Affiliation:
1Nuerology Service, Hospital Central “Dr. Ignacio Morones Prieto”, San Luis Potosi 78290, Mexico
ORCID: https://orcid.org/0000-0001-8809-0714
Affiliation:
1Nuerology Service, Hospital Central “Dr. Ignacio Morones Prieto”, San Luis Potosi 78290, Mexico
ORCID: https://orcid.org/0000-0002-0185-509X
Affiliation:
2Hospital Central “Dr. Ignacio Morones Prieto” and Medicine Faculty of Universidad Autónoma de San Luis Potosí, Av. Venustiano Carranza, Zona Universitaria, San Luis Potosi 78290, Mexico
ORCID: https://orcid.org/0000-0001-8285-383X
Affiliation:
1Nuerology Service, Hospital Central “Dr. Ignacio Morones Prieto”, San Luis Potosi 78290, Mexico
Affiliation:
2Hospital Central “Dr. Ignacio Morones Prieto” and Medicine Faculty of Universidad Autónoma de San Luis Potosí, Av. Venustiano Carranza, Zona Universitaria, San Luis Potosi 78290, Mexico
ORCID: https://orcid.org/0000-0003-4131-093X
Affiliation:
1Nuerology Service, Hospital Central “Dr. Ignacio Morones Prieto”, San Luis Potosi 78290, Mexico
2Hospital Central “Dr. Ignacio Morones Prieto” and Medicine Faculty of Universidad Autónoma de San Luis Potosí, Av. Venustiano Carranza, Zona Universitaria, San Luis Potosi 78290, Mexico
Email: Ildefonso.rodriguez@uaslp.mx
ORCID: https://orcid.org/0000-0002-3316-1471
Explor Neuroprot Ther. 2023;3:131–138 DOI: https://doi.org/10.37349/ent.2023.00042
Received: October 18, 2022 Accepted: February 06, 2023 Published: April 28, 2023
Academic Editor: Noureddin Nakhostin Ansari, Tehran University of Medical Sciences, Iran
Aim: To compare the efficacy of memantine with that of valproate as a prophylactic treatment for episodic migraine within three months. The efficacy, safety, and response rate were evaluated.
Methods: Prospective, randomized, double-blind, controlled clinical trial randomized participants were divided into two groups. The memantine group received memantine 10 mg twice daily, and the valproate group received valproate 500 mg twice daily.
Results: Thirty-three patients participated in the study; 27 completed the treatment protocol, 14 in the memantine group, and 13 in the valproate group. The mean number of migraine attacks per month in the memantine group was 5.31 [standard deviation (SD) ± 1.54] initially and 0.93 (SD ± 1.49) at the end of treatment, noting a decrease of 4.21 (SD ± 1.76; P < 0.001). In the valproate group, the mean number of migraine attacks per month was 5.35 (SD ± 1.11) initially and 0.77 (SD ± 1.16) at the end of treatment, with a difference of 4.5 (SD ± 1.39; P < 0.001). All 27 patients had excellent response rates. Adverse effects were infrequent and mild in severity.
Conclusions: A clinical trial compared the efficacy of memantine with that of valproate (first-line drug) as a prophylactic treatment. A significant reduction in attacks was noted in both drugs. Memantine could be a new preventive treatment option for migraine (ClinicalTrials.gov identifier: NCT04698525).
Migraine is one of the three most debilitating diseases worldwide [1]. Its 1-year prevalence is approximately 15–18% in women and 6% in men, thus creating a sizeable socioeconomic problem [2].
Prophylactic treatment aims to decrease the frequency, duration, and severity of migraine attacks [3]. The first-line, evidence-based drugs for migraine treatment with established efficacy are sodium valproate, topiramate, propranolol, and metoprolol [4]. However, oral preventive medications showed adherence rates of 26–29% at 6 months, which diminished to 17–20% at 12 months. There are new preventive therapies for migraine, such as calcitonin gene-related peptide (CGRP) monoclonal antibodies, administered subcutaneously or intravenously on a monthly to quarterly basis. However, their cost is significantly higher than that of oral therapies, and unfortunately, public health systems in developing countries need access to them [5, 6].
One of the first evidence points to use the memantine was the role of glutamate in cortical spreading depression, an extreme depolarization of neuronal membrane that results in the release of this neurotransmitter, and a transient increase of this followed by a decrease in cerebral blood flow [7]. Levels of glutamate are also increased in the trigeminocervical complex after stimulation of dural structures. Other studies have found high levels of glutamate in the blood of patients with migraine and cerebrospinal fluid in patients with chronic migraine [8]. Memantine is a moderate-affinity uncompetitive N-methyl-D-aspartatic acid (NMDA) receptor antagonist [8, 9]. Charles et al. [10] reported a series of 71 patients with refractory migraine who received memantine as a preventive treatment for two months, 54 of whom reported a 50% reduction in headache frequency and proposed it as an excellent therapeutic possibility given the safety of memantine as a preventive treatment for refractory migraine. Noruzzadeh et al. [11] conducted, to our knowledge, the first randomized, double-blind, placebo-controlled clinical trial aimed at evaluating the efficacy of memantine as a prophylactic treatment for migraine with aura. Patients in the memantine group showed a significant reduction in the monthly frequency of attacks than those in the placebo group [11].
Our study aimed to evaluate the efficacy and safety of memantine and compare it with that of sodium valproate in the prophylactic treatment of migraine. Considering comparing a different therapeutic possibility with a standard one.
This study was conducted under the hypothesis that “the frequency of migraine attacks under prophylactic treatment with memantine is equal to or lower than that observed with sodium valproate for three months in adult patients with episodic migraine”.
Randomized, double-blind, controlled pilot trial. It was conducted at the Neurology Service, Central Hospital “Dr. Ignacio Morones Prieto” at San Luis Potosi, Mexico, from July 2019 to October 2020. The study was presented to be approved by the research and ethics committees of Hospital Central. It was also registered in the National Institutes of Health Clinical Trials of the United States (registration No. 74-19 in the HC and NCT04698525 for clinical trials).
Inclusion criteria: adults aged 18–65 years with the antecedent of episodic migraine with or without aura in the least 12 months that met the requirements specified by the International Classification of Headache Disorders, 3rd edition (beta version) [12]. Besides, patients were required to have four or more migraine attacks and less than fifteen days per month in the last three months before the study; patients were not under prophylactic treatment, and to be considered, they needed to sign an informed consent.
Participants were automatically excluded from the study if they were pregnant or lactating, had ≥ 15 headache days per month during the last three months before the study (chronic migraine), had other types of primary or secondary headaches, and had an allergy to memantine or valproate.
Recruitment was carried out from July 2019 to August 2020 in the Neurology Service, Central Hospital “Dr. Ignacio Morones Prieto” at San Luis Potosi, Mexico. Patients who met the inclusion criteria and signed the informed consent form participated in the study.
Computer-generated numbers that perform random assignments were used to create a random assignment sequence. Patients were randomized into the memantine or valproate group. Investigators and patients were blinded, and the assignment was randomized at study entry, except for the study technician who performed the randomization.
The protocol included five clinic visits follow-up appointments Neurology Outpatient Service. The first was a screening and run-in period of 4 weeks, and patients were randomized at the second visit; each subsequent visit was performed by the investigator every four weeks until the participant completed a period of treatment of 12 weeks, being double-blinded. The memantine group started with one 10 mg memantine tablet per night for one week, followed by an increase to one 10 mg tablet morning and night with a maximum dose of 20 mg (divided into two doses). The valproate group started with one 250 mg valproate tablet per night for one week, followed by an increase to one 250 mg tablet morning and evening with a maximum dose of 500 mg. Both memantine and valproate were donated by Torrent Pharma Mexico, which remained on the sidelines of the research.
Therapeutic efficacy and response were assessed based on information recorded by the participant in the migraine diary provided, in which they recorded the frequency, intensity, duration, triggers, and clinical features of their migraine. In addition, the Migraine Disability Assessment (MIDAS) test was performed at the second and fifth visits.
The primary outcome was to assess the change in attack frequency and intensity from baseline to week 12, comparing the two groups (using a migraine diary). Secondary outcomes evaluated the treatment response rate in each group, defined as a ≥ 50% reduction in migraine days; before and after treatment, migraine disability was evaluated using the MIDAS test, and the intensity of migraine was assessed through the visual analog scale (VAS), besides to identify adverse effects to sodium valproate and memantine.
A descriptive statistical analysis of the variables of interest was done. Student’s t test was used for the analysis of continuous variables. The aim was to reach a number of participants (n) of 20 for each group and did the final analysis using R software for data analysis. A P ≤ 0.05 was set to avoid an error type 1. In addition, the power was set to 0.2 to avoid an error type 2. Since the study was limited to a small number of participants per treatment, the delta was estimated with this restriction.
A total of 87 participants were assessed for eligibility. Thirty-three patients underwent randomization: 16 were assigned to the memantine group and 17 to the valproate group. Among them, 14 completed the protocol in the memantine group and 13 in the sodium valproate group. The consort flow diagram can be reviewed in the Figure S1.
The participants’ characteristics were similar between both groups (Table 1). The patients’ ages ranged from 18 years to 53 years (mean = 31.36 years), and 78.78% were women, and 77.86% had migraine without aura.
Baseline demographic and clinical characteristics
| Characteristic | Memantine | Sodium valproate | P |
|---|---|---|---|
| Female sex—no (%) | 13 (81.25%) | 13 (76.47%) | 0.54 |
| Male sex—no (%) | 3 (18.75%) | 4 (23.52%) | |
| Age | 31.18 ± 10.94§ | 31.58 ± 7.51§ | 0.91 |
| A family history of migraine (%) | 10 (62.5%) | 9 (52.94%) | 0.82 |
| Clinical characteristicsPhotophobia—no (%) | 14 (87.50%) | 16 (94.12%) | 0.48 |
| Phonophobia—no (%) | 16 (100%) | 10 (58.82%) | 0.60 |
| Nausea—no (%) | 14 (87.5%) | 17 (100%) | 0.23 |
| Disability activities of daily living (%) | 15 (93.75%) | 16 (94.12%) | 0.74 |
| Migraine without aura (%) | 13 (81.25%) | 13 (76.47%) | 0.54 |
| Migraine with aura (%) | 3 (18.75%) | 4 (23.52%) | 0.54 |
Fisher’s exact test; § plus-minus values are means ± standard deviation (SD)
Before treatment, the average migraine attacks in the memantine group were 5.31 (SD ± 1.54) per month and then 0.93 (SD ± 1.49) per month after 12 weeks of treatment, noting a decrease of 4.21 (SD ±1.76) migraine attacks (P < 0.001). The average migraine attacks per month in the sodium valproate group was 5.35 (SD ± 1.11) before treatment and 0.77 (SD ± 1.16) after 12 weeks of treatment, noting a decrease of 4.5 (SD ± 1.39) migraine attacks (P < 0.001) (Figure 1).
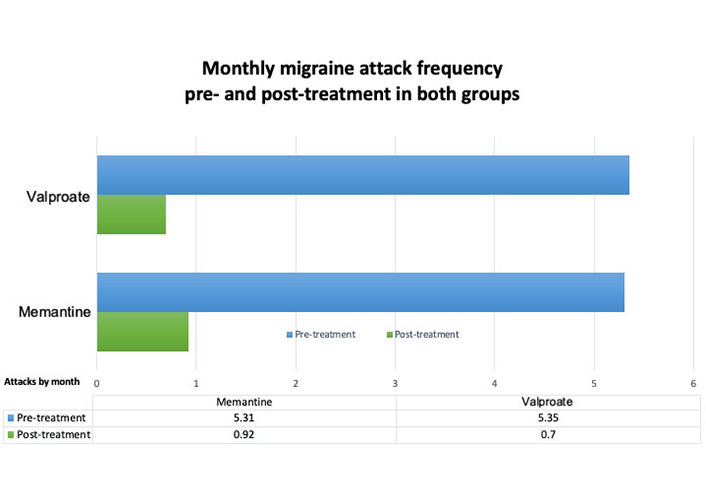
Difference between the frequency of migraine attacks pre- and post-treatment in both groups. A clear difference in both groups is observed
A total of 4.3 (SD ± 1.93) days of migraine attacks before treatment and 0.23 (SD ± 0.44) days of migraine attacks after 12 weeks of treatment were noted in the memantine group, showing a significant reduction of 4.3 (SD ± 1.93) days of migraine attacks (P < 0.001). In the valproate group, 5.5 (SD ± 1.25) days of migraine attacks before treatment and 0.27 (SD ± 0.55) days of migraine attacks after 12 weeks of treatment were observed, showing a significant reduction of 4.88 (SD ± 1.93) days of migraine attacks (P < 0.001). The response rate to treatment was obtained for all 27 patients (Figure 2).
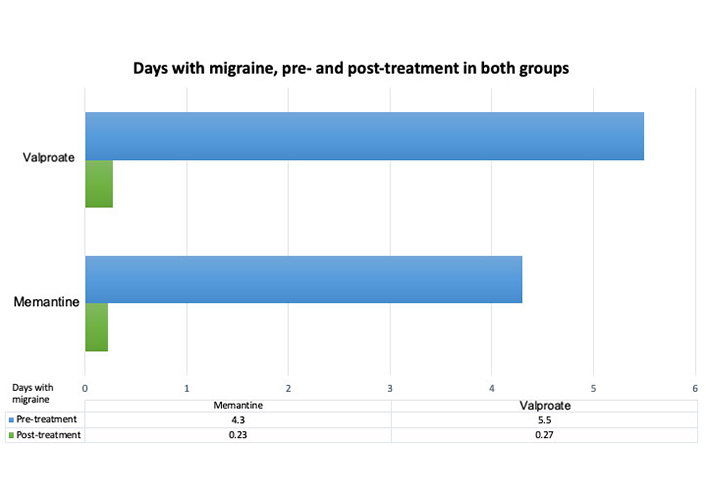
The average number of migraine days before and after treatment. Clearly, a difference in the days of migraine pre- and post-treatment, an average over the past 3 months and after 3 months at the start of treatment, is observed with either the memantine or valproate group
The intensity of the migraine was evaluated using the VAS pre- and post-treatment. In the memantine group, the VAS score was 8.5 (SD ± 1.36) pre-treatment and 4.28 (SD ± 3.65) post-treatment (P < 0.00014). In the valproate group, the VAS score was 8.94 (SD ± 0.87) pretreatment and 2.5 (SD ± 0.87) post-treatment (P < 0.0000012).
For the migraine disability assessed using the MIDAS test, the MIDAS mean score was 60.87 (SD ± 25.22) pre-treatment and 15.57 (SD ± 14.32) post-treatment in the memantine group (P < 0.000004). The MIDAS score was 51.92 (SD ± 22.67) pre-treatment and 10.53 (SD ± 19.97) post-treatment in the sodium valproate group (P < 0.00002) (Figure 3).
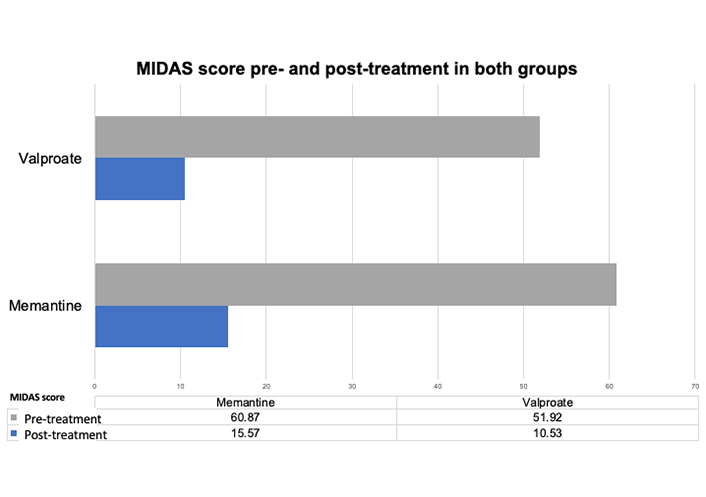
Disability measured by MIDAS before and after treatment. A difference in the MIDAS scores for migraine pre- and post-treatment, an average over the past 3 months and after 3 months at the start of treatment, can be observed in either the memantine or valproate group
The patients presented with non-severe side effects; eight in the memantine group and seven in the sodium valproate group showed non-severe side effects (Table 2), with somnolence being the most frequent side effect in both groups.
Adverse events according to group
| Memantine(n = 17) | Sodium valproate(n = 16) | Clinical severity CTCAE | |
|---|---|---|---|
| 8 | 7 | Grade 1 mild | |
| 4 | 6 | Grade 1 mild | |
| Poor concentration | 2 | 0 | Grade 1 mild |
| Parasomnia | 0 | 1 | Grade 1 mild |
| Dizziness | 2 | 0 | Grade 1 mild |
CTCAE: common terminology criteria for adverse event (from US Department of Health and human services)
The present research is an original pilot, double-blind, randomized clinical trial comparing the efficacy of memantine with first-line treatment (sodium valproate) as a prophylactic therapy for migraine. Considering that valproate is a first-line treatment in migraine prophylaxis.
Intending to recruit 40 patients because this was a pilot study, the Neurology Outpatient Clinic was suspended due to the coronavirus disease 2019 (COVID-19) pandemic. Finally, 33 patients were recruited, three lost follow-ups, and 3 abandoned the study due to COVID-19, with 27 patients completing the treatment protocol.
Compared to the study by Noruzzadeh et al. [11], in which the memantine group had a migraine frequency of 5.4 + 2.5 per month after three months of treatment, the baseline migraine frequency was 1.9. These results were similar to our study in the memantine group, with a mean number of migraine attacks of 5.31 before treatment and 0.92 after three months of treatment, with a difference of 4.39 migraine attacks (P < 0.001). Concerning the results in those taking sodium valproate, a Class 1A drug, our research observed a reduction of 4.5 migraine attacks (P < 0.001). Therefore, both drugs have oral administration in their presentation and are pretty good in acceptance; titration does not seem necessary, but taking a dose one step lower might be good looking for better tolerance. Valproate in a low dosage as used in this study had no undesired side effects such as weight gain, tremors, or alopecia. Memantine, on the other hand, offered a reduction in pain days, a lowering in intensity and duration of pain, and had lower disability effects and it is possible that memantine is safe enough that its use in pregnancy (Class B) has been proposed [13]. Both memantine and valproate have different action mechanisms, so their combined use is also possible; these can also be combined with monoclonal antibodies as a different possible therapy for refractory patients.
In conclusion, memantine can be a new prophylactic treatment option in migraine patients; it is safe, easy to administer, and effective as valproate. However, further clinical trials with a more significant number of samples are required to corroborate the findings of this study. In synthesis, migraine predominates in women. Its prevalence follows a bimodal pattern, with a peak at around 35 years of age and another at 50 years of age, affecting the productive and reproductive age population [14]. Prophylactic treatment aims to decrease migraine attacks’ frequency, duration, and severity. Memantine is a moderate-affinity uncompetitive NMDA receptor antagonist, an ionotropic glutamate receptor. Memantine could be a new prophylactic treatment option in patients with migraine. Our study showed that the efficacy and safety of memantine are comparable to those of sodium valproate, both considered excellent and safe.
MIDAS: Migraine Disability Assessment
SD: standard deviation
VAS: visual analog scale
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100442_sup_1.pdf.
Thanks are due to Torrent Pharmaceuticals, Mexico for providing both memantine and valproate for this study.
DVG: Conceptualization, Resources, Data curation, Writing—original draft, Writing—review & editing. IRL: Conceptualization, Writing—review & editing. AON: Investigation, Writing—review & editing. FRR: Resources. HGHR and JMSM: Formal analysis. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The protocol study was approved by the Research and Ethics committees of Hospital Central (no. 74-19). It was also registered in the National Institutes of Health Clinical Trials of the United States (registration NCT04698525 for clinical trials) and complied with the Declaration of Helsinki and its later amendments.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
The datasets for this study can be found in the Clinical Trials of the National Institutes of Health of the United States (https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT04698525).
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
