Abstract
It is widely known that each tissue has unique mechanisms to respond to injury and maintain homeostasis effectively. Although peripheral nerves have limited regeneration capacity, they conduct a complicated regeneration process by orchestrating multiple cell complexes after injury. In addition to drawing attention to anterograde and retrograde transportation, the absence of a cell body in the damaged area also points to the significance of immune and glial cells in the environment. Cellular reorganization following injury in the dorsal root ganglion, which takes place in the cell bodies of sensory peripheral nerve fibers, has attracted much attention. Growing research has been focused on investigating the molecular and cellular interactions occurring in sensory neurons and glial cells within the dorsal root ganglia after injury. It is clearly becoming that the sensory neurons and glial cells in the dorsal root ganglion are derived from the same embryological origins. Therefore, this information attracts attention to the potential of these two cells to differentiate into each other in case of injury. The focus of these studies is to illuminate the genes and pathways responsible for an increase in the plasticity of the neurogenic cell line following nerve injury. This review explores and discusses the underlying mechanisms responsible for maintaining homeostasis in the dorsal root ganglion and regeneration of peripheral nerves and how neuronal plasticity functions in the regeneration of the injury.
Keywords
Dorsal root ganglia, neuronal plasticity, sciatic nerve injury, ratSensory neurons of the rat sciatic nerve
Peripheral nerves include sensory, motor, and autonomic nerve fibers. While cell bodies of motor fibers enter the spinal cord via the ventral root, cell bodies of sensory axons are inside the dorsal root ganglia (DRG) located in the intervertebral foramina of the vertebrae [1]. The DRGs are located along dorsal spinal roots, surrounded by a collagen tissue capsule isolating them [2]. The information that cells bodies of sensory nerves in rats originated from L4–L6 spinal segments appears frequently in the literature [3, 4]. However, some anatomical variations in which the spinal nerve fibers from L6 do not participate in the formation of the sciatic nerve are also mentioned often in literature [5] (Figure 1). Nerve fibers originating from L4–L6 segments continue unifascicular until they are at the level 5–7 mm distal to the trochanter after which the nerve undergoes a process of bifurcation, initially splitting into two, and subsequently dividing into four fascicles. The peroneal portion of the nerve gives rise to the peroneal nerve and a cutaneous branch that passes through the lateral hamstring muscles to innervate the proximolateral face of the calf. On the other hand, the tibial portion of the nerve gives rise to the sural and tibial nerve [6]. Multiple studies have established that approximately 20% of the neurons in the sciatic nerve’s DRG supply the muscle afferents. Most of the remaining neurons are primarily involved in the innervation of the skin [7].
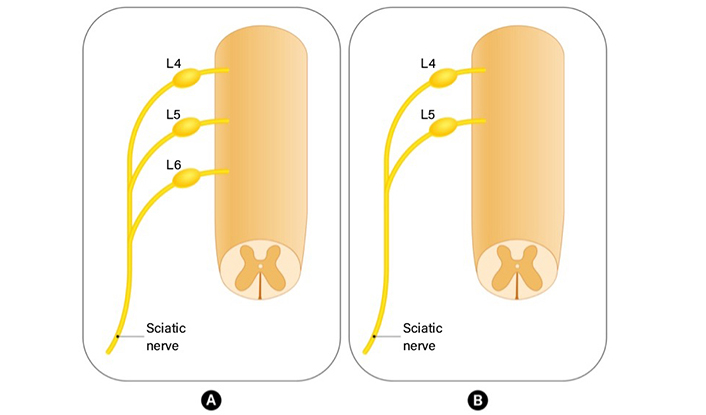
The anatomical variation in rat sciatic nerve anatomy. (A) Nerve fibers originating from L4–L6 segments continue unifascicular and form sciatic nerve; (B) L6 does not contribute to the formation of the sciatic nerve
It is thought that in middle-aged rats, the number of sensory neurons in the DRGs, which includes the cell bodies of the sciatic nerve axons, lies somewhere between 10,500 and 11,000 ± 2,000 [7]. In a study, the average total neuron count was calculated in DRG following peripheral nerve damage with the physical disector method, one of the unbiased stereological techniques. While the control group’s data differed between 10,512 and 11,006, numerical proliferation was observed in DRG neurons of the injured axon [2].
Processes following the peripheral nerve injury
Although the peripheral nerve system has innate regeneration and repair ability, returning to pre-injury level functionally and physically is almost impossible. As a result of nerve transection, the distal part of the nerve loses its connection with the cell body, and the axon part distal to the injury is surrounded by glial cells in 4 days and removed from the environment. This process is called the “Wallerian degeneration” (WD) [8]. Myelin sheath destruction starts before the axon and ends between the 30th and 40th days after the injury. Schwann cells leave the degenerated distal part but continue to exist in the environment [9]. During WD, molecular changes in the neuronal body and surrounding neuroglial cells make the source of the positive and negative signals required for inflammatory reactions. The signals flowing to axons from the cell body or vice versa trigger the processes of new neuron formation or neuron death and establish an inflammatory balance [10]. In addition to pointing out factors such as interleukin-6 (IL-6), IL-1β, IL-12, tumor necrosis factor α (TNF-α), and TNF-β which have crucial roles in maintaining balance, studies focus on the importance of the amounts of TNF receptor-I (TNF-RI) and TNF-RII molecules which are the receptors of these agents in sensory neuron cell bodies [11, 12].
The inflammation that occurs following injury is a complicated and multifactorial process that has both useful and harmful consequences [13] (Figure 2). While acute inflammation and WD play a role in the clearance of cellular debris, the transition of inflammation to the chronic phase can impede the healing process, leading to nociception and cell death [14, 15]. These inflammatory mediators participate in the inflammatory response and play a role in regulating necrotic and apoptotic processes [16, 17]. While Schwann cells, Satellite cells, lymphocytes, and macrophages have distinct roles in the inflammatory process, the primary chemical regulators of this response are TNF-α, nuclear factor-kappa B (NF-κB), IL-6, and IL-1β [16]. The activated nuclear protein-beta (NF-β) increases the expression of gene families associated with the inflammatory response (NF-κB, TNF-α, caspases, ILs, neuropeptides). Although the receptors of TNF-α and IL-1 proteins are very different from each other, they function in the same way. These two proteins increase NF-κB activation through phosphorylation and ubiquitination and consequently by triggering the signal pathway destroying I kappa beta (IκB) suppressor protein. Gene expression levels of these inflammatory agents begin to increase as of the ninth hour following damage and rise gradually until day 14 and show a pleiotropic effect [18, 19].
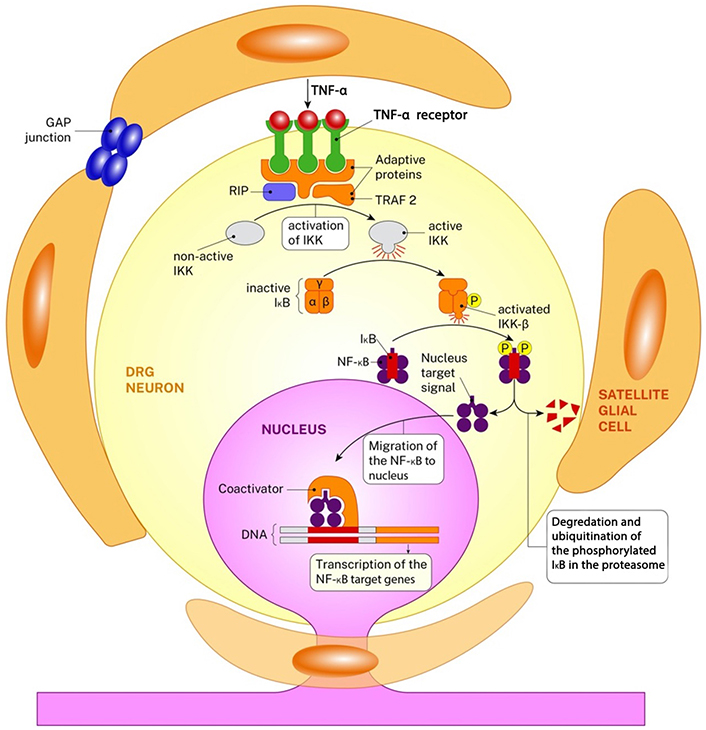
The inflammation cascade in DRG neurons after peripheral nerve injury is seen. The image shows the activation of the NF-κB via TNF-α. Both TNF-α and its receptors are in the form of protein trimer. The connection of the TNF-α and its receptors changes the protein structure of the receptors in cytosolic side and this leads to the recruitment of the receptor-interacting protein-1 (RIP1) and tumor necrosis factor receptor associated factor-2 (TRAF 2). This gives rise to the sequential activation of IκB kinase-β (IKK-β). IKK-β then phosphorylates (P) the IκB at serine residues and this leads to the degradation of itself. The nucleus target signal of the free NF-κB directs the protein towards nucleus and this leads to the transcription of the NF-κB target genes. NF-κB transcription factors induce the expression of genes related the inflammation, cell proliferation/differentiation, the immune response, genes that suppress stress-induced apoptosis, and death receptor [20]
It is important to shed light on the changes in DRG, including cell bodies of axons and the changes in peripheral nerve after injury. Previous studies have shown that adult peripheral nerve axotomy increases the level of IL-6 in DRG. The increase in the level of IL-6 is closely related to the increase in growth-associated genes and the related intrinsic factors. In axotomized neurons, the gp130/JAK/STAT3 signal pathway is activated by neuropoietic cytokines, which was indicated in a study by showing the increase in STAT3 phosphorylation in the environment via immunohistochemical methods [21, 22]. After STAT3 is primarily synthesized and activated in the damaged nerve area, it is transported retrogradely to the sensory neuron body, which promotes axon growth by modifying transcription genes [23]. In addition, STAT3 molecules in DRG are synthesized locally and activated through the neuropoietic cytokines field [12]. Studies are showing that bilateral activation of the STAT3 molecule protects neurons from death [23]. The regeneration of the axons is disrupted in the absence of STAT3 expression and STAT3 works as a regulator specific to different phases of neuronal outgrowth in both the central nervous system (CNS) and peripheral nervous system (PNS). Through the utilization of conditional deletion of STAT3 in conjunction with in vivo imaging, it has been demonstrated that STAT3 expression is not solely linked to axonal regeneration but is indeed essential for the timing of the initiation of axonal growth subsequent to a PNS injury. Nevertheless, it is noteworthy that axons originating from neurons lacking STAT3 are still capable of initiating a growth response, albeit with an extended “lag” phase in comparison to their STAT3-competent counterparts. The molecular mechanisms responsible for the initiation of the axonal growth program by STAT3 are unknown. However, given that STAT3 functions as a transcription factor, it is probable that the downstream effects are facilitated through the induction of gene expression. A considerable number of genes that are influenced by STAT3 have already been recognized. Among these downstream targets, certain genes such as the cell cycle inhibitor p21/Cip1/Waf1 [24] and the small proline-rich protein 1a (SPRR1A) [25] have the capacity to directly impact neuronal outgrowth [26, 27]. The recent study involving transcriptional profiling has revealed several genes specifically regulated by STAT3 in DRG neurons, including the [interferon (IFN) regulatory factor 1 (IRF1)] [28]. This finding is significant as IRF1 has been shown to enhance neuronal outgrowth in cultured cerebellar neurons [28]. The study also demonstrated that sciatic nerve transection leads to the phosphorylation and activation of STAT3 in DRG neurons [22]. Furthermore, it has been established that leukemia inhibitory factor (LIF) signals through a receptor containing the signaling subunit gp130, leading to the activation of STAT3 by inducing its phosphorylation and nuclear translocation [29]. Additionally, IRF1 has been identified as a transcriptional regulator that is induced by various extracellular factors, including type I and II IFN, and viral infection [30]. Moreover, a subgroup of genes induced by IFN-γ requires both STAT1 and IRF1 for transcriptional activation [31]. These findings collectively highlight the intricate regulatory network involving STAT3 and IRF1 in the transcriptional control of genes related to neuronal outgrowth and regeneration.
Changes in neuronal plasticity as a result of mechanical stress
Profound plasticity changes are evident in the PNS and the CNS [2]. The information that new neurons are generating in the DRG is based on very old studies [32, 33]. Previous studies showed that DRG satellite cell number increased after nerve injury and that they changed their differentiation lineage to give rise to new DRG neurons [2].
Gene expression in DRG has a dynamic structure that can respond to chronic or acute injury. Changes in various gene expression levels and protein levels are expected in response to injury. These alterations in the protein level following the changes in the gene expression level have influence on inflammation, cell death, and nociception [16]. Following the stress response that starts half hours after damage, a progress to pre-regeneration phase takes place with the up-regulation of genes such as atf3, jun, smad1, and stat4. The beginning of the regeneration phase occurs with the upregulation of several genes such as cdkn1a, il6, atf3, igf1, runx3, socs3, jun, and agtr2, pld2, npy, gap43, and prad genes [34–36].
Previous studies demonstrated that following injury; the expression of the transcription factor c-jun is essential for the initiation of regeneration along a peripheral nerve graft. Furthermore, they have observed that their response is prolonged with the help of a growth-promoting substrate. Previous studies have also indicated that these neuronal populations exhibit an upregulation of growth associated protein-43 (GAP-43), when subjected to similar lesion and grafting conditions [37, 38] The failure to upregulate both GAP-43 and c-jun subsequent to axonal injury appears to correspond with the inability to regenerate axons. This suggests that c-jun may play a pivotal role in governing the gene programs that regulate the cell body response, thereby enabling CNS neurons to regenerate in the presence of a favorable growth substrate. Additionally, our results indicate that the discrepancies observed in the regenerative capacity of CNS neurons along a peripheral nerve graft [39, 40] may be linked to variations in their intrinsic molecular response to axotomy.
In a study conducted by Li et al. [19], a transcriptome analysis was done to shed light on genes that are up and down-regulated in DRGs following peripheral nerve injury [19]. Although a previously conducted similar study reported that the expression of differentially expressed (mainly up-regulated) genes increased in time. Li et al. [19] showed that the expression of these genes increased and decreased alternately. On the contrary, the gene expression profile during processes of response to stimulus, such as wound healing, inflammatory response, immune response, defense response, and apoptosis, demonstrated a consistent increase, reaching its peak seven days after peripheral nerve injury.
Primary sensory neurons change their phenotype in response to endogenous and exogenous stimuli. DRG neurons try to adapt to changing conditions by repressing exogenous stimuli and simultaneously activating survival and regeneration mechanisms. It has been shown that substance P and calcitonin gene-related peptide (CGRP), which participate in synaptic permeability in the dorsal horn in small DRGs under normal circumstances, are down-regulated following axotomy [41, 42]. The reason for this phenomenon is the inability of target-derived nerve growth factor (NGF) to affect the cell body. However, controversial results also show that the production of substance P increases in large-sized neuron in DRG following partial injury [43, 44]. Other peptides such as histidine-isoleucine, vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), and galanin are expressed in low levels in sensory neurons; however, they increase dramatically following axotomy [41, 42]. Under normal circumstances, although galanin is a substance that is synthesized in small quantities in small-sized neurons in DRG, galanin expression in primary afferents is upregulated following peripheral nerve injury [45]. The increase in galanin synthesis following damage occurs due to the increase in LIF production, and it is thought that all these are encountered due to the rise in IL-6 level [46]. Although it is very difficult to detect NPY under normal conditions, the NPY-Y1 receptor is expressed strongly in the cell body. Following axotomy, it is seen in NPY levels in small- and middle-size neurons in the DRG [5, 47].
Glial cells within the DRGs and their response to injury
In addition to providing support to sensory neurons, glial cells in DRG have essential metabolic and structural characteristics [48]. New neuron-glial communications are built after damage, and the altered gene expression profiles cause phenotypic changes. Regarding their shapes and sizes, two different types of glial cells surround DRG neurons. First-type satellite cells extend flat plates to envelop the somata of DRG neurons. The initial region of the dendro-axon of large-diameter DRG neurons is also covered by another type of satellite glial cells [49, 50].
Under normal circumstances, satellite glial cells provide vital support to neurons and play a role in homeostasis and immune response [48]. It is known that these cells change their phenotypes following peripheral nerve damage and release chemicals such as TNF-α and nitrous oxide in vitro [5, 51, 52]. In parallel with the numerical increase in the satellite glial cells following damage, there is also an increase in the release of inflammatory cytokines and neurotrophic factors. For this reason, it is safe to say that the satellite glial cells have an active role in nociception and cell death [51]. Secondly, Schwann cells surround the dendro-axon area except for the starting region. Schwann cells in the myelinated nerve fibers surround dendro-axons with large diameters and axons extending from big-sized DRGs. Schwann cells in the non-myelinated nerve fibers cover a few thin axons and form Remak bundles. All these glial cells in DRG are in direct contact with DRG neurons (Figure 3).
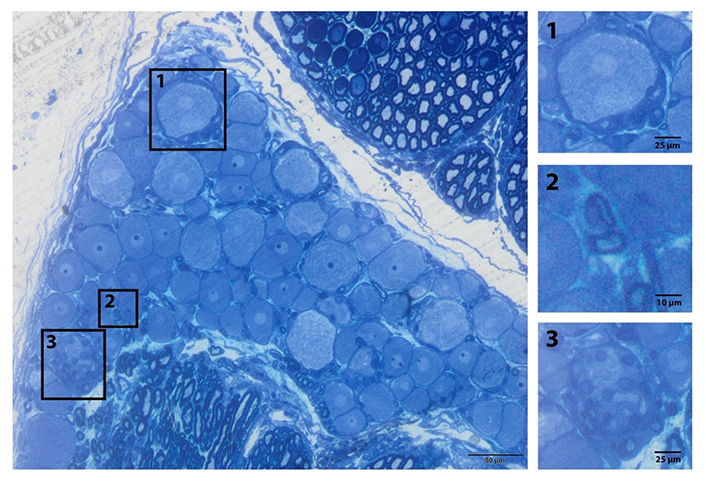
Glia subtypes of the peripheral ganglia. General view of the L5 DRG. (1) Satellite cells surround the sensory nerve; (2) myelinated Schwann cell; (3) non-myelinated cell (Remak bundle) resin section and toluidine blue staining [20]
It is known that peripheral glial progenitor cells originate from neural crest cells in the embryonic development period and that they differentiate into Schwann cell precursors (SCPs). SCPs reside in relation with embryonic nerves and share remarkable similarities with their maternal population, as they can generate autonomic neurons, melanocytes, peripheral glial cells, and other cell types [53–55]. Peripheral glial cells and neurons, coming from similar embryologic origin, can also differentiate in the postnatal period. Laranjeira et al. [56] showed that SCPs present in the enteric nervous system play a role not only in normal gliogenesis and neurogenesis during the initial postnatal weeks but also contribute to injury-induced neurogenesis during later stages [56, 57]. Studies also show that peripheral glial cells can differentiate into neurons in case of damage [2]. Similarly, when glial progenitor cells in optical nerves are cultured [without paired box gene 2 (Pax2) signal], they can also differentiate into neuron cells [58]. The optic nerve exhibits the expression of the homeodomain transcription factor Pax2, which is sustained in differentiated astrocytes. Pax2 undergoes rapid down-regulation in explanted optic nerves that give rise to neurons. Furthermore, overexpression of Pax2 through electroporation in the optic nerve, or in the neural tube, effectively impedes neuronal differentiation and facilitates glial development. This underscores the significant role of Pax2 in governing cell fate within the optic nerve. In vitro and ex vivo experiments additionally demonstrate that a signaling cascade, involving sonic hedgehog and fibroblast growth factor is essential for maintaining Pax2 expression in optic nerve precursors. This cascade serves to inhibit neuronal fate and promote astroglial differentiation. It is plausible that under the influence of potent neurogenic and proliferative signals in culture, most enteric glia can differentiate into neurons, while in vivo neurogenic potential may only be activated in a subset of SRY-box transcription factor 10 (Sox10)-expressing glial cells. The high differentiation potential of these two cell types (glial cells and neurons) into each other suggests that trying new glial cell-based treatment methods may be beneficial.
Conclusions
Peripheral nerves display regenerative properties that allow the repair of injured nerves; however, weakening the connection with the cell bodies negatively affects the repair process. At this stage, adult glial cells exhibit an extraordinary level of plasticity that allows them to undergo reprogramming during injury. This reprogramming enables them to coordinate peripheral nerve repair or migrate to injured tissue to contribute to the repair process. Understanding these complex processes’ mechanisms, including the molecular pathways and gene regulations, has important therapeutic implications for improving tissue and nerve regeneration. The studies focusing on triggering the regulation of the cellular differentiation signals to improve plasticity can be helpful in understanding and facilitating regeneration.
Abbreviations
| CNS: | central nervous system |
| DRG: | dorsal root ganglia |
| IFN: | interferon |
| IL-6: | interleukin-6 |
| IRF1: | interferon regulatory factor |
| IκB: | I kappa beta |
| NF-κB: | nuclear factor-kappa B |
| NPY: | neuropeptide Y |
| Pax2: | paired box gene 2 |
| PNS: | peripheral nervous system |
| SCPs: | Schwann cell precursors |
| TNF-α: | tumor necrosis factor α |
| WD: | Wallerian degeneration |
Declarations
Author contributions
BD: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. SK: Supervision, Conceptualization, Investigation, Writing—original draft, Writing—review & editing. AAEE: Supervision, Validation, Writing—review & editing. All authors granted their final clearance for this version of the work to be published after revising it critically for its intellectual content, and gave their final approval for this version of the paper to be published.
Conflicts of interest
Süleyman Kaplan who is a Guest Editor of Exploration of Neuroprotective Therapy had no involvement in the decision-making or the review process of this manuscript.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.