Abstract
Astrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in supporting functions of the central nervous system (CNS), including blood-brain barrier (BBB) development and maintenance, water and ion regulation, influencing neuronal synaptogenesis, and stimulating the immunological response. In terms of epidemiology, glioblastoma (GB), the most common and malignant astrocytoma, generally occur with higher rates in Australia, Western Europe, and Canada, with the lowest rates in Southeast Asia. Additionally, significantly higher rates of GB are observed in males and non-Hispanic whites. It has been suggested that higher levels of testosterone observed in biological males may account for the increased rates of GB. Hereditary syndromes such as Cowden, Lynch, Turcot, Li-Fraumeni, and neurofibromatosis type 1 have been linked to increased rates of astrocytoma development. While there are a number of specific gene mutations that may influence malignancy or be targeted in astrocytoma treatment, O6-methylguanine-DNA methyltransferase (MGMT) gene function is an important predictor of astrocytoma response to chemotherapeutic agent temozolomide (TMZ). TMZ for primary and bevacizumab in the setting of recurrent tumor formation are two of the main chemotherapeutic agents currently approved in the treatment of astrocytomas. While stereotactic radiosurgery (SRS) has debatable implications for increased survival in comparison to whole-brain radiotherapy (WBRT), SRS demonstrates increased precision with reduced radiation toxicity. When considering surgical resection of astrocytoma, the extent of resection (EoR) is taken into consideration. Subtotal resection (STR) spares the margins of the T1 enhanced magnetic resonance imaging (MRI) region, gross total resection (GTR) includes the margins, and supramaximal resection (SMR) extends beyond the margin of the T1 and into the T2 region. Surgical resection, radiation, and chemotherapy are integral components of astrocytoma treatment.
Hereditary risk factors, genetic mutations, and imaging modalities are discussed in reference to astrocytoma staging and mechanism of growth. In terms of the treatment of astrocytomas, chemotherapy, radiation therapy, and strategic surgical interventions are discussed
Keywords
Astrocytoma, surgical resection, chemotherapy, radiation, glioblastoma, diagnostic imagingIntroduction
As astrocytes are the most plentiful cell in the human central nervous system (CNS), it is not entirely surprising that astrocytomas are the most common form of primary malignant brain tumors in both adults and children [1–3]. Lacking the ability to produce electrical depolarization, astrocytes are often described as star-shaped glial cells that support the function of neurons [4]. Astrocytes have a plethora of roles in the CNS, including blood-brain barrier (BBB) development and maintenance, water and ion regulation, influencing neuronal synaptogenesis, and stimulating immunological response [4, 5]. The exact function and morphology of the astrocyte may be primarily determined by the spatial domain the cell occupies within the brain and the surrounding population of astrocytes [6, 7].
The clinical presentation of astrocytoma symptoms is similar to most brain tumors with both focal and generalized neurologic signs. Focal symptoms include weakness, numbness, seizures, and aphasia, while generalized symptoms due to edema and increased intracranial pressure can cause headaches, vision deficits, and emesis [8, 9]. Diffuse headaches present at diagnosis in 50% of patients, with seizures observed in 20–40% of patients [10].
Radiation, chemotherapy, and surgical resection play a role in the medical management of both low- and high-grade astrocytomas to alleviate symptoms, improve prognosis, and prevent malignant transformation [11, 12]. By summarizing the current understanding of astrocytomas, this paper strives to consolidate and clearly explain the accepted medical and surgical management of this condition to better educate the reader.
Epidemiology
Glioblastoma (GB) is both the most common astrocytoma and the most malignant, accounting for roughly 65% of astrocytomas [8, 13]. Based on 2003–2007 data gathered from region-specific populations over 40 years old, the age-adjusted incidence rate of astrocytic tumors per 100,000 is highest in Australia [9.58, 95% confidence interval (CI) = 9.30–9.86], Western Europe (8.45, 95% CI = 8.30–8.59), and Canada (8.26, 95% CI = 8.04–8.48), with lowest rates in Southeast Asia (1.91, 95% CI = 1.75–2.08) [14]. These trends in astrocytoma incidence based on the region are summarized in Figure 1.
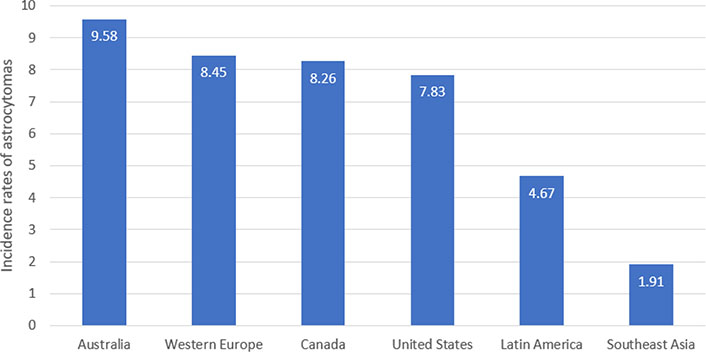
The age-adjusted incidence rates of astrocytomas per 100,000 in adult populations (> 40 years old) around the world. The highest rates are observed in Australia (9.58/100,000) and the lowest are observed in Southeast Asia (1.91/100,000) [14]
When examining 244,808 patients with gliomas from 2000–2014, there was a higher incidence of astrocytomas in non-Hispanic whites compared to Hispanic whites (30% lower), blacks (52% lower), or Asians (52% lower) [15]. Overall, epidemiological studies have indicated an increased incidence of astrocytomas in males compared to female populations [16]. This sex preference is especially prominent in the development of GB, with an incidence 60% higher in both male children and adults [15–17]. Elevated levels of testosterone and activation of androgen receptors on cancer cells are believed to play significant roles in the increased incidence of male GB, while the protective benefit of progesterone and estrogen has also been theorized [18–20]. With the exception of low-grade pilocytic astrocytomas (PAs) that typically present in pediatric populations, the majority of astrocytomas are observed sporadically in adulthood after the age of 40 without a familial association [14, 21, 22]. While the mortality rate varies with different grades of astrocytomas and mutations present, GB has a median survival of 15 months with treatment and a 3-year survival rate of 3–5% [23]. There are numerous factors associated with improved overall survival rate, including female sex, younger age of diagnosis, lower comorbidity burden, presence of O6-methylguanine-DNA methyltransferase (MGMT) methylation in tumor genetics, reduced tumor size, single lesion, complete tumor resection, chemotherapy, and radiation treatment [24]. Although it could be explained by the patient population and socioeconomic elements, there appears to be a trend towards superior 30–90 day mortality and reduced readmission rates in academic research and high-volume facilities compared to community and integrated network cancer programs [24].
Astrocytoma hereditary and environmental risk factors
Therapeutic ionizing radiation exposure is one of the few environmental risk factors with a strong correlation with increased incidence of astrocytomas and other glioma formation [25]. Surprisingly, cigarette smoking and alcohol consumption have not been associated with higher rates of astrocytomas as observed in many other forms of cancer [26]. Although the risk factors of astrocytoma are poorly understood, germline genetic variants contribute significantly to the risk of developing gliomas like astrocytomas. For example, some genetic syndromes [Cowden, Lynch, Turcot, Li-Fraumeni, neurofibromatosis type 1 (NF1)] have been identified as potential risk factors. Conditional inactivation of NF1, phosphatase and tensin homolog (PTEN), and tumor protein p53 (TP53) tumor suppressor genes in murine models has induced the formation of malignant astrocytomas [27, 28]. Carlos-Escalante et al. [29] found an association between genetic variants in angiotensinogen (AGT, involved in angiogenesis), TP53 (tumor suppressor), and MGMT (DNA repair) and the risk of developing astrocytoma in a case-control study performed in 49 Mexican patients.
Another genetic association involves B-Raf proto-oncogene (BRAF) abnormalities, in which tandem duplication-rearrangements and BRAF V600E mutations are observed in PA and pleomorphic xanthoastrocytoma, respectively [30]. The most common glial tumor in children is PA. While sporadic cases are associated with BRAF rearrangements, 15–20% of children develop PA in the context of NF1 [31]. The most common tumor in NF1 patients is the optic pathway tumor, also known as optic glioma. Although these are rare tumors (2–5% of childhood brain tumors), up to 70% are associated with NF1 [32]. Most of these tumors are grade I PAs. These tumors form within the optic pathway and brainstem to then expand into the optic nerve, producing a fusiform mass [27, 33, 34].
Certain genetic markers have been associated with worse prognosis. Perdomo-Pantoja et al. [35] conducted a prospective study on 48 patients diagnosed with astrocytoma and proposed that the genetic variant AGT reference single nucleotide polymorphism 5050 (rs5050) GG-genotype can be detected safely and practically in blood/saliva samples and was identified as a risk factor for lower survival rates. Other genes [ST6 beta-galactoside alpha-2,6-sialyltransferase 1 (ST6GAL1), cytochrome P450 family 19 subfamily A member 1 (CYP19A1)] have also shown an association with worse prognosis [36].
Astrocytoma genetics and mechanisms of growth
As astrocytomas, like all cancers, are the unregulated overgrowth of cells brought about by changes in genetic expression, genetics play a significant role in astrocytoma advent, evolution, and treatment [37]. While some cancer states are strongly linked to specific genes, such as Li-Fraumeni with TP53 and breast and ovarian cancers with breast cancer 1 (BRCA1)/BRCA2, other cancers, such as astrocytomas rest at the intersection of a combination of genes [38, 39]. Significant research on the genetics of astrocytomas focuses on isocitrate dehydrogenase 1 (IDH1) and MGMT.
IDH1 and IDH2 are genes that encode nicotinamide adenine dinucleotide phosphate (NADP+)-dependent IDHs which convert isocitrate to alpha-ketoglutarate [40, 41]. This activity plays a role in cellular processes affecting lipid synthesis, oxidative respiration, and signal transduction [42, 43]. The R132H mutation in IDH1 can lead to the transformation of alpha-ketoglutarate into 2-hydroxygluterate (2HG) [44]. Aberrations in IDH1/2 as well as the accumulation of 2HG have been linked to a multitude of cancers, including astrocytomas like GB [42, 44, 45]. While their presence is not ubiquitous in primary GB (5%), mutations in IDH1 are prevalent in secondary GB (80%) [46].
Extensive exploration into the link between IDH1 and gliomas has led to a deeper understanding of the influence of IDH1 on current treatments. Investigation of this relationship has also opened the door for targeted therapeutic interventions. Mutation of IDH1 has proven to be a strong prognostic indicator in gliomas [45, 46]. Studies have shown better responses to treatment and improved outcomes in patients with IDH1 mutated GB and anaplastic astrocytoma (AA) [46, 47]. Therapies targeting IDH1-specific mutations including hypomethylating agents, IDH enzyme inhibition, and vaccination are being investigated with promising early findings [42].
MGMT is another gene that’s been shown to influence patient response to treatment in astrocytoma. MGMT encodes a protein that repairs alkylated DNA via the transfer of a methyl group [48]. Temozolomide (TMZ) is an alkylating agent that has been a stalwart therapy in the treatment of astrocytoma [49]. As such, functional MGMT repairs damage caused by the alkylation of DNA by drugs like TMZ and can lead to drug resistance in tumors [50]. While the loss of function of MGMT via methylation is associated with different cancers, it is also associated with improved patient outcomes in the treatment of astrocytomas [48, 51, 52]. Studies have shown a significant association between methylated MGMT and longer survival as well as decreased tumor size over the course of treatment [51, 52]. Of note, there is no current consensus on the amount of methylation that distinguishes methylated from unmethylated MGMT. The mechanism of the improved outcome of TMZ treatment in MGMT-inhibited tumors is summarized in Figure 2 [53].
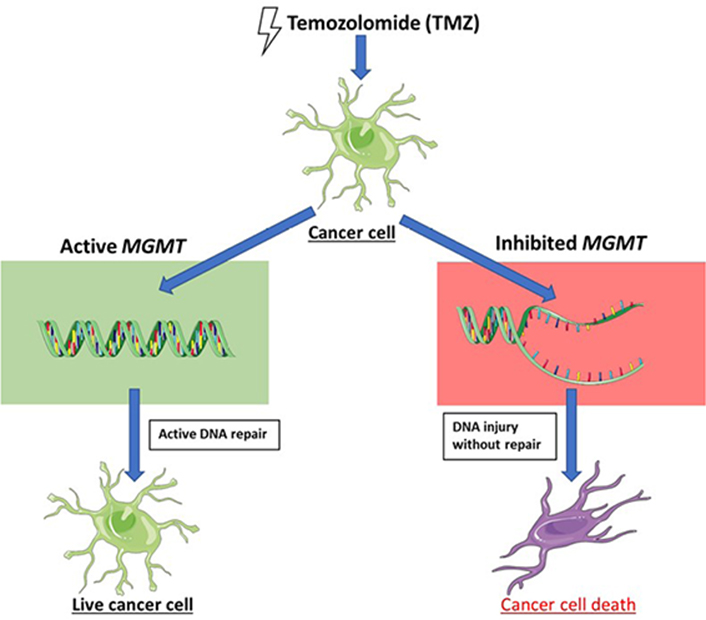
In the setting of dysfunctional MGMT DNA repair, TMZ has an improved capacity to promote astrocytoma cellular injury
Note. This figure contains (modified) images from Servier Medical Art (https://smart.servier.com). CC BY.
While the BBB normally restricts immune cell access to the brain, malignancy in the brain often creates a privileged environment where leukocytes are granted access [54]. Despite that, often times immune response in brain tumors is limited by patient immunodeficiency or immunological escape of the cancer [54]. Immunological escape is a phenomenon whereby tumors elude immune action [55]. There are a few forms of immune escape exhibited by astrocytomas, including the restriction of antigen presentation via the downregulation of major histocompatibility complex (MHC) expression [56]. Other methods include the facilitation of cell death in leukocytes by increasing Fas-ligand expression, and secretion of immunosuppressive cytokines like transforming growth factor-β (TGF-β), and interleukin-10 (IL-10) [57, 58]. As immunological escape and immune suppression increase the difficulty of treating astrocytomas, genetically modified immune cells and augmentation of the immune response are of particular interest in the treatment of astrocytomas [59]. Of note, these methods may run the risk of autoimmunity.
When examining GB mutation distribution based on sex, there appears to be a trend toward higher subclonal mutation burden in the majority of glioma subtypes of biological females over males [60]. Additionally, some mutations observed in astrocytomas appear to express some level of male or female preference. For example, mutations in the signaling pathway of mitogen-activated protein kinase (MAPK) tend to occur more often in the GB of biological female patients [60]. In the case of gliomas with mutations of the phosphoinositide 3-kinase (PI3K) pathway, these genetic changes occur more frequently in the helical domain for females and the kinase domain for males, demonstrating a sex preference for mutation location [61]. The increased mutation burden could be explained by increased mutations on the X chromosome, or potentially by different environmental exposures that may be more sex-specific.
As genetics play a significant role in the development and progression of astrocytomas, further investigation into the influence of specific genes may illuminate a pathway to better understand and more effectively treat the disease.
Grading astrocytomas
The grading of astrocytomas is based on histological observations and cellular markers [62]. The following presents a discussion on some of the most common locations for each lesion type and prognosis based on tumor grading. The grading type and location are summarized in Table 1.
While different types of astrocytomas can be found throughout the CNS, there are regions of the brain where specific astrocytomas tend to present more commonly
| Grade | Astrocytoma type | Common location |
|---|---|---|
| 1 | PA | Cerebellum |
| Subependymal giant cell astrocytoma (SEGA) | Walls of the lateral ventricles or foramen of Monro | |
| 2 | Diffuse astrocytoma | Frontal, parietal, or temporal lobes |
| Pleomorphic xanthoastrocytoma | Temporal lobes | |
| 3 | AA | Frontal, parietal, or temporal lobes |
| 4 | GB | Frontal, parietal, or temporal lobes |
Grade 1
Grade 1 astrocytomas are slow-growing lesions, allowing these tumors to grow quite large without inducing symptoms as the brain gradually adapts to the tumor growth [63]. Grade 1 tumors include PA, which is the most common primary CNS tumor in children aged 5–14 [64]. Though commonly localized to the cerebellum, PAs can also be found in the brainstem, hypothalamus, spinal cord, and optic chiasm, with the latter having the least ideal outcome [65–67]. With a 10-year survival over 90%, pediatric PAs are amenable to surgery and hold an excellent prognosis if completely excised [68]. PA in adults is not fully understood, though recurrence is still substantially less prevalent in those with complete resection than those without (4% vs. 38%) [69]. SEGAs are rarer, slow-growing pediatric astrocytomas that are typically localized in the walls of the lateral ventricle or foramen of Monro [70]. Similar to PAs, SEGAs also carry a favorable prognosis and are generally considered curative if complete resection is achieved [71]. Finally, though diffuse astrocytomas are typically considered grade 2, the recent fifth edition of World Health Organization (WHO) classifications has updated those pediatric diffuse astrocytomas with alterations in proto-oncogenes myeloblastosis (MYB) and MYB proto-oncogene like 1 (MYBL1) to grade 1, likely stemming from their improved prognosis (10-year overall survival of 95% with MYB/MYBL1 alterations compared to 38% otherwise) [72–75].
Grade 2
Diffuse grade 2 astrocytomas present a much greater challenge for clinicians as tumor borders are no longer well-delineated, complicating resection and increasing the likelihood of recurrence [76]. Though prognostication can be complicated by the inability of histopathological features to accurately predict the disease course, clinical features such as the preoperative performance and extent of resection (EoR) may better inform physicians [77]. Nevertheless, some novel analyses focusing on oxidative enzymes and their products have established new methods of generating a prognosis based on histopathological evidence [78]. IDH mutations, for example, are thought to have a protective effect and display improved survival in glioma [79, 80]. Interestingly, in some instances mutant IDH grade 2 astrocytomas have displayed a 21% reduction in 5-year progression-free survival (PFS) but an improved survival following recurrence when compared to wild-type IDH tumors [81]. Nevertheless, evidence points to an overall limited prognostic value of IDH1 mutations in grade 2 astrocytomas [81, 82]. Overall, grade 2 astrocytomas display markedly poorer outcomes (65% 2-year survival) when compared to grade 1 tumors (98% 2-year survival) [83]. Diffuse astrocytomas are localized to the cerebrum in the frontal, parietal, or temporal lobes in 60% of cases [83]. Pleomorphic xanthoastrocytoma is another tumor with the potential to be grade 2 or 3 depending on the degree of anaplastic changes present. Presenting most frequently in the temporal lobes, grade 2 pleomorphic xanthoastrocytomas carry a favorable 3-year overall survival of 84% [84, 85].
Grades 3 and 4
Similar to grade 2 astrocytomas, grades 3 and 4 are largely localized to the supratentorial region, with 62% (grade 3) and 68% (grade 4) found in one of the frontal, parietal, or temporal lobes [83]. Despite this similarity, these tumors display significantly higher rates of metastasis and progression when compared to their low-grade counterparts. AA is a grade 3 astrocytoma and around half of the individuals with AA display progression at 3 years [86]. For grade 4 astrocytomas (GB), 2-year PFS is an abysmal 10% [87]. With this comes an especially poor 2-year survival prognosis of 50% for grade 3 and 19% for grade 4 tumors [83]. IDH mutations in high-grade astrocytomas are extremely relevant to prognosis, with wild-type IDH AAs typically displaying worse prognosis than IDH-mutated GB [47, 88]. Taken together, these findings communicate the diverse set of pathologies that grade 1 through 4 astrocytomas represent with their resulting prognosis.
When evaluating depth within the brain, tumor location can impact prognosis. Especially in elderly populations, the closer the margin of a high-grade glioma is to the 3rd ventricle, generally the worse the prognosis [89, 90]. Specifically, periventricular and ventricular high-grade gliomas were associated with the worst outcome in elderly patients, when compared to more superficial tumors [89].
It is important to note that the new 2021 WHO fifth edition classification of gliomas differs significantly from the previous 2016 fourth edition standards. Under the new system, diffuse gliomas are subcategorized into pediatric-type diffuse low-grade gliomas, pediatric-type diffuse high-grade gliomas, adult-type diffuse gliomas, and circumscribed astrocytic gliomas [91].
Adult-type diffuse gliomas are further subcategorized into astrocytoma IDH-mutant (grade 2–4), oligodendroglioma IDH-mutant and 1p/19q-codeleted (grade 2–3), and GB IDH-wild-type (grade 4) [75, 91].
Astrocytoma (IDH-mutant), which includes the now obsolete categories of GB (IDH-mutant), diffuse astrocytoma, and AA, can be classified as a grade 2, 3, or 4 tumor [75, 92]. Grading within this tumor type is based on both genetics and histology with higher proliferative tumors receiving a grade of 3 and more ominous findings such as microvascular expansion or necrosis receiving a grade of 4 [75, 92]. Additionally, cyclin dependent kinase inhibitor 2A (CDKN2A)/CDKN2B homozygous deletion tumors receive a default grade of 4 regardless of histological findings [75, 92]. Of note, astrocytoma (IDH-mutant) often includes a TP53, alpha-thalassemia/mental retardation, X-linked (ATRX), or CDKN2A/B mutation, but must not include a 1p/19q codeletion [75, 91].
As the name implies, oligodendroglioma IDH-mutant and 1p/19q-codeleted must include both an IDH mutation and 1p/19q-codeletion [75]. It can be a grade 2 or 3 tumor based on histology and image findings [75, 92]. Common co-mutations include a telomerase reverse transcriptase (TERT) promoter mutation and a notch receptor 1 (NOTCH1) mutation [75, 92].
GB IDH-wild-type is by default a grade 4 tumor [75, 92]. Defining features of this diffuse glioma tumor type are the presence of IDH-wild-type and the histological finding of necrosis or microvascular expansion or one of three key genetic features—epidermal growth factor receptor (EGFR) amplification, +7/−10 chromosome copy number changes, or a TERT promoter mutation [75, 91, 92].
Under this new system, a number of tumor types are now obsolete including GB IDH-mutant, AA, diffuse astrocytoma, anaplastic pleomorphic xanthoastrocytoma, and anaplastic oligodendroglioma [75].
Diagnostic imaging
Magnetic resonance imaging (MRI) maintains a significant role in the standard of care among patients diagnosed with astrocytomas, especially as MRI has evolved to incorporate functional, hemodynamic, metabolic, cellular, and cytoarchitectural modalities [93]. Malignant gliomas typically demonstrate strong contrast enhancement, peritumoral edema [evident on T2-fluid-attenuated inversion recovery (FLAIR) sequences], mass effects, heterogeneity, central necrosis, and intratumoral hemorrhage on conventional MRI [94–97]. In contrast, low-grade astrocytomas often present as well-defined homogeneous masses with little or no mass effect, vasogenic edema, or enhancement after contrast administration on conventional MRI [94–96]. Yet, distinguishing low-grade astrocytomas from high-grade astrocytomas and metastases using conventional MRI findings alone is not always possible, as overlapping features may persist [98, 99]. The distinctions that exist between high- and low-grade astrocytomas when observed on different MRI modalities are summarized in Table 2.
Differences in low- and high-grade astrocytomas among various diagnostic MRI sequences that are currently utilized in astrocytoma diagnoses
| Imaging modality | Low-grade astrocytomas | High-grade astrocytomas |
|---|---|---|
| MRI | Well-defined homogeneous masses with little or no mass effect, vasogenic edema, or enhancement after contrast administration | Strong contrast enhancement, peritumoral edema (evident on T2-FLAIR sequences), mass effects, heterogeneity, central necrosis, and intratumoral hemorrhage on conventional MRI |
| SWI | Low ITSS grade | High ITSS grade |
| DWI | High ADC | Low ADC |
| PWI | Low rCBV | High rCBV |
| DCE | High Ktrans | Low Ktrans |
| MRS | Low Lip-Lac/Cr, low Cho/Cr | High Lip-Lac/Cr, high Cho/Cr |
SWI: susceptibility-weighted imaging; ITSS: intratumoral susceptibility score; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; PWI: perfusion-weighted imaging; rCBV: relative cerebral blood volume; DCE: dynamic contrast enhancement; Ktrans: volume transfer coefficient; MRS: magnetic resonance spectroscopy; Lip-Lac/Cr: lipid-lactate to creatine; Cho/Cr: choline to creatine
Within GB management, functional MRI (fMRI) has utility pre-operatively, particularly when resection may disrupt eloquent areas [97]. Notably, patients with tumors previously deemed unresectable have become candidates for a more aggressive surgical resection following the utilization of advanced MRI sequences and functional mapping. Diffusion tensor imaging (DTI) can assist in distinguishing between postoperative vascular damage and residual enhancing tumor [100, 101]. Serving a predictive role, DCE can pre-operatively measure contrast uptake, which may be associated with early disease progression [102]. Dynamic susceptibility contrast (DSC) can indicate tumor capillary permeability and neoangiogenesis, factors useful in characterizing glioma type, grade, and tumor recurrence after treatment [103].
In 2019, Bulakbaşı and Paksoy [104] utilized advanced MRI techniques including SWI, DWI, PWI, MRS, and fMRI to differentiate low- and high-grade gliomas. ADC is determined using DWI, rCBV is approximated using PWI, Ktrans is determined using DCE, ITSS is calculated using SWI, and metabolic markers (i.e., Lip-Lac/ Cr and Cho/Cr) can be measured using MRS. Their study suggested low-grade gliomas have lower cellularity (high ADC), decreased angiogenesis (low rCBV), decreased capillary permeability (high Ktrans), and decreased mitotic activity (Cho/Cr), thus supporting the use of advanced MRI techniques in the differentiation of astrocytomas [104]. Challenges in utilizing these advanced MRI techniques include the diversity of imaging equipment and techniques available, as standardization is currently lacking across facilities [104].
de Fatima Vasco Aragao et al. [99] evaluated the application of advanced MRI sequences using DWI, PWI, and MRS in differentiating PAs from high-grade astrocytomas. Their study suggested lower rCBV from PWI and higher ADC on DWI were suggestive of PAs, whereas higher Lip-Lac/Cr and Cho/Cr ratios on MRS favored high-grade astrocytomas [99, 104]. The accuracy of MRS in glioma grading is controversial, particularly as many studies have not included the broad range of low-grade gliomas (particularly PAs) [99]. Additionally, MRS patterns do not necessarily translate between adults and children, as is particularly notable in PAs [105].
Per a 1991 study by Krouwer et al. [106], as low as a 5% gemistocytic component within a patient’s astrocytoma is sufficient to negatively impact prognosis. In 2016, Simkin et al. [107] described the use of MRI in diagnosing gemistocytic astrocytomas. Specifically, gemistocytic astrocytoma evaluation should be considered in patients with heterogeneous cystic lesions that display contrast enhancement and partial FLAIR suppression [107]. Recent literature has emerged on the use of MRI to predict tumor grade in IDH-mutant astrocytomas and oligodendrogliomas [108]. Joyner et al. [108] suggest a positive association between contrast enhancement and the grade of astrocytomas as well as oligodendrogliomas. Additionally, their study suggests a negative association with ADC and a positive association with rCBV in predicting astrocytoma grade, but not oligodendroglioma grade [108].
Whole-brain radiotherapy vs. stereotactic radiosurgery
Over the last 50 years, radiotherapy (RT) has been a key therapy in astrocytoma management, as an integral component of multimodal treatment alongside surgical and medical considerations [109]. Initial clinical trials in patients with malignant gliomas, including high-grade astrocytomas, utilized whole-brain RT (WBRT) in conjunction with surgical resection. The combination of WBRT using 5,000–6,000 rad in addition to surgical resection of malignant gliomas revealed significant survival benefit in comparison to surgical resection alone [110, 111]. Studies led by the Brain Tumor Study Group (BTSG) from the National Cancer Institute (NCI) examined the relationship between increasing WBRT dose titration and median survival [112]. Patients receiving doses of 5,000–6,000 rad demonstrated significantly longer median survival, compared to patients randomized to no radiation [112].
With the advent of more targeted approaches in radiation oncology such as stereotactic radiosurgery (SRS), new techniques, protocols, and recommendations have emerged for studying and treating astrocytoma. SRS differs from WBRT in numerous ways, but the most important distinction is the high degree of precision SRS has directed against cancerous lesions while sparing healthy tissue [113]. SRS typically refers to multisource cobalt-60 gamma radiation or linear accelerator (LINAC)-based photon therapy [114]. Radiation Therapy Oncology Group (RTOG) 93-05 trial evaluated the use of standard RT with TMZ compared to SRS with TMZ and results demonstrated comparable overall survival between both arms. PFS, complete and partial response rates did not significantly differ, but significantly higher toxicity was observed in the standard RT with TMZ arm [113, 115]. Like other focal regimens, SRS has not produced a significantly different survival advantage relative to standard RT [116]. Other roles for SRS may exist, such as salvage therapy for recurrent astrocytoma and as an adjuvant to systemic immunotherapy, but further elucidation of this role is necessary through pre-clinical studies and randomized controlled trials (RCTs) [117, 118].
Evidence-based guidelines for use of RT in treating high-grade astrocytoma have been established by the American Society for Radiation Oncology (ASTRO). Regarding ideal target volumes for RT against GB, ASTRO issued a “strong recommendation” for partial brain radiation therapy directed against brain areas with the highest recurrence risk, namely within 2 cm of the primary site [119]. This recommendation was supported by evidence indicating that partial brain RT did not decrease survival relative to WBRT [119]. Additionally, a “strong recommendation” was issued for two RT strategies for high-grade astrocytoma:
Two-phase strategy utilizing RT against the primary target volume encompassing edema and gross residual tumor/resection cavity plus a boost target volume encompassing gross residual tumor/ residual cavity [119].
One-phase single target volume including gross residual tumor/resection cavity with wide margins [119].
Guidelines issued by the European Association for Neuro-Oncology (EANO) present similar recommendations as ASTRO for various subtypes of astrocytoma. Depending on various prognostic factors, the mainstay radiation recommendation is focused RT with a 1–2.5 cm margin, with SRS suggested as an option in the recurrent setting to improve the delivery of therapy against cancerous lesions and sparing healthy structures [120].
Guidelines for RT for low-grade astrocytoma were examined and issued by the Spanish Society of Medical Oncology, regarding timing and dosage. Early RT demonstrated a significant increase in PFS, but no difference in overall survival. Based on level I evidence, high and low doses of radiation were demonstrated to be equivalent, with lower doses minimizing radio-necrosis and toxicity [121].
Chemotherapies
TMZ
TMZ is part of a class of drugs known as alkylating agents and was first approved for use in the treatment of GB and AA in 1999 by the Food and Drug Administration (FDA) [122]. Landmark RCTs were conducted in 2005, demonstrating significant survival benefit when TMZ was administered as concomitant and adjuvant chemotherapy with RT. Compared to patients randomized to the RT-alone arm, patients in the TMZ with radiation arm at 28 months displayed a 37% death risk reduction and an overall 2.5-month survival benefit [123]. Follow-up assessing 2-year survival rate revealed that patients who had received the combination arm had a 26.5% survival rate compared to 10.4% for patients who had only received RT with significant survival benefit persisting through the fifth year of follow-up [123, 124]. The methylation status of MGMT further stratified patients with GB. Methylation of this gene in tumor tissue was associated with significant increases in median survival for patients receiving combinatorial therapy of 21.7 months compared to patients lacking methylation of MGMT which had a median survival of 12.7 months [50]. CeTeG/ NOA-09 trial investigated the use of TMZ with lomustine, another alkylating agent, in addition to RT in patients with newly diagnosed GB and who had methylation of the MGMT. The lomustine-TMZ combination treatment arm demonstrated a significant overall survival benefit of 16.7 months, compared to patients randomized to the TMZ-alone treatment arm [125]. The use of TMZ was also assessed in patients with low-grade glioma (astrocytoma, oligoastrocytoma, or oligodendroglioma) compared to RT-alone. Median overall survival has not been reached in this study and no significant differences in PFS were observed between TMZ-alone and RT-alone treatment groups [126]. American Society of Clinical Oncology (ASCO)-Society for Neuro-Oncology (SNO) guidelines issued recommendations for treating various molecular subtypes of astrocytoma graded II–IV using RT with adjuvant TMZ [127]. Additionally, TMZ offers greater tolerability relative to other chemotherapies such as the lone chemotherapeutic agent l,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) or the triple combination therapy procarbazine, lomustine [1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU)], and vincristine (PCV), further supporting TMZ’s role a first-line treatment for patients with astrocytoma [86].
Bevacizumab
Bevacizumab is a monoclonal antibody that is believed to neutralize vascular endothelial growth factor A (VEGF-A). Bevacizumab is FDA-approved for use in adult patients diagnosed with recurrent GB, following promising phase II clinical trials [128]. A double-blind placebo-controlled trial comparing the use of bevacizumab to placebo with standard RT-TMZ therapy in adult patients diagnosed with GB revealed no overall survival benefit [129]. While PFS was longer in patients receiving bevacizumab, there was a coinciding increase in adverse events such as thromboembolic events, neutropenia, worsening quality of life, and increased symptom burden [129]. Another phase III, randomized, double-blind placebo-controlled study elucidated similar findings as seen in previous literature, in which patients with newly diagnosed GB received bevacizumab compared to placebo following standard RT-TMZ [130]. PFS was found to be significantly increased in patients receiving bevacizumab compared to placebo, but no significant differences in overall survival emerged [130]. Several studies have examined the use of bevacizumab in combination with other agents [131–136]. The addition of lomustine with bevacizumab was evaluated but did not confer a significant survival advantage compared to lomustine alone in patients with progressive GB [137].
Other chemotherapies
In early trials of adjuvant chemotherapy with RT for astrocytoma treatment, one of the first chemotherapies to demonstrate a survival advantage was carmustine, or BCNU [138, 139]. Meta-analysis of early clinical trials evaluated the use of BCNU in combination with RT, showing a 10% increase in survival at 1 year [139]. Additional trials were conducted by comparing BCNU with a cocktail of cytotoxic agents known as PCV [140, 141]. When compared to BCNU, patients treated with PCV demonstrated significant increases in time to progression and overall survival [140, 141]. These drugs remain second-line with respect to TMZ due to limited tolerability. Other classes of cytotoxic chemotherapies, such as platins, phosphoramides, and topoisomerase inhibitors, have been studied in the context of treating primary and recurrent high-grade astrocytomas. These classes have demonstrated minimal or limited activity and poor efficacy, with various clinical guidelines suggesting the use of these compounds only after trials of RT with TMZ or PCV have failed [109, 142].
Radiation and chemotherapy treatment side effects
Despite survival benefits with multimodal management of astrocytomas, iatrogenic toxicities and adverse events do exist and afflict patients both short- and long-term. Among the various clinical trials cited in this review, RT-related toxicities and adverse events include but are not limited to infection, worsening neurologic status and late cognitive decline, and significantly worsening Karnofsky performance scores [115, 123, 143]. Related to the use of chemotherapies such as TMZ, toxicities and adverse events include, but are not limited to hematologic toxicities and myelosuppressive effects such as neutropenia, anemia, thrombocytopenia, and lymphopenia [115]. Other adverse effects include moderate-to-severe fatigue, deep vein thrombosis, pulmonary embolism, and severe pneumonia [115]. The use of bevacizumab in patients with GBs exhibited numerous toxicities such as hypertension, leukopenia, thromboembolic events, non-CNS, and CNS hemorrhage [144]. The combination of lomustine and bevacizumab demonstrated similar toxicities to other chemotherapies used to treat astrocytomas [145]. These toxicities include leukopenia, intracranial hemorrhage, serious thromboembolic events, hypertension, liver toxicity, and limited gastrointestinal side effects [145]. In patients that are candidates for re-irradiation due to recurrence, some studies have found rates of neurologic toxicity related to radiation necrosis ranging from 5–20% among patients following SRS, while others have found high rates of therapy tolerance and few adverse events [143, 146]. Overall, each treatment comes with its own unique risks and potential reduction in quality of life.
Surgical options
Resection extent
Resection of low-grade astrocytomas can be curative, but for higher-grade astrocytomas such as GBs, surgery serves a more palliative function [147–149]. In addition, EoR plays a critical role in the outcome and prognosis after surgery. Gross total resection (GTR) is a term that has generally come to mean the resection of an entire lesion mapped with T1 enhancement on MRI [150]. In contrast, subtotal resection (STR) is the partial ablation of a lesion that spares the margins of the T1 enhancement, as seen in Figure 3.
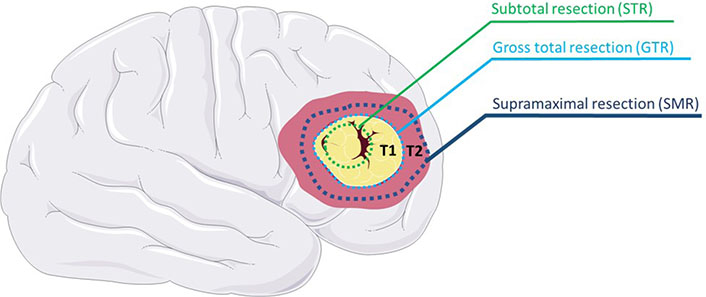
STR spares T1 MRI region margins, GTR includes the T1 margins, and SMR extends into the T2 MRI region
Note. This figure contains (modified) images from Servier Medical Art (https://smart.servier.com). CC BY.
A meta-analysis of IDH wild-type low-grade gliomas by Di Carlo et al. [151] demonstrated a significant inverse correlation between EoR and the hazard ratio of death. Similarly, a meta-analysis of low-grade gliomas by Brown et al. [152] showed that GTR compared with STR resulted in clinically and statistically significant reductions in relative risk of death and progression at the 2-, 5-, and 10-year mark. This suggests that GTR when compared to STR has the potential to improve both mortality and morbidity of patients with low-grade astrocytomas. Studies have shown that GTR provides similar benefits for spinal cord astrocytomas [153, 154]. However, given the delicate nature of the region, GTR is often not feasible for high-grade spinal cord astrocytomas [153, 154].
Recent research has focused on expanding the zone of ablation even further than the bounds of GTR in what is now called SMR, as seen in Figure 3 [155]. This expanded zone of resection is typically mapped using MRI T2 FLAIR instead of T1 [150, 156]. SMR may be more indicative of the extent of GB infiltration given that studies have demonstrated tissue samples beyond the T1 weighted MRI region comprise as much as 89% cancerous tissue [157–159]. A number of studies have suggested ideal average ranges of FLAIR resection ranging from 20–53% [160–163]. A more recent study by Tripathi et al. [164] suggests that the ideal SMR volume for GBs depends on the physiology of the tumor, with a range of 10–29% for nodular, 10–59% for moderately diffuse, and 30–90% for highly diffuse. One retrospective analysis of 102 patients with GBs showed a statistically significant 6.06-month median overall survival improvement in SMR when compared with GTR [165]. An improvement in overall survival and PFS for SMR compared with GTR has been repeatedly supported by a plethora of studies [150, 156, 166–168].
Neuro navigation
While the survival benefits of increased EoR are clear, advanced techniques and equipment are necessary to avoid collateral damage to functioning regions of the CNS when achieving GTR or SMR.
One such commonly utilized system is intraoperative navigation technology. Integrating pre-operative images and tract mapping [magnetoencephalography (MEG), DTI, and fMRI] as a reference point, optical or electromagnetic sensors can locate surgical equipment in real-time in relation to the patient’s brain viewed as a three-dimensional (3D) field [169, 170]. While this allows for more precise ablation, the benefit is often constrained by intraoperative brain shift due to edema, tumor mass reduction, and loss of colony stimulating factor (CSF) [169, 170]. To overcome the limitations introduced by brain shift, intraoperative imaging has been introduced. While intraoperative MRI (iMRI) offers advantages in image resolution it can significantly increase operation time [171]. By comparison, interoperative ultrasound is less costly and may have similar levels of specificity and sensitivity to iMRI, but may require a higher level of training and experience [172]. Another method of monitoring eloquent regions of the brain intraoperatively is through motor evoked potentials (MEPs). In MEP, the brain and primary motor cortex are stimulated intraoperatively to produce corresponding action potential in muscles [173]. Using this method, the motor function of the patient can be monitored and eloquent regions of the brain can be more readily avoided [171, 173].
Fluorescence
The two most utilized fluorophores for astrocytoma visualization are 5-aminolevulinic acid (5-ALA) and sodium fluorescein (SF). A porphyrin precursor, 5-ALA accumulates in cancer cells and is metabolized to protoporphyrin IX (PpIX), a fluorophore that is best visualized under a violet, 370–440 nm wavelength light [174, 175]. A systemic review and meta-analysis by Gandhi et al. [176] demonstrated that the addition of 5-ALA to high-grade glioma resections was associated with a 26% increase in GTR rates. SF functions by binding proteins within the blood which collect in regions of tumor-induced BBB perturbation [175]. In this way, SF allows for tumor visualization under 465–490 nm wavelength light [175, 177]. Naik et al. [178] performed a meta-analysis comparing various resection guiding modalities and found that iMRI combined with 5-ALA produced the highest rates of GTR followed by iMRI, SF, and 5-ALA alone. However, another recent meta-analysis by Smith et al. [175] suggested that SF produced a 29.5% increase in GTR which was functionally equivalent to 5-ALA when compared to surgeries without fluorescent guidance. Looking forward, the combined use of both fluorophores may offer some cumulative advantages and may be the focus of future research [179].
Awake surgery
While numerous studies have demonstrated the safety of awake craniotomy (AC) compared to general anesthesia (GA), whether AC offers a clear advantage over GA remains a matter of debate [180–182]. Some studies have suggested that AC increases the EoR or decreases hospital stay after surgery [180, 182–188]. Furthermore, a recent multicenter cohort study by Gerritsen et al. [189] examined surgical outcomes of 1,047 patients with GBs in eloquent regions. They found that AC was associated with significant reductions in postoperative neurological deficits, an increase in overall survival, and an increase in PFS when compared with GA [189]. Currently, a number of prospective studies designed to answer the question of AC vs. GA efficacy (including NCT03861299 and NCT04708171) are underway [190, 191].
Future treatment
With unique intrinsic factors and a privileged microenvironment, astrocytomas continue to remain an elusive target for new therapeutic options. One of the reasons it has been challenging to develop new treatments for astrocytomas is the need to penetrate the BBB [192]. The BBB consists of a highly selective semipermeable border of endothelial cells, through which most conventional drugs cannot pass [193]. The selectivity of the BBB is one of the reasons that TMZ remains the standard chemotherapeutic agent in the treatment of astrocytomas. TMZ is able to cross the BBB due to its small size and lipophilic properties [194]. Two recent developments have shown promise in facilitating transport through the BBB [195, 196]. One of these is convection-enhanced delivery (CED), a technique in which a pressure gradient is generated at the tip of an infusion catheter allowing therapeutics to be delivered through the interstitial space into the CNS [195]. Since the development of CED in the 1990s, there have been many advancements over the years. Although CED in its current state has limited anti-tumor effects for astrocytomas, it may show promise in the near future with many current clinical trials seeking to improve the technique [197, 198]. Another method to cross the BBB employed by Gregory et al. [196] is nanoparticle technology. Researchers engineered a synthetic protein nanoparticle (SPNP) based on human serum albumin along with internalizing arginine-glycine-aspartic acid (iRGD) for cell penetration. An in vitro study using this SPNP against signal transducer and activator of transcription 3 inhibitor (STAT3i) in mice found that tumor regression and long-term survival were increased in mice with GB [196]. STAT3i downregulates signal transducer and activator of transcription 3 (STAT3), which impairs anti-tumor immunity and enhances tumor progression [196, 199]. As such, this technology shows promise for the treatment of high-grade astrocytomas.
Another challenge in developing new therapies for astrocytomas is the tumor microenvironment. Astrocytomas invade the CNS, an immune-privileged site. This has been a significant obstacle in the development of effective immunotherapies. In recent years, immunotherapy treatments such as chimeric antigen receptor (CAR)-T cell therapy, monoclonal antibodies against programmed cell death protein 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have been approved by the FDA for the treatment of various cancers [200–202]. However, currently, there are no immunotherapy drugs approved by the FDA for the treatment of astrocytomas [203]. With current clinical trials that seek to improve the shortcomings of CAR-T cell, anti-CTLA-4, anti-PD-1, and anti-PD-L1 therapies in regard to the treatment of astrocytomas, these immunotherapy drugs may show promise in the future. Additional immune checkpoints that are being explored as potential targets for treatment are cluster of differentiation 47 (CD47) and CD73 [203]. CD47 blockade has shown to be effective against GB in vitro and in animal models, however, clinical trials have not yet been conducted on this immunotherapy target [204]. CD73 pathway targeting agents are now reaching clinical trials and may prove successful as a target for immunotherapy [205]. Other immunotherapy options that are being developed for the treatment of astrocytomas are vaccine therapies including dendritic cell-based vaccines, heat shock protein vaccines, peptide vaccines, and viral-based vaccines [192].
Astrocytomas often have high recurrence rates and may be resistant to chemotherapy and radiation therapy. One of the reasons behind this high recurrence rate may be glioma stem cells (GSCs) which allow for tumorigenicity and stem-cell renewal [203]. Additionally, different types of astrocytomas present with molecular heterogeneity, making it difficult to develop targeted therapeutics [206]. Despite this molecular heterogeneity, targeted therapies may show promise against specific astrocytomas. As discussed previously, some astrocytomas have been found to contain the BRAF V600E mutation, with the highest rate of the mutation found in pleomorphic xanthoastrocytomas [30]. Studies evaluating the use of BRAF V600E kinase inhibitors in various astrocytomas have had interesting results [207, 208]. A basket trial by Kaley et al. [207] looking at the BRAF V600E kinase inhibitor vemurafenib found that patients with pleomorphic xanthoastrocytomas responded best to the drug, while patients with other astrocytomas had variable response. Unfortunately, high-grade astrocytomas tend to have low rates of the BRAF V600E mutation, which may limit the application of vemurafenib and other BRAF V600E kinase inhibitors to a subgroup of astrocytomas [209]. An additional targeted therapeutic that shows potential against high-grade astrocytomas is Erdafitinib, a tyrosine kinase inhibitor of fibroblast growth factor receptor 1–4 (FGFR1–4) [208]. This drug is currently in phase II clinical trials (NCT04083976). Inhibitors of the mutated form of IDH may also prove beneficial against astrocytomas that display this mutation. Potential therapeutics against astrocytomas with mutant IDH1 include AG-5198 and ivosidenib. Additionally, enasidenib may prove to be a potential therapeutic against astrocytomas with mutant IDH2 [206].
Conclusions
Staging based on physical and genetic characteristics, imaging, RT, chemotherapy, surgical management, and targeted therapies all serve an integral function in the understanding and management of astrocytomas. Genetic mutations and a variety of imaging modalities are playing an increasingly more important role in the staging and consideration of treatment approaches for astrocytomas. With the ever-increasing interest in targeting the mechanism of each mutation that drives tumor formation, genetic characterization and targeted therapies for astrocytoma treatment will likely continue to develop in conjunction with surgical and radiation therapy. While not always improving the speed and efficiency of surgery, the newest surgical techniques may dramatically improve the field of resection and most importantly improve mortality and quality of life.
Abbreviations
| 5-ALA: | 5-aminolevulinic acid |
| AA: | anaplastic astrocytoma |
| AC: | awake craniotomy |
| ADC: | apparent diffusion coefficient |
| ASTRO: | American Society for Radiation Oncology |
| BBB: | blood-brain barrier |
| BCNU: | l,3-bis(2-chloroethyl)-1-nitrosourea |
| BRAF: | B-Raf proto-oncogene |
| CD47: | cluster of differentiation 47 |
| CDKN2A: | cyclin dependent kinase inhibitor 2A |
| CED: | convection-enhanced delivery |
| Cho/Cr: | choline to creatine |
| CI: | confidence interval |
| CNS: | central nervous system |
| DCE: | dynamic contrast enhancement |
| DWI: | diffusion-weighted imaging |
| EoR: | extent of resection |
| FDA: | Food and Drug Administration |
| FLAIR: | fluid-attenuated inversion recovery |
| fMRI: | functional magnetic resonance imaging |
| GA: | general anesthesia |
| GB: | glioblastoma |
| GTR: | gross total resection |
| IDH1: | isocitrate dehydrogenase 1 |
| iMRI: | intraoperative magnetic resonance imaging |
| ITSS: | intratumoral susceptibility score |
| Ktrans: | volume transfer coefficient |
| Lip-Lac/Cr: | lipid-lactate to creatine |
| MGMT: | O6-methylguanine-DNA methyltransferase |
| MRI: | magnetic resonance imaging |
| MRS: | magnetic resonance spectroscopy |
| NF1: | neurofibromatosis type 1 |
| PAs: | pilocytic astrocytomas |
| PCV: | procarbazine, lomustine, and vincristine |
| PFS: | progression-free survival |
| PWI: | perfusion-weighted imaging |
| rCBV: | relative cerebral blood volume |
| RT: | radiotherapy |
| SEGA: | subependymal giant cell astrocytoma |
| SF: | sodium fluorescein |
| SMR: | supramaximal resection |
| SRS: | stereotactic radiosurgery |
| STR: | subtotal resection |
| SWI: | susceptibility-weighted imaging |
| TMZ: | temozolomide |
| TP53: | tumor protein p53 |
| WBRT: | whole-brain radiotherapy |
Declarations
Author contributions
MW: Conceptualization, Writing—original draft, Writing—review & editing. JW: Writing—original draft, Writing—review & editing. JF: Writing—original draft. ED: Writing—original draft. SS: Writing—original draft. BO: Writing—original draft. KC: Writing—original draft. JH: Writing—original draft. BLW: Conceptualization, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.