian target of rapamycin (mTOR) inhibition. It also modulates silent information regulator sirtuin 1 (SIRT1) activity, a key regulator of autophagy-related genes, thereby influencing autophagy [75]. EGCG, a polyphenol, has been found to increase the expression of key autophagy-related proteins, leading to improved clearance of cellular debris and damaged organelles. This regulation is crucial in neurodegenerative conditions. EGCG promotes selective autophagy of damaged mitochondria through the PTEN-induced kinase 1 (PINK1)/Parkin pathway, maintaining neuronal health. It also enhances protein aggregate clearance through autophagy while protecting neurons from excessive autophagy, which could lead to cell death. This balance is maintained through its effects on oxidative stress and calcium homeostasis [76]. Recent research has revealed that EGCG regulates endoplasmic reticulum (ER) stress responses in neural cells by modulating the UPR pathways. It interacts with stress sensors like PERK and IRE1α, coordinating cellular responses to stressors. EGCG also influences the crosstalk between apoptotic and autophagic pathways through p53 and NF-κB, allowing coordinated responses. This dual regulation of apoptosis and autophagy has therapeutic implications for neurodegenerative conditions and acute neural injury. The compound’s concentration-dependent ability suggests potential applications in neurological conditions [77].
EGCG in specific neurodegenerative disorders
Recent research shows that EGCG has therapeutic potential in various neurodegenerative disorders, with distinct mechanisms tailored to each condition’s pathophysiology. In AD, EGCG interferes with Aβ aggregation, promotes non-toxic oligomers, and reduces tau hyperphosphorylation. It also activates AMPK signaling pathways in neural tissues, enhancing autophagy and improving neuronal energy metabolism. In PD, EGCG protects dopaminergic neurons through multiple pathways [5]. EGCG, a polyphenol, interacts with α-synuclein to prevent aggregation and promote autophagy clearance. It preserves mitochondrial function in dopaminergic neurons by reducing oxidative stress and maintaining membrane potential. It activates the Nrf2 pathway, increasing antioxidant enzyme expression. EGCG also modulates iron homeostasis, particularly in PD. It regulates mutant huntingtin protein aggregation and enhances protein quality control systems. It promotes BDNF expression in Huntington’s disease (HD) through cAMP-response element binding protein (CREB) signaling pathways [78]. EGCG improves mitochondrial function and reduces oxidative stress in striatal neurons, maintaining cellular energy homeostasis. It shows promise in amyotrophic lateral sclerosis (ALS) by reducing SOD1 and transactive response DNA binding protein 43 (TDP-43) aggregation, enhancing protein quality control, and reducing microglial activation and pro-inflammatory cytokine production. It also protects against glutamate excitotoxicity through calcium signaling and N-methyl-D-aspartate (NMDA) receptor activity [79]. EGCG is a compound used in multiple sclerosis (MS) treatment, promoting immunomodulation and neuroprotection. It reduces T-cell activation and proliferation, regulates cytokine balance, and protects oligodendrocytes. It also promotes remyelination through signaling pathways and reduces oxidative stress-induced damage. EGCG enhances mitochondrial function, reduces oxidative stress, modulates protein aggregation, and regulates inflammation [80]. EGCG regulates DNA methyltransferases and histone deacetylases, providing long-term protective effects. It promotes adult neurogenesis and synaptic plasticity, potentially aiding in neurodegenerative conditions. It enhances neurotrophic factor expression, supports neural progenitor cell survival, and modulates microglial phenotypes for an anti-inflammatory state, promoting neural repair and regeneration [81].
Alzheimer’s disease
EGCG has shown potential in treating AD by targeting multiple pathological aspects. It directly interferes with Aβ aggregation through its polyphenolic structure, binding to unfolded Aβ monomers and oligomers, preventing their assembly into toxic fibrils. EGCG can redirect the aggregation pathway towards non-toxic oligomers by stabilizing off-pathway conformations. It also remodels toxic Aβ fibrils into benign aggregates, reducing pathogenic species in neural tissues. Additionally, EGCG modulates secretase activity to favor the non-amyloidogenic pathway in APP processing [82]. EGCG, a compound, enhances α-secretase activity and inhibits β-secretase, reducing Aβ production. This dual action addresses the source of Aβ accumulation, not just managing existing aggregates. It also increases the expression of neprilysin and insulin-degrading enzyme, contributing to reduced Aβ loads in neural tissues. EGCG also reduces tau hyperphosphorylation through multiple mechanisms, inhibiting kinases responsible for tau phosphorylation and activating protein phosphatase 2A. It can also influence tau aggregation directly, preventing toxic tau oligomers and promoting aggregate clearance through enhanced autophagy pathways [83]. EGCG, a compound, plays a crucial role in AD pathology by activating macro-autophagy and chaperone-mediated autophagy through AMPK-dependent pathways. This leads to improved protein clearance and damaged organelles. EGCG also enhances lysosomal function and promotes heat shock proteins, acting as molecular chaperones to prevent protein misfolding. Its modulation of the UPR helps maintain protein homeostasis and protect against ER stress-induced neuronal death [84]. EGCG, a compound, activates the Nrf2 pathway, promoting antioxidant enzymes and reducing oxidative damage. It also chelates metal ions, particularly copper and iron, which contribute to oxidative stress and Aβ aggregation in AD. EGCG targets inflammation and synaptic dysfunction in AD by modulating microglial activation, reducing pro-inflammatory cytokines and promoting the release of anti-inflammatory factors. It enhances synaptic plasticity by promoting BDNF expression and protecting against Aβ-induced synaptic toxicity through NMDA receptor activity and calcium homeostasis. EGCG influences DNA methylation patterns and histone modifications, affecting genes involved in AD pathogenesis. It regulates microRNA expression, particularly those involved in APP processing and tau phosphorylation, providing additional therapeutic action. However, bioavailability and stability remain important considerations for clinical applications. Recent research focuses on developing modified delivery systems and EGCG derivatives with enhanced stability and brain penetrance [85].
Parkinson’s disease
EGCG has shown potential in PD through its complex neuroprotective mechanisms. It interacts with α-synuclein, a protein involved in PD pathogenesis, preventing aggregation and redirecting it towards non-toxic conformations. EGCG’s unique molecular structure allows it to remodel toxic α-synuclein fibrils into benign forms, reducing pathogenic species in dopaminergic neurons. It also enhances the clearance of α-synuclein aggregates through multiple protein degradation pathways [5]. EGCG is a compound that significantly improves mitochondrial function, which is crucial in PD. It enhances mitochondrial biogenesis and improves electron transport chain efficiency, protecting against complex I inhibition and electron leakage. EGCG also promotes mitophagy through the PINK1/Parkin pathway, removing damaged mitochondria before cell death. It also manages oxidative stress, particularly in dopaminergic neurons, by activating the Nrf2-antioxidant response elements (ARE) pathway and increasing the expression of antioxidant enzymes. It also chelates iron, which accumulates abnormally in PD brains and contributes to oxidative damage, providing additional protection. EGCG’s influence on neuroinflammation in PD is particularly significant [86]. EGCG, a polyphenol, has been shown to modulate microglial activation towards an anti-inflammatory phenotype, reducing the production of pro-inflammatory cytokines and promoting the release of anti-inflammatory factors. It inhibits the NF-κB pathway and reduces nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome activation, key mediators of neuroinflammation in PD. EGCG also protects against astrogliosis and maintains blood-brain barrier integrity, reducing peripheral immune cell infiltration. It also plays a role in dopamine homeostasis and signaling, protecting dopaminergic neurons by enhancing dopamine transporter function and reducing dopamine oxidation. It also enhances macroautophagy and chaperone-mediated autophagy through AMPK-dependent pathways, improving the clearance of protein aggregates and damaged organelles [87]. Polyphenol (EGCG) is a compound that regulates heat shock proteins, preventing protein misfolding and aggregation. It also modulates the UPR, maintaining protein homeostasis under stress. EGCG’s neuroprotective effects extend to synaptic function and axonal transport, promoting the expression of neurotrophic factors like BDNF and GDNF. It enhances axonal transport by maintaining microtubule stability and regulating motor protein function, which are often impaired in PD. However, bioavailability and stability remain important considerations [88].
Huntington’s disease
HD is a genetic disorder characterized by the accumulation of toxic self-propagating, amyloidogenic mutant huntingtin (mHTT) aggregates in neurons. These aggregates disrupt essential cellular functions, especially in medium spiny neurons. mHTT interferes with transcriptional regulation, axonal transport, synaptic transmission, and mitochondrial function. EGCG, a compound, has emerged as a promising therapeutic for HD through multiple mechanisms. EGCG can modulate protein aggregation, affecting the accumulation of mHTT, by promoting proper protein folding and enhancing cellular protein quality control systems. It also activates molecular chaperones to prevent protein misfolding and aggregate formation [89]. EGCG’s interaction with cellular pathways is crucial in HD treatment, as it activates AMPK signaling, which plays a vital role in cellular energy homeostasis and autophagy regulation. Additionally, EGCG’s antioxidant properties help combat increased oxidative stress in HD neurons by directly scavenging ROS and upregulating endogenous antioxidant systems through Nrf2 pathway activation. Recent molecular studies have shown that EGCG can influence transcriptional regulation in head direction cells, potentially normalizing gene expression patterns disrupted in HD. This includes restoring BDNF expression, a crucial neurotrophic factor typically reduced in HD patients. EGCG also modulates calcium signaling and glutamate receptor function, potentially preventing excitotoxicity, a major contributor to neuronal death in HD. Its anti-inflammatory properties in HD have been shown to reduce microglial activation and decrease pro-inflammatory cytokine production in the brain. EGCG’s ability to cross the blood-brain barrier makes it an attractive therapeutic candidate. Laboratory studies have demonstrated that EGCG treatment can improve mitochondrial function in HD models, maintaining membrane potential and enhancing ATP production. Additionally, EGCG regulates the UPR, helping cells manage protein folding stress without triggering apoptotic pathways, which is particularly relevant in HD [90].
Other disorders
EGCG has shown significant therapeutic potential in treating neurodegenerative disorders like HD and ALS. It prevents mutant huntingtin protein aggregation and enhances cellular quality control systems. It interacts with polyglutamine-expanded huntingtin protein, promoting clearance through autophagy and SIRT1-mediated pathways. EGCG also enhances BDNF expression, which is typically depleted in HD, through CREB signaling pathways. Overall, EGCG has multiple protective effects [91]. EGCG is a polyphenol that reduces SOD1 and TDP-43 aggregation in ALS pathology, while enhancing protein degradation pathways. It protects motor neurons from glutamate excitotoxicity by modulating calcium signaling and NMDA receptor activity. EGCG’s anti-inflammatory properties help reduce microglial activation and pro-inflammatory cytokine production, contributing to motor neuron death in ALS. In MS, EGCG modulates T-cell responses, protects oligodendrocytes, and promotes remyelination through specific signaling pathways. It also reduces oxidative stress and inflammation in the central nervous system, preserving axonal integrity and reducing disease progression. In Prion diseases, EGCG exhibits anti-aggregation properties against pathogenic prion proteins, promoting the clearance of existing aggregates. Its ability to modulate protein folding and enhance cellular quality control mechanisms provides additional protection against prion-induced neurodegeneration [92]. EGCG has potential for treating age-related cognitive decline and mild cognitive impairment by enhancing synaptic plasticity, promoting adult neurogenesis, improving cerebral blood flow and glucose metabolism, reducing oxidative stress and inflammation, and promoting cellular repair mechanisms. It also has potential in treating rare neurodegenerative disorders, such as spinocerebellar ataxias, by reducing polyglutamine protein aggregation and improving protein quality control systems, and hereditary spastic paraplegias by improving mitochondrial function and axonal transport [93]. EGCG, a compound with common protective mechanisms, has been shown to enhance autophagy, reduce oxidative stress, modulate mitochondrial function, and regulate inflammation. Its ability to cross the blood-brain barrier makes it relevant for therapeutic applications. EGCG also has epigenetic effects, modifying DNA methylation patterns and histone modifications, influencing gene expression in neuroprotection and cellular repair. Its regulation of microRNA expression provides additional therapeutic action in neurological disorders. Recent studies have focused on optimizing EGCG delivery and bioavailability for various neurological conditions, exploring modified delivery systems like nanoparticle formulations and EGCG derivatives [94].
Challenges
In clinical applications
EGCG faces challenges in clinical application due to its complex molecular interactions and systemic limitations. Despite its biological properties, EGCG’s inherent characteristics create barriers to effective systemic absorption and cellular delivery. Its pharmacokinetic profile reveals metabolic transformations, leading to reduced bioavailability and diminished therapeutic potential due to rapid metabolism and conjugation processes in the intestinal epithelium and liver [95]. Metabolomic analyses reveal EGCG undergoes glucuronidation and sulfation, reducing its concentration in circulation. Innovative delivery strategies, including nanotechnology-based formulations, lipid-based nanoparticles, polymeric micelles, and molecular carriers, are being explored to improve EGCG’s stability, extend circulation time, and facilitate targeted cellular delivery, overcoming limitations in the compound’s structure and metabolic behaviour [96]. Computational modelling and molecular simulation techniques have provided insights into EGCG’s absorption mechanisms, revealing intricate molecular behaviour and suggesting chemical modifications and innovative delivery mechanisms. Clinical trials have highlighted challenges in achieving therapeutically relevant EGCG concentrations, with conventional oral administration strategies often resulting in low systemic concentrations.
Researchers are exploring controlled-release formulations, alternative routes of administration, and combination approaches with absorption-enhancing compounds to address these limitations [97]. Metabolic variability in EGCG’s clinical applications is a challenge due to individual genetic differences in metabolic enzymes. Pharmacogenomic research is exploring these variations, suggesting personalized approaches. Advanced analytical techniques, such as high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy, provide detailed insights into complex metabolic pathways [98]. Technological advancements are improving our understanding of EGCG’s systemic behavior and potential therapeutic limitations. Interdisciplinary research collaborations are integrating perspectives from pharmaceutical sciences, molecular biology, nanotechnology, and computational modeling to develop sophisticated strategies for enhancing its clinical utility. Alternative administration strategies, such as sublingual delivery and advanced transdermal formulations, are being explored to circumvent traditional absorption limitations. Advanced chemical engineering techniques are being used to develop derivatives with enhanced stability and cellular penetration. Despite the promising evidence, researchers advocate for rigorous clinical trials and advanced technological interventions [5].
Therapeutic applications
EGCG, a compound with diverse biological activities, has shown potential in treating various diseases, including cancer, cardiovascular diseases, metabolic disorders, and neurodegenerative conditions. It modulates cell proliferation, survival, and metastasis through mechanisms like inhibiting DNA methyltransferases, regulating microRNA expression, and modulating key signaling molecules like NF-κB and MAPK. EGCG’s significant therapeutic application lies in treating metabolic disorders, enhancing insulin sensitivity and regulating glucose metabolism through activation of AMPK signaling pathways [4]. EGCG has shown promise in reducing lipid accumulation and improving metabolic parameters in obesity-related conditions through mechanisms like increased fat oxidation, reduced lipogenesis, and enhanced energy expenditure. However, its poor bioavailability when administered orally is a major challenge. Recent research focuses on developing novel delivery systems like nanoencapsulation techniques and targeted delivery systems to improve EGCG’s bioavailability. Additionally, EGCG’s stability is affected by environmental factors like pH, temperature, and oxidative conditions, posing challenges for formulation and storage [99]. EGCG’s therapeutic window varies based on target condition and patient factors, with high doses potentially leading to adverse effects like hepatotoxicity. Recent studies aim to identify biomarkers to predict individual responses and optimize dosing strategies. Drug interactions are another significant challenge, as EGCG interacts with medications through various mechanisms, including effects on drug-metabolizing enzymes and transport proteins. For example, EGCG can inhibit certain cytochrome P450 enzymes, potentially affecting drug metabolism [20]. Understanding interactions is crucial for safe and effective therapeutic use. Advances in delivery technology have led to the development of modified EGCG derivatives with improved pharmacokinetic properties, aiming to enhance stability, bioavailability, and target specificity while maintaining therapeutic efficacy. Researchers are also investigating combination therapies incorporating EGCG with other therapeutic agents to enhance treatment efficacy. EGCG’s potential in preventive medicine is promising, but determining optimal dosing strategies and duration of use remains challenging. Research is ongoing to establish evidence-based guidelines for preventive applications, considering individual genetic variations and environmental factors [100].
Current preclinical and clinical studies
EGCG treatment has shown promise in neurodegenerative disease studies, particularly in transgenic mouse models of AD and PD. Studies show that EGCG treatment reduces protein aggregation and improves cognitive function, particularly in AD, and PD models, due to its antioxidant properties and ability to modulate alpha-synuclein aggregation [93]. EGCG has shown potential as a preventive and therapeutic agent in cancer treatment. In vitro studies have shown that EGCG can inhibit cancer cell proliferation through various mechanisms, including cell cycle arrest and apoptosis induction. Studies in breast cancer cell lines have shown EGCG’s ability to target cancer stem cells and enhance chemotherapy effectiveness. Animal studies have also shown reduced tumor growth and metastasis in various cancer models. Clinical studies have shown mixed but promising results, with Phase I trials establishing safety profiles and maximum tolerated doses. A multicentre trial in early-stage prostate cancer showed modest benefits in preventing disease progression [101]. Clinical investigations are exploring the potential of EGCG in cognitive decline and neurodegeneration. A double-blind, placebo-controlled trial showed improved memory and attention task performance in mild cognitive impairment participants. This is significant due to the growing interest in preventive strategies for age-related cognitive decline. EGCG is also being investigated for its anti-inflammatory effects in inflammatory conditions like rheumatoid arthritis and inflammatory bowel disease. It is also being explored in combination therapy approaches, particularly in cancer treatment protocols, to enhance treatment effectiveness and reduce side effects [102]. Pharmacokinetic studies have provided valuable insights into EGCG’s behavior in humans, with recent clinical investigations focusing on optimizing delivery methods to improve bioavailability. Novel formulations, like nanoparticle-based systems, have shown promising results in improving EGCG absorption and tissue distribution. Safety monitoring in clinical trials has generally shown EGCG to be well-tolerated, but some studies have reported mild adverse effects at higher doses. Current research is identifying patient populations more susceptible to adverse effects and establishing appropriate dosing guidelines. Long-term safety studies are also ongoing [103].
Advances in formulation: nanoparticles, liposomes, and prodrugs
Recent research has shown that EGCG delivery systems have been significantly improved by nanoparticle formulations using biocompatible materials like poly (lactic-co-glycolic acid) (PLGA), chitosan, and zein. These nanocarriers enhance stability and cellular uptake by controlling size distribution and surface modification capabilities. PLGA-based nanoparticles can increase EGCG’s cellular uptake by up to 10-fold compared to free EGCG, while maintaining biological activity. Surface modification with targeting ligands enhances their selective delivery to target tissues. Liposomal formulations are also promising for EGCG delivery [104]. PEGylated liposomes containing EGCG have been developed to improve circulation time and stability, achieving up to 3−4 times higher plasma concentrations than free EGCG. These liposomal formulations can also selectively release EGCG in acidic tumor environments, enhancing therapeutic efficacy while reducing systemic exposure. Prodrug development has also advanced EGCG’s pharmacokinetic properties by creating EGCG prodrugs through strategic chemical modifications. Acetylated EGCG derivatives have shown improved cellular uptake and metabolic stability and are designed to release active EGCG through enzymatic or chemical hydrolysis at target sites, providing sustained therapeutic effects. Certain EGCG prodrugs can achieve up to 5-fold higher bioavailability compared to unmodified EGCG [105]. Hybrid delivery systems, such as nanoparticle-in-liposome systems and prodrug-loaded nanocarriers, have been developed to provide both protection and controlled release properties. These systems have shown promising results in preclinical studies, improving pharmacokinetic profiles and therapeutic efficacy. Recent innovations in smart delivery systems have also incorporated stimuli-responsive materials into EGCG formulations. New materials, such as pH-sensitive polymers, thermosensitive materials, and enzyme-responsive carriers, have been developed to target specific tissues. Temperature-sensitive liposomes containing EGCG release their cargo at slightly elevated temperatures, while enzyme-responsive nanoparticles release EGCG in response to disease sites. Novel surface modification techniques have enhanced targeting capabilities, and recent studies have explored cell-penetrating peptides, aptamers, and receptor ligands to improve cellular uptake and tissue specificity. These modifications have shown promise in crossing biological barriers, including the blood-brain barrier, expanding EGCG’s therapeutic applications [106].
Potential for synergistic effects with other therapies
EGCG has shown promising synergistic effects when combined with conventional chemotherapeutic agents in cancer treatment, enhancing the efficacy of drugs like doxorubicin and cisplatin by inhibiting drug efflux pumps, particularly P-glycoprotein. This can reduce drug doses by up to 50% while maintaining therapeutic efficacy, potentially minimizing adverse effects. EGCG also shows promising synergistic effects in neurodegenerative disease therapy when combined with established treatments [107]. Studies show that EGCG can enhance neuroprotective effects in AD models by complementing memantine or cholinesterase inhibitors through its antioxidant properties and regulation of amyloid processing pathways. This combination can improve cognitive outcomes more effectively than either treatment alone. EGCG also has potential to combat antibiotic resistance by disrupting bacterial cell membranes and inhibiting efflux pumps, allowing antibiotics to accumulate more effectively within bacterial cells. This synergistic effect is particularly strong against methicillin-resistant Staphylococcus aureus (MRSA) and other resistant pathogens [108]. EGCG has shown potential in cardiovascular therapy when combined with standard treatments like statins or antihypertensive medications. It provides antioxidant and anti-inflammatory benefits, potentially reducing the dose of conventional medications. Combination therapy improves endothelial function and reduces inflammation markers. EGCG’s role in enhancing immunotherapy outcomes has also gained attention, as it can improve treatment responses in various cancer types by modulating the tumor microenvironment and enhancing T-cell responses [109]. EGCG, when used as an adjuvant therapy, has been shown to increase tumor infiltration and improve treatment outcomes. Combining EGCG with anti-inflammatory agents has shown promise in treating chronic inflammatory conditions. The synergy works through complementary anti-inflammatory pathways, with EGCG providing additional benefits through NF-κB pathway inhibition and antioxidant effects. This approach reduces inflammation and may lower the required doses of traditional anti-inflammatory medications [110].
Prevalence and impact of neurodegenerative disorders (e.g., Alzheimer’s, Parkinson’s)
Neurodegenerative disorders, like Alzheimer’s and Parkinson’s, are a global health challenge due to progressive neuronal degeneration and impaired cognitive and motor functions. Green tea’s primary polyphenol, EGCG, has shown potential in mitigating oxidative stress, neuroinflammation, and protein misfolding, which are key pathological processes in these diseases [111]. EGCG has shown promising neuroprotective effects in AD by inhibiting Aβ aggregation and reducing neuroinflammatory responses. It interacts with Aβ peptides, preventing their oligomerization and neurotoxic cascade. EGCG’s ability to cross the blood-brain barrier allows direct neuronal protection and potentially modulates neuroinflammatory pathways [3]. For PD, EGCG exhibits remarkable antioxidant and anti-apoptotic properties, particularly in protecting dopaminergic neurons. Research published in the Journal of Neurochemistry demonstrates EGCG’s capacity to counteract mitochondrial dysfunction, reduce ROS generation, and modulate key signaling pathways involved in neuronal survival. Experimental models have consistently shown that EGCG can mitigate α-synuclein aggregation, a hallmark of Parkinson’s pathogenesis [87, 112]. EGCG’s neuroprotective effects involve multiple pathways, including activation of Nrf2, inhibition of pro-inflammatory cytokines, and modulation of cellular stress response proteins. Recent meta-analyses suggest sustained, moderate EGCG consumption may contribute to long-term neurological health and slow disease progression. Advanced research is exploring advanced delivery mechanisms and synthetic derivatives to enhance EGCG’s bioavailability and neurological targeting, using nanotechnology-based approaches and targeted drug delivery systems [78]. Clinical trials are advancing to understand optimal dosage, long-term effects, and potential combinations with existing pharmacological interventions. While preliminary research is promising, more comprehensive human trials are needed to definitively establish EGCG’s therapeutic efficacy, as it represents a potential complementary strategy in comprehensive neurological management [113]. Recent review papers highlight the potential of natural compounds like EGCG in managing neurodegenerative diseases, emphasizing the need for further research into nutritional and phytochemical interventions to develop novel therapeutic strategies for these challenging neurological conditions [114].
Novel therapeutic approaches
Researchers are exploring innovative strategies for neurodegenerative disorders, with EGCG emerging as a promising compound. Advances in nanomedicine have revolutionized EGCG’s therapeutic potential, particularly in targeted drug delivery systems. Advanced nanoformulations are being developed to enhance bioavailability and neurological targeting, allowing precise delivery of EGCG to specific neural regions affected by neurodegenerative processes [115]. Innovative encapsulation techniques, such as lipid-based nanoparticles and polymeric nanocarriers, optimize EGCG’s therapeutic efficacy. Precision medicine is another frontier where EGCG shows promise. Personalized therapeutic strategies integrate genetic profiling, metabolomic analysis, and individual neurological risk assessments. Understanding genetic variations and molecular mechanisms underlying neurodegenerative disorders allows for tailored interventions using EGCG’s unique properties, allowing for personalized treatment protocols [116]. Computational biology techniques are revolutionizing EGCG research by using advanced molecular modeling and artificial intelligence-driven predictive analyses. Machine learning algorithms simulate EGCG interactions with complex neurological protein structures, predicting therapeutic interventions with unprecedented accuracy. This allows researchers to explore molecular interactions, design effective derivatives, and understand intricate neurological pathway modulations [117]. EGCG’s therapeutic application in immunomodulatory approaches is promising, as it can regulate neuroinflammatory responses, a key factor in neurodegenerative progression. By regulating microglial activation, reducing pro-inflammatory cytokine production, and enhancing neuroprotective immune responses, EGCG can manage neurological inflammation and address underlying disease mechanisms. It also has potential in supporting neurogenesis and neural stem cell proliferation in regenerative medicine [56]. EGCG, a substance that promotes neuroplasticity and supports cellular regeneration, could be used to develop therapeutic strategies that slow disease progression and potentially reverse neurological damage. Interdisciplinary collaboration between experts from neuroscience, pharmacology, nanotechnology, and computational biology is driving these approaches. Recent scientific literature highlights the transformative potential of EGCG in developing novel therapeutic paradigms [118]. Advanced technology, molecular understanding, and research methodologies offer unprecedented opportunities for developing personalized neurodegenerative interventions. However, these approaches are complex and multifaceted. Researchers are cautiously optimistic, recognizing the need for continuous refinement, clinical validation, and interdisciplinary collaboration to translate promising scientific insights into tangible clinical interventions [119].
Future directions and research gaps
The research gap lies in understanding the precise molecular mechanisms of EGCG’s action in various disease conditions. Despite numerous pathways identified, there is uncertainty about their interaction and their significance for therapeutic effects. Future research should use advanced molecular techniques like proteomics and metabolomics to map the cellular interactions, optimizing therapeutic applications and predicting drug interactions. Developing reliable biomarkers for monitoring EGCG’s efficacy is also crucial [120]. Current studies lack standardized methods for measuring EGCG’s biological effects in vivo, making it difficult to optimize dosing regimens and predict treatment outcomes. Future research should focus on identifying biomarkers that reliably indicate EGCG’s therapeutic effectiveness across different conditions, develop novel imaging techniques, and incorporate pharmacogenomic approaches to identify genetic markers that predict treatment response. This could lead to personalized therapeutic approaches and improved patient outcomes [121]. Long-term safety data for EGCG is lacking, especially in specific patient populations with compromised liver function or multiple medications. Future research should include extended follow-up periods and diverse patient populations. Optimizing delivery systems remains a challenge, despite advances in formulation technology, to overcome biological barriers while maintaining EGCG’s stability [122]. Future research should focus on developing novel delivery platforms that enhance bioavailability and precise targeting capabilities, exploring new nanocarrier materials and tissue-specific targeting strategies. Translation of promising preclinical findings into clinical applications is crucial, as many studies have failed to demonstrate efficacy in clinical trials [123]. EGCG’s potential for drug interactions and resistance development is understudied, especially in combination therapy approaches. Future research should focus on comprehensive drug interaction studies and resistance mechanisms, especially in cancer therapy. The role of EGCG in preventive medicine is emerging, but optimal timing, dosing, and duration remain unclear. Large-scale, long-term preventive trials with well-defined endpoints and consideration of various population groups are needed [124].
Large-scale, randomized clinical trials
Current research underscores the critical need for large-scale randomized clinical trials (RCTs) of EGCG for neurodegenerative disorders. While preclinical studies show promise, existing clinical evidence primarily comes from small-scale trials with methodological limitations. Recent meta-analyses highlight significant heterogeneity in study designs, dosing protocols, and outcome measures, making it difficult to draw definitive conclusions about EGCG’s therapeutic efficacy. Comprehensive RCTs are needed to establish optimal dosing regimens, evaluate long-term safety profiles, and determine EGCG’s effectiveness across different disease stages and patient populations [100]. Multi-center trials with standardized protocols would help assess biomarkers, cognitive outcomes, and disease progression markers. Additionally, studies should investigate EGCG’s interaction with standard treatments and potential synergistic effects. Key areas requiring investigation include bioavailability enhancement strategies, therapeutic window identification, and patient stratification based on genetic and environmental factors. Modern trial designs incorporating advanced imaging techniques and molecular biomarkers could provide deeper insights into EGCG’s mechanisms of action in human subjects. These large-scale studies would also help establish evidence-based guidelines for EGCG supplementation in clinical practice [125].
Exploration of EGCG derivatives for enhanced efficacy
Recent research has focused on developing EGCG derivatives with improved pharmacological properties. Structure-activity relationship studies have identified key molecular modifications that enhance stability, bioavailability, and blood-brain barrier penetration. Novel synthetic derivatives demonstrate increased half-life and reduced susceptibility to oxidation compared to natural EGCG. Promising modifications include the addition of lipophilic groups to improve membrane permeability and targeted delivery systems using nanocarriers. Studies show certain derivatives exhibit enhanced neuroprotective effects through stronger binding to therapeutic targets and improved cellular uptake [126]. Computational modeling has guided the design of derivatives with optimized interactions with amyloid proteins and key signaling molecules. Current research gaps include limited understanding of derivatives’ long-term safety profiles and metabolic fate. Future work should focus on comparative efficacy studies between natural EGCG and its derivatives, optimization of synthesis methods for large-scale production, and investigation of potential side effects. Development of site-specific derivatives targeting particular aspects of neurodegenerative pathways could lead to more effective therapeutic strategies [127].
Long-term safety and toxicity studies
Research on EGCG’s long-term safety reveals complex considerations for therapeutic applications. While generally recognized as safe, high-dose studies indicate potential hepatotoxicity risks, particularly in susceptible individuals. Recent investigations highlight dose-dependent effects on liver enzymes and the importance of monitoring hepatic function during extended EGCG supplementation. Clinical data suggests variation in individual tolerance levels, with factors like genetic polymorphisms and pre-existing conditions affecting susceptibility to adverse effects. Studies recommend establishing personalized dosing guidelines based on patient characteristics. Current research emphasizes the need for standardized safety protocols and identification of biomarkers for early detection of toxicity [128]. Key areas requiring further investigation include cumulative effects of chronic exposure, drug interactions, and impact on various organ systems. Emerging evidence points to the importance of considering circadian rhythms and timing of administration in safety profiles. Development of more sensitive toxicity screening methods and establishment of clear safety thresholds across different patient populations remain crucial research priorities. Recent findings also underscore the need to evaluate potential interactions with common medications and supplements used in treating neurodegenerative disorders. This includes understanding how EGCG’s antioxidant properties might affect other therapeutic interventions and identifying any contraindications [129].
Integrating EGCG into combination therapies for naturopathic doctors
Recent studies demonstrate promising synergistic effects when combining EGCG with established treatments for neurodegenerative disorders. Research shows EGCG enhances the efficacy of conventional medications through complementary mechanisms, particularly in targeting multiple pathological pathways simultaneously. Clinical investigations reveal positive interactions between EGCG and cholinesterase inhibitors in Alzheimer’s treatment, showing improved cognitive outcomes compared to monotherapy [130]. Studies also indicate EGCG’s potential to reduce side effects of standard treatments while enhancing their therapeutic benefits through antioxidant and anti-inflammatory mechanisms. Current research focuses on optimal combination strategies, including timing of administration and dosage ratios. Emerging evidence suggests EGCG may enhance drug delivery across the blood-brain barrier when used in combination therapies. Investigation of EGCG’s role in targeting multiple aspects of neurodegeneration, including protein aggregation and oxidative stress, shows promise for developing more effective therapeutic approaches. Future directions include determining ideal drug combinations, understanding potential interactions, and developing standardized protocols for combination therapy. This includes evaluating EGCG’s impact on drug metabolism and identifying patient populations most likely to benefit from combined treatment approaches [131].
Conclusions
Our comprehensive review of EGCG advances neurological research by synthesizing critical insights into its multifaceted neuroprotective mechanisms. By integrating molecular, cellular, and clinical perspectives, we illuminate EGCG’s potential as a sophisticated therapeutic intervention for neurodegenerative disorders. The review critically examines EGCG’s complex interactions with neurological pathways, highlighting its unique ability to modulate neuronal survival, mitigate inflammation, and inhibit protein aggregation. Our analysis bridges preclinical research with emerging clinical evidence, providing a strategic roadmap for future investigations that could transform current approaches to neurodegenerative disease management. By emphasizing the need for advanced research methodologies, personalized treatment strategies, and comprehensive clinical trials, we offer a nuanced framework for translating EGCG’s promising neuroprotective potential into practical, targeted therapeutic applications.
Neuroprotective insights into EGCG for neurodegenerative disorders future research directions should strategically expand the investigative scope beyond current neurological paradigms. Promising unexplored avenues include evaluating EGCG’s potential in rare neurodegenerative conditions like HD, multiple system atrophy, and progressive supranuclear palsy. Additionally, research should target understudied patient populations such as early-onset neurodegenerative patients, individuals with genetic predispositions, and populations with complex comorbidities. Comprehensive investigations should also explore EGCG’s therapeutic potential across diverse demographic groups, considering age, genetic variations, and distinct neurological disease progression patterns. The research trajectory must integrate advanced molecular techniques, longitudinal clinical trials, and personalized medicine approaches to unlock EGCG’s comprehensive neuroprotective mechanisms and translate preclinical promises into robust clinical interventions.
Abbreviations
| AD: | Alzheimer’s disease |
| ALS: | amyotrophic lateral sclerosis |
| AMPK: | adenosine monophosphate-activated protein kinase |
| Aβ: | amyloid-beta |
| BDNF: | brain-derived neurotrophic factor |
| CREB: | cAMP-response element binding protein |
| EGCG: | epigallocatechin gallate |
| ER: | endoplasmic reticulum |
| HD: | Huntington’s disease |
| IL-1β: | interleukin-1β |
| mHTT: | self-propagating, amyloidogenic mutant huntingtin |
| MS: | multiple sclerosis |
| NLRP3: | nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 |
| NMDA: | N-methyl-D-aspartate |
| Nrf2: | nuclear factor erythroid 2-related factor 2 |
| PD: | Parkinson’s disease |
| PINK1: | PTEN-induced kinase 1 |
| PLGA: | poly (lactic-co-glycolic acid) |
| RCTs: | randomized clinical trials |
| ROS: | reactive oxygen species |
| SIRT1: | silent information regulator sirtuin 1 |
| SOD: | superoxide dismutase |
| TDP-43: | transactive response DNA binding protein 43 |
| TNF-α: | tumor necrosis factor-alpha |
| UPR: | unfolded protein response |
Declarations
Acknowledgments
All authors thank the Graphic Era (Deemed to be University), for supporting.
Author contributions
NK and SS: Writing—original draft, Investigation, Writing—review & editing. RK: Writing—original draft, Investigation, Writing—review & editing, Conceptualization, Supervision, Validation. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.