Abstract
The patient was a 6-year-old child with spastic quadriplegic cerebral palsy (CP) categorized with the gross motor function classification system (GMFCS) as a level IV and a Modified Modified Ashworth Scale (MMAS) of 2 for the bilateral hamstring and hip adductor muscles, and 3 for the bilateral gastrocnemius muscles. This patient’s limited range of motion significantly affected the caregiver’s ability to perform activities of daily living (ADLs). Dry needling (DN) is considered a standard treatment (TX) when treating adults with poor range of motion. This article aims to place intramuscular electrical stimulation (IMES), the delivery of an electrical current through a monofilament needle into targeted trigger points (TrPs) within the context of treating children with spastic CP. Following IMES TXs over 32 months that totaled 12 left hamstring TXs, 13 right hamstring TXs, 13 hip adductor TXs, 21 left gastrocnemius TXs, and 18 right gastrocnemius TXs, the patient demonstrated an increase in passive range of motion (PROM) of the hamstring, hip adductors, and gastrocnemius muscles. These gains equated to ease in ADLs. Both the Pediatric Evaluation of Disability Inventory (PEDI, PEDI-Caregiver Assistance Scale) and the Goal Attainment Scale (GAS) demonstrated decreased caregiver burden. The child’s GMFCS level and the MMAS did not change. Further data collection related to treating children with spasticity using IMES is indicated to validate this type of TX with this patient population.
Keywords
Spasticity, cerebral palsy, dry needling, electrical stimulation, case reportIntroduction
Cerebral palsy (CP) is the most common cause of spasticity, defined as muscle overactivity, or an excessive or sustained involuntary muscle contraction. Spasticity is the result of an upper motor neuron injury. Injury to the upper motor neuron reduces descending reticulospinal and corticospinal tract function, which affects inhibitory pathways leading to muscle hyperactivity [1]. Cerebral palsy results from brain damage yet symptoms of the condition manifest themselves in muscles [2]. Spasticity corresponds to a structural change in the muscle as muscle actin and myosin filaments overlap decreasing muscle sarcomere length [3]. The changes in muscle may occur at the protein, single-fiber, and whole-muscle levels, and include a decreased mitochondrial volume fraction, a reduction in muscle fiber length, and a decrease in the number of serial sarcomeres. It is conceivable that at least some of the increased muscle tone in subjects with spasticity could be caused by structural changes in the musculature [4].
Prolonged contractures lead to decreased passive range of motion (PROM) and the formation of latent trigger points (TrPs), defined as hyperirritable spots that are painful upon stimulation. Latent TrPs may develop into active TrPs and become spontaneously painful. TrPs affect overall muscle function and further restrict PROM [5]. TrPs are associated with an excess release of acetylcholine (ACh) into the neuromuscular junction, inhibition of acetylcholinesterase, and an increase in the number and sensitivity of ACh receptors [6], which is particularly problematic for those with spasticity. Children with CP already have abnormal neuromuscular junctions with extra junctional ACh receptors [7]. Furthermore, spastic movement patterns constitute repetitive muscle loading which is a risk factor for the development of TrPs [5, 8, 9].
The combination of decreased PROM and hypertonicity affects a child’s ability to move and negatively impacts a caregiver’s ability to perform activities of daily living (ADLs), including bathing, dressing, diaper changing, carrying, and stand pivot transferring a child [1, 10]. Knee and hip PROM are important for easily abducting and extending a child’s lower extremities (LEs) for carrying, bathing, dressing, and diapering. Hip and knee extension and ankle dorsiflexion are important for LE weight bearing, allowing for greater child participation during assisted stand pivot transfers.
Physical therapists (PTs) play a key role in the rehabilitation and habilitation of children with CP [11]. Though there is no standardized PT approach to treating children with CP [1], it is considered best practice to develop a comprehensive treatment (TX) plan that includes increasing PROM, maximizing muscle strength, improving motor control, and teaching home exercise programs [11]. Ultimately, goals focus on improving a child’s function while teaching the caregiver how to assist the child. Decreasing caregiver burden [12] is especially important when treating children classified as level IV and V on the gross motor function classification system (GMFCS) [13]. A child with CP categorized as a level IV and V on the GMFCS requires much assistance with ADLs [13]. Caregiver burden is defined as the caregiver’s perceived subjective and objective evaluation of the difficulty performing ADLs [14, 15].
Managing spasticity is a major focus when treating children with CP [1]. Research validates that dry needling (DN) decreases spasticity and muscle stiffness and increases muscle extensibility and PROM [5]. Though PTs currently use DN to treat patients with spasticity related to stroke [3, 14, 16–18] and spinal cord injury [19], to the best of our knowledge, there is only one study describing the use of DN to treat a child with spasticity. Gallego and del Moral [20] studied resistance to PROM in a child’s upper extremities and reported that TX with DN decreased resistance to PROM.
For the same reasons why DN is a valid TX option for adults with spasticity, it is also a valid TX for children with spastic CP. DN has an immediate positive impact on temporarily reducing spasticity and increasing PROM by lowering ACh levels in neuromuscular junctions [3, 16, 18, 21]. Stopping the cycle of muscle hyperactivity caused by elevated ACh levels is key to successfully treating patients with spasticity. Research shows that DN changes the biochemical makeup of TrPs by increasing local blood flow and oxygenation, and by elevating low pH [9, 22]. Low pH levels are associated with hypoxia and may cause other problems, including vasoconstriction and the inhibition of acetylcholinesterase, the enzyme that breaks down ACh leading to decreased spasticity [5].
Transcutaneous electrical nerve stimulation (TENS), the application of surface electrodes on affected muscles, decreases neuron excitability and is also used to decrease spasticity in CP [10, 23, 24]. TENS increases PROM [23] and improves localized oxygenation [25] and muscle blood research demonstrates that patients with spastic CP benefit from TENS to our knowledge, only one study combined DN with surface electrode electrical stimulation (ES) to treat a stroke patient [23]. The authors did not identify any intramuscular ES (IMES), research combining DN with ES administered through a monofilament needle into targeted TrPs to treat a patient with spasticity. One case report used IMES to treat a patient with low back pain [26].
This case report investigated the effects of IMES on LE PROM in a child with spastic CP. Using IMES to treat children with spastic CP is a new TX model.
Case report
Patient information
The patient is a 6-year-old child with spastic quadriplegic CP categorized with the GMFCS as a level IV and a Modified Modified Ashworth Scale (MMAS) of 2 for the bilateral hamstring muscles and adductor muscles, and 3 for the bilateral gastrocnemius muscles [13, 27–32, 33].
She was born at 24 weeks gestation, with an intraventricular hemorrhage, hemorrhagic hydrocephalus, and seizures. The patient received a ventricular-peroneal shunt placed, and was diagnosed with spastic CP, diplegia, and superimposed right hemiplegia affecting her right upper extremity (UE).
Clinical findings
The patient was dependent on her caregivers for all ADLs. The patient’s receptive language skills were excellent, and she verbalized basic thoughts and feelings. She commando crawled forward with effort and sat briefly in both rings sitting and in a chair without back support, with her hips and ankles positioned at 90 degrees. She took high marching short steps with a scissoring gait pattern when pushed forward in her gait trainer. She rode an adapted trike when assisted to propel forward.
Timeline
This patient was seen over 32 months in the home setting for weekly physical therapy services that were modified to twice-monthly interventions as the patient’s and family’s needs decreased. When the patient or caregiver complained of muscle tightness that caused discomfort or impacted ADLs, TXs included IMES.
The caregiver completed the Goal Attainment Scale (GAS) before and after each IMES session and the Pediatric Evaluation of Disability Inventory (PEDI) once every 6 months [33, 34]. Spasticity and PROM were assessed before and immediately after each IMES session using the same pre- and post-TX measurements.
Diagnostic assessment
PROM
PROM measurements were always taken in the same positions, using a digital goniometer. Measurements were taken in supine according to the following standards:
To measure knee extension (hamstring PROM), the PT placed the patient in supine, extended the patient’s hip and knee, applied pressure to the anterior thigh in the anterior-posterior direction, and measured the degree of knee extension on the right and left.
To measure hip abduction (adductor PROM), the PT placed the patient in hook lying (supine with knees flexed and feet flat on the floor), positioned the goniometer head on the umbilicus, moved the legs into abduction, applying overpressure, and measured maximum hip abduction PROM.
To measure ankle dorsiflexion (gastrocnemius PROM), the PT placed the patient in supine, extended the patient’s hip and knee, and then moved each foot into dorsiflexion and measured end-range dorsiflexion.
PEDI
The PEDI is a valid and comprehensive clinical assessment for children 6 months to 7.5 years [33]. The PEDI is an interview-based assessment tool that measures self-care, mobility, and social function in two formats: functional skill performance and caregiver assistance.
To determine caregiver burden defined as the effort required by the caregiver to perform self-care, mobility, and social skills [33] the PEDI’s Caregiver Assistance Scale was completed. The Caregiver Assistance Scale measures perceived assistance provided during self-care, mobility, and social skills. The PT interviewed the patient’s primary caregiver, and the primary caregiver reported her perceived level of caregiver assistance needed to complete self-care, mobility, and social skills.
The PEDI’s Caregiver Assistance Assessment is scored on a 5-point scale. A child is scored 0 when caregivers perceive that they do almost all the activity. Levels 1–3 note that the caregiver assists from a maximum to a minimum amount, respectively. Level 4 requires verbal cues and caregiver setup, whereas level 5 denotes perceived independence.
GAS
The GAS is an outcome-based measurement of individually identified goals [34]. Caregivers or patients set goals and the therapist assesses goal attainment using the GAS. Though the GAS allows patients to develop personalized outcome measures, goal scoring is standardized. Goals are rated by the caregiver or patient using a 5-point scale.
This patient’s caregiver chose to focus on three ADL goals and rated the ease of performing these goals after each IMES TX session, and also rated the length of TX benefit at the following visit. The goals were as follows:
The child assists with stand pivot transfers by bearing weight through LEs, requiring moderate rather than maximum caregiver assistance.
Caregiver easily abducts the child’s LEs within seconds vs. minutes to allow for quick diaper changes.
The caregiver easily abducts the child’ LEs to place the child on her (caregiver’s) back to carry the child, legs remain open and relaxed, so the legs do not squeeze and hurt the caregiver.
The caregiver rated the child’s performance and when the child achieved the expected progress, the caregiver reported a score of 0. When the caregiver perceived the child performed better than expected, the rated score was +1, and much better was +2. Likewise, when the caregiver perceived that the child performed worse than expected, the caregiver reported a score of –1, less than expected, or –2, much less than expected.
Functional goals
In addition to recording the above outcome measures, the PT also set functional goals based on the patient’s and caregiver’s needs and desires.
The patient will prop sit at the edge of the bed for at least 10 s without assistance.
The patient will ring and sit for at least 3 min without assistance.
The patient will commando crawl forward up to 5 feet without assistance.
The patient will propel an adapted trike at least 50 feet without assistance, on an outdoor level surface.
The patient will walk 5 feet by herself in the gait trainer with ankle prompts.
GMFCS
The GMFCS is a valid and reliable classification system for children with CP [13]. Level I describes the highest functional level, meaning the child walks without issues. The child classified as a level II walks in most settings but may experience difficulty walking long distances. A level III classification defines children that walk with a hand-held mobility device and use wheeled mobility for long distances. Children classified as level IV walk short distances with assistance and require a wheelchair. Level V describes the fully dependent child with limited head and trunk control. The level V child is wheelchair-dependent.
MMAS
The degree of hamstring, hip adductor, and gastrocnemius muscle spasticity was determined using the MMAS, which is a valid and reliable test for spasticity measurements [27–32].
The MMAS grades the intensity of spasticity from 0–4 through a rapid passive stretch test. Zero indicates no increase in muscle tone or resistance to movement and four indicates that the affected joint is rigid in flexion and extension [16, 27, 28]. Respectfully, grades 1–3 note slight, marked, and a considerable increase in muscle tone. The MMAS showed excellent reliability for the assessment of LE muscle spasticity in children with CP [32].
To measure gastrocnemius and hip adductor spasticity, the PT followed guidelines set by Ghotbi et al. [31]. To measure gastrocnemius spasticity, the patient was placed supine, with the head in midline, arms alongside the trunk, and LEs extended. The PT placed one hand under the ball of the foot to be evaluated, while the other hand stabilized the ankle joint. The PT moved the ankle from maximum plantarflexion into maximum dorsiflexion.
To measure hip adductor spasticity, the patient was kept supine with the head in midline. The PT placed the patient on a hook lying, rather than in supine with LE extension. The PT placed one hand on the medial surface of one knee and the other hand on the medial surface of the other knee. The PT measured maximum hip abduction.
To measure hamstring spasticity, the PT followed the guidelines set by Khalifeloo et al. [16], that being to position the patient in prone, hips extended, with a pillow placed under the patient’s chest. The knee being evaluated was flexed and the PT stabilized the patient’s hip on the testing side before passively moving the knee from full flexion to full extension.
During the above tests, the patient was instructed to relax. The joints were moved into the maximum position with a fast velocity stretch over 1 s, counted as one thousand-and-one. The passive movement was repeated three times at all joints [31].
Therapeutic intervention
IMES intervention
At the onset of each TX when the patient or caregiver subjectively reported muscle tightness, the PT assessed spasticity, and PROM, and palpated the hamstrings, hip adductors, and gastrocnemius muscles for TrPs. Latent TrPs were identified and treated according to the standard practice of care. This patient did not present with active TrPs.
Identified latent TrPs were treated with IMES in a submaximal stretched position [35]. To palpate and treat the hamstrings, the PT placed the patient in prone with the LEs extended to a comfortable position. To palpate and treat the hip adductors, the PT placed the patient in a hook lying with her LEs abducted to a comfortable position. To palpate and treat the gastrocnemius, the PT placed the patient in prone and positioned the ankle in dorsiflexion to a comfortable degree.
DN procedure: The needle was inserted into the spastic taut band where the PT palpated the latent TrP. Needle insertion was slow. Local twitch responses (LTRs) were not sought given the current debate in the literature as to whether DN and the commonly used pistoning technique, whereby the needle is moved in an up-and-down motion, may cause post-TX muscle soreness [36–38]. Likewise, eliciting LTR may be uncomfortable [39]. Finally, whether obtaining an LTR is necessary to obtain positive outcomes is under debate [37, 39, 40]. Considering these issues, seeking an LTR was deemed contraindicated to functional goal attainment for fear the patient may refuse the IMES TX if she experienced discomfort during or post-TX.
Detailing the exact needle insertion points is challenging as points were chosen through TrP palpation without association with acupuncture points. Once TrPs were located, the TrP was bracketed, tissues compressed, and the needle inserted perpendicular. Or the PT grasped the affected muscle group and threaded the needle through the group, horizontal to the bone. In general, TrPs were palpated in the middle of the belly of the gracilis and adductor magnus muscles. Hamstring points were generally palpated proximal to the muscle belly of the biceps femoris, semimembranosus, and semitendinosis muscles. Gastrocnemius TrPs were typically palpated in the bellies of the medial and lateral gastrocnemius.
Once the needle was inserted into a TrP, IMES was administered with a Pointer Excel II LT™ Stimulator (Lhasa OMS, Weymouth, MA, USA). A metal-to-metal technique was used by placing the grounding pole (bar) on a needle while placing the metal pointer of the stimulator on a different needle within the same muscle, or single-needle stimulation was used with the bar grounded on the skin. The Excel II LT™ Stimulator has set parameters including, one output channel, a pulse width of 260 microseconds (MS), an asymmetric biphasic square wave, and a continuous pulse mode. The PT chose positive polarity to increase patient comfort [41] and a frequency of 10 Hz (1–16 Hz options) to fall as closely in line with low-frequency TENS parameters considering the fixed pulse width [42].
Muscles were stimulated from 30 s to 90 s. Since no guidelines are available for optimal TX times for IMES when treating spasticity [26], the PT prioritized observable tissue change before removing the needle, which, on average, took between 30 s and 90 s. The stimulation intensity progressed until a short duration or sustained contraction was obtained. Muscle contractions were preferred to maximize blood flow [43]. However, patient comfort was prioritized and when the patient reported the stimulation was strong, stimulation was not increased any further. When the patient reported the stimulation was “too strong” the PT decreased the stimulation intensity to tolerance. As soon as the needle was removed from the skin, the site was firmly compressed for at least 3 s. Spasticity and PROM were re-assessed immediately after IMES intervention.
The IMES TX was followed by other therapeutic interventions related to functional goal attainment, including PROM exercises, positioning in developmental positions to gain range and strength, sitting balance exercises, facilitation of static standing, stand pivot transfer training, gait training, and trike riding.
Outcomes
The number of IMES TXs over 32 months totaled 12 left hamstring TXs, 13 right hamstring TXs, 13 hip adductor TXs, 21 left gastrocnemius TXs, and 18 right gastrocnemius TXs. The PT completed 75 sessions in total during these 32 months.
The patient demonstrated an increase in PROM of the bilateral hamstrings, adductors, and gastrocnemius muscles treated (Tables 1 and 2; Figures 1–3). The PEDI’s Caregiver Assistance Scale demonstrated decreased caregiver burden (Table 3), and the caregiver reported ease in ADLs noted by the improved GAS score (Table 4). Noteworthy was the caregiver’s report that the ease of ADLs lasted approximately 2 weeks. The patient achieved five out of six functional goals (Table 5). Finally, the patient’s GMFCS level did not change pre- and post-TX and remained a level IV. Likewise, the patient’s MMAS scores did not change pre- and post-TX and remained 2 in bilateral hamstring muscles and adductor muscles and 3 in bilateral gastrocnemius.
PROM average increase after each IMES TX
| Muscle tested | Right hamstring | Left hamstring | Combined hip abduction | Right gastrocnemius | Left gastrocnemius |
|---|---|---|---|---|---|
| Average degrees of increased PROM | 6.55° | 4.43° | 11.92° | 10.74° | 8.02° |
Trend of PROM gain in degrees following IMES TX
| Muscle treated | PROM gain in degrees following IMES TX | ||
|---|---|---|---|
| Hamstring PROM gains: measured each LE in supine | Sequential order of sessions | Right hamstring degrees gained | Left hamstring degrees gained |
| Session 1 | 4.5° | 7° | |
| Session 2 | 4.6° | 11.3° | |
| Session 3 | 1.4° | 2.4° | |
| Session 4 | 8.8° | -- | |
| Session 5 | 6.7° | 10.8° | |
| Session 6 | 10.8° | 3.2° | |
| Session 7 | 1.2° | 7.1° | |
| Session 8 | 9.7° | 11.3° | |
| Session 9 | 9.6° | -- | |
| Session 10 | 5.4° | 8.9° | |
| Session 11 | 2.8° | 1.8° | |
| Session 12 | -- | 13 | |
| Session 13 | 9.4° | 2.2° | |
| Session 14 | 4.7° | 4.1° | |
| Number of TXs: | 13 TXs | 12 TXs | |
| Hip adductor PROM gains: measured right and left LE range with a combined stretch, in hook lying | Sequential order of sessions | Combined adductor degrees gained | |
| Session 1 | 17.9° | ||
| Session 2 | 5.7° | ||
| Session 3 | 3° | ||
| Session 4 | 3° | ||
| Session 5 | 8° | ||
| Session 6 | 4.7° | ||
| Session 7 | 9° | ||
| Session 8 | 12.2° | ||
| Session 9 | 24.1° | ||
| Session 10 | 13° | ||
| Session 11 | 26° | ||
| Session 12 | 23.4° | ||
| Session 13 | 5° | ||
| Number of TXs: | 13 TXs | ||
| Gastrocnemius PROM gains: measured each LE in supine | Sequential order of sessions | Right gastrocnemius degrees gained | Left gastrocnemius degrees gained |
| Session 1 | 24° | 10.6° | |
| Session 2 | 9.5° | 10° | |
| Session 3 | 12.9° | .7° | |
| Session 4 | 1.9° | -- | |
| Session 5 | 2° | 9.7° | |
| Session 6 | 11.7° | 4.5° | |
| Session 7 | 6.8° | 15.2° | |
| Session 8 | -- | 7.5° | |
| Session 9 | 3.6° | 20.6° | |
| Session 10 | 15.8° | 2° | |
| Session 11 | 4.9° | 5° | |
| Session 12 | 10.1° | 21.4° | |
| Session 13 | -- | 4.5° | |
| Session 14 | -- | 1.9° | |
| Session 15 | -- | 17.4° | |
| Session 16 | 5° | 11.3° | |
| Session 17 | 6.5° | 2.4° | |
| Session 18 | 12.3° | 10.1° | |
| Session 19 | 25.5° | 2.8° | |
| Session 20 | -- | 6.7° | |
| Session 21 | 16.8° | -- | |
| Session 22 | 7.1° | 1.2° | |
| Session 23 | 17° | 3° | |
| Number of TXs: | 18 TXs | 21 TXs | |
The list of DN sessions is sequential though TXs were not performed in specific intervals, rather IMES was utilized to address patient or parent complaints of tightness
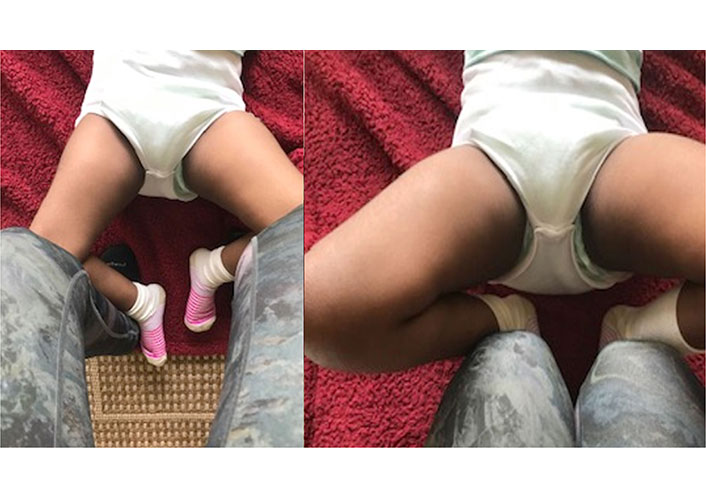
Left image was taken pre-IMES TX and the right image was taken post-IMES TX to the adductor muscles

Left image was taken pre-IMES TX and the right image was taken post-IMES TX to the gastrocnemius muscles

Left image was taken pre-IMES TX and the right image was taken post-IMES TX to the hamstring and gastrocnemius muscles
PEDI: initial evaluation compared to the final assessment (TX after 32 months): scaled scores
| Performance domains | Self-care | Mobility | Social function |
|---|---|---|---|
| Pre assessment score | 44.4 | 45.8 | 67.6 |
| Post assessment score | 51.1 | 49.8 | 72.5 |
Scaled scores range from 0–100. Scores do not compare a child to peers, rather scores describe where the child’s performance falls relative to the maximum possible scaled score: low score = low capability, high score = high capability
GAS
| Caregiver driven goals | Goal 1: stand pivot transfer goal | Goal 2: diaper changing goal | Goal 3: carrying goal |
|---|---|---|---|
| Initial evaluation score | –2 | –2 | –2 |
| Score post each TX | 0 | 0 | 0 |
-2: baseline score taken at the initial evaluation (described as much less than the expected ability to assist with ADLs); 0: expected level of ease completing ADLs after each TX session. Caregiver-driven goals defined: goal 1: patient has sufficient LE PROM to extend legs toward the floor to bear weight and help withstand pivot transfers; goal 2: patient’s legs are relaxed and have sufficient PROM so the caregiver can easily open the legs during diapers changes; goal 3: patient’s legs are relaxed and have sufficient PROM so the caregiver can lift the patient, open the patient’s legs, and maneuver the patient onto the caregiver’s back so the caregiver can carry the patient
Functional goals
| Functional goals | Objective measurement |
|---|---|
| Achieved functional goals |
|
| Functional goal not achieved |
|
Discussion
In this case report, IMES improved the PROM of the hamstrings, hip adductors, and gastrocnemius muscles. Though spasticity results from an upper motor neuron lesion [1], involuntary contractions lead to structural changes in the muscle including a decrease in muscle fiber length [4]. By elevating low pH levels [9, 22], DN changes the biochemical makeup in the area of a TrP. This change includes disinhibiting the release of acetylcholinesterase thereby decreasing muscle fiber depolarization [5] and the vicious cycle of muscle hyperactivity. Furthermore, DN improves neuromuscular junction synapse function [44] which may help to decrease spasticity.
Increased PROM decreased caregiver burden by immediately decreasing resistance to PROM. Therefore, the caregiver was more easily able to complete diaper changing, carrying, and stand pivot transferring the child. Per the parent report, PROM increases lasted approximately two weeks.
The patient’s GMFCS and MMAS scores did not change. Though many studies demonstrated decreased muscle spasticity after DN [3, 10, 14, 16–20, 23, 24], in a study by Mendigutia-Gómez et al. [45] spasticity did not decrease. There are many plausible explanations as to why this patient’s MMAS scores did not change after IMES. The rapid velocity MMAS test may have been alarming to the patient, whereby she could not help but resist the movement. This patient also loved to talk, and all verbal expressions were accompanied by motor overflow which affected the ability to relax during testing. It is also conceivable that the MMAS is not the most sensitive tool for every case since the MMAS does not recognize that hypertonia is a combination of spasticity resulting from upper motor neuron lesions and local changes related to altered muscle viscoelastic properties. The MMAS does not distinguish between these different components of hypertonia [20, 45]. Though this patient’s spasticity did not change the viscoelastic properties of her muscles did improve, as demonstrated by greater PROM when she was stretched slowly. Another hypothesis might be that since DN changes synaptic transmission, needle insertion may decrease spinal reflex excitability rather than spasticity [45]. Finally, the only study the authors can directly compare results with is a case report written by Gallego and del Moral [20], who evaluated the effectiveness of DN on a patient with CP. They found that UE spasticity decreased following DN. Since it is not known whether weight-bearing factors affect LE responses to DN in children with CP [20], further investigation is indicated to understand why MMAS scores improved with UE DN TXs, but not with LE IMES TXs.
Encouraging information from a recent case report found that DN improved the brain activity of a stroke patient with right UE spasticity and an MMAS score of 1. This patient’s post-TX functional magnetic imaging (fMRI) revealed improvements in the activation of the sensory and motor areas in the affected hemisphere, and activation and intensity in the primary motor cortex of the unaffected hemisphere [17]. Further research is recommended to determine if treating children with CP and DN improves brain activity.
Although the efficacy of treating children with spasticity using IMES requires additional data, this case report demonstrates that this TX has the potential to be a valid alternative TX option for some children with spasticity. Traditional TX options include both non-invasive and invasive means of lengthening tight muscles associated with spasticity. Traditional non-invasive therapy includes PROM exercises, active assisted range of motion exercises, positioning, and casting, among others [1, 11], but these do not always produce immediate results [1]. Traditional invasive therapies include botulinum-A toxin injections, oral or intrathecal baclofen, tendon lengthening surgery, and selective dorsal rhizotomy surgery [1], but these have inherent risks. Botulinum toxin-A injections decrease contractile elements causing muscle weakness and atrophy [46], a child may have an adverse reaction to baclofen [47], and undergoing orthopedic and dorsal rhizotomy surgery has specific risks [48]. On the other hand, the risks of DN are minimal when targeting LE muscles and the results are immediate [3, 5, 14, 16, 18–20, 26, 35, 49].
Further research is recommended to validate using IMES to treat children with spastic CP. In this case, the results of each TX, subjectivity reported by the patient’s caregiver, lasted approximately two weeks. One study described a decrease in endplate noise for two weeks following latent TrP DN TX [21]. This study explored DN TX in latent TrP sites and non-TrP regions in model rats. Further research related to DN’s effect on endplate noise in spastic muscles is indicated. More research is also needed to establish the optimal needle insertion TX time with IMES, ADL changes following IMES TX, and the age groups of children with spastic CP who could benefit from IMES. Research comparing PROM gains attained through an IMES program vs. a standard stretching program, a stretching program with only DN, and a stretching program with only TENS are indicated. Finally, research is recommended to understand whether DN with or without ES improves the brain activity of a child with spastic CP and whether DN changes muscle spasticity.
Two other important points to consider related to choosing IMES for children with spastic CP are whether IMES could be an adjunct or replacement to botulinum toxin-A injections [13, 14] and whether treating latent TrPs may prevent the development of active TrPs common to patients with spastic CP. In conclusion, the authors propose that IMES may increase the PROM of children with spastic CP thereby decreasing caregiver burden during assisted ADLs.
This case report provides an initial framework for treating children with diplegic CP using IMES. To the best of our knowledge, there are no studies investigating the use of IMES in children with spastic CP, however, treating patients with spasticity using DN is well supported in the literature, as is treating spasticity with ES. Further investigation related to treating children with spasticity using IMES is required.
Abbreviations
| ACh: | acetylcholine |
| ADLs: | activities of daily living |
| CP: | cerebral palsy |
| DN: | dry needling |
| ES: | electrical stimulation |
| GAS: | Goal Attainment Scale |
| GMFCS: | gross motor function classification system |
| IMES: | intramuscular electrical stimulation |
| LEs: | lower extremities |
| LTRs: | local twitch responses |
| MMAS: | Modified Modified Ashworth Scale |
| PEDI: | Pediatric Evaluation of Disability Inventory |
| PROM: | passive range of motion |
| PTs: | physical therapists |
| TENS: | transcutaneous electrical nerve stimulation |
| TrPs: | trigger points |
| TX: | treatment |
| UE: | upper extremity |
Declarations
Acknowledgments
The authors thank SM and her family for their unwavering cooperation.
Author contributions
TO: Conceptualization, Investigation, Methodology, Project Administration, Validation, Visualization, Writing—original draft, Writing—review & editing. JD: Resources, Writing—original draft, Writing—review & editing. Both authors read and approved the final manuscript that was submitted to Exploration of Neuroprotective Therapy.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Human Research Protection Office of the University of Maryland, Baltimore Campus reviewed the study, Dry needling with electrical stimulation for the treatment of a pediatric patient with spastic cerebral palsy: a case report, and determined that the project met the definition of Not Human Subjects Research (NHSR). Institutional Review Board (IRB) oversight was therefore not required.
Consent to participate
Parent of under 16-year-old child signed the consent to participate-attached.
Consent to publication
The informed consent to publication was obtained from the parent of the patient.
Availability of data and materials
ROM data is available at the web address https://1drv.ms/w/s!AnsjmUSrka7QgYAE_WX-SyrxhK5xbA?e=PPaoWR
Funding
Not applicable.
Copyright
© The Author(s) 2022.