Affiliation:
1Department of Basic Medical Sciences, Faculty of Medicine and Health Sciences, Simad University, Mogadishu 999128, Somalia
ORCID: https://orcid.org/0000-0003-1213-9846
Affiliation:
2Department of Histology and Embryology, Faculty of Medicine, Recep Tayyip Erdoğan University, 53020 Rize, Türkiye
ORCID: https://orcid.org/0000-0001-6277-1255
Affiliation:
3Department of Histology and Embryology, Faculty of Medicine, Ondokuz Mayıs University, 55139 Samsun, Türkiye
ORCID: https://orcid.org/0000-0001-8922-9642
Affiliation:
3Department of Histology and Embryology, Faculty of Medicine, Ondokuz Mayıs University, 55139 Samsun, Türkiye
4Department of Health and Biomedical Sciences, School of Life Science and Bioengineering, Nelson Mandela African Institution of Science and Technology, Arusha 23311, Tanzania
Email: skaplanomu@yahoo.com; skaplan@omu.edu.tr
ORCID: https://orcid.org/0000-0003-1477-5002
Explor Neuroprot Ther. 2024;4:288–299 DOI: https://doi.org/10.37349/ent.2024.00083
Received: January 01, 2024 Accepted: May 21, 2024 Published: June 21, 2024
Academic Editor: Lucio Marinelli, University of Genoa, Italy
Peripheral nerve injuries (PNIs) constitute a significant concern as they predominantly affect young and productive age groups of the population, causing social and economic pressure on patients. PNIs are a global problem that can result in disability because of the disruption of nerve function. PNI leads to a reduction in nerve conduction velocity, which worsens or impairs the mobility of the innervated area. Managing PNI remains a major clinical challenge. Coenzyme Q10 (CoQ10) is a lipid-soluble antioxidant first identified in 1957. It is an important antioxidant necessary for the organs to maintain their normal function and the body’s chemical processes. It scavenges free radicals and reduces oxidative stress. Studies showed that antioxidants such as CoQ10 a potent antioxidant, help the regeneration of PNIs. It has been observed to increase the myelination process in nerve fibres and promote nerve regeneration in rats after injury. Therefore, this review handles the current positive effects of CoQ10 on peripheral nerve regeneration following injury.
Peripheral nerve injuries (PNIs) have been mostly reported during the wartimes. The nerve injuries can be caused by gunfire, car crashes, or crushing traumas [1]. The World Health Organization (WHO) stated that car crashes are the leading cause of injury among these and that they also result in a higher number of disabled people. According to reports, the radial and peroneal nerves in the upper and lower limbs are most susceptible to auto accidents [2]. Despite advances in prevention and emergency medical care, morbidity in road accidents is a challenging and growing issue of concern for people all over the world. In most developing nations, the number of injuries and death rates are rising, leading to severe problems. According to WHO statistics, there are 1.5 injuries per second and two deaths every minute. States with the least developed economies account for nearly two-thirds of the victims [3]. The leading cause of mortality among people between the ages of 1 and 34 is trauma [4]. PNI is one of the most common medical conditions in clinics [5]. In America, twenty million people face PNI through medical issues or trauma [6]. Every year, PNI results in neuropathic pain for about 300,000 individuals in Europe, and despite treatment, nerve regeneration is disrupted in almost half of the patients [7].
PNIs are prevalent problems where the nerves are affected, and the extent of damage determines the predominant symptoms. Treatments are inadequate despite a depth of knowledge regarding nerve injury and the regeneration mechanisms [8]. PNIs are severe medical conditions for which there are few options for treatment. One of the most active cases in medical studies up to this point has been PNIs and their treatments [9]. About 3% of all trauma cases encountered in emergency rooms require surgical intervention due to severe PNIs [10].
PNI may result in aberrant axon degeneration, demyelination, or occasionally both. Anytime the myelin sheath is damaged, and the Schwann cells (SCs) are eliminated, demyelination occurs. The nerve conduction velocity is decreased as a result of this demyelination. The axonal integrity of the remaining fibres will allow the conduction velocity to continue normally. Wallerian degeneration (WD) is a crucial biological process based on chemical alterations in the distal nerve following injury. Myelin sheath degradation and axon degeneration occur [11, 12]. The interactions between macrophages and SCs influence the onset of WD. According to research, WD cannot begin until macrophages are drawn into a peripheral nerve that has been injured. Furthermore, macrophages with debris were often found adjacent to intact myelin sheaths, demonstrating that myelin degradation is not only initiated close to degenerated axons. The mechanisms causing myelin disintegration and mitosis, which trigger the secondary responses of SCs, do not take place in WD [13].
Regarding PNIs, various categorizations are occasionally used to describe the fundamental causes of medical loss, such as simple, partly demyelination, or complete axon loss [14]. PNIs are classified into the Seddon and the Sunderland classifications, as shown in Table 1 [15].
Standard classifications of nerve injury mechanisms [15]
| Sunderland | Seddon | Features |
|---|---|---|
| 1st degree | Neurapraxia | Segmental demyelination (damage is limited to myelin sheath alone) |
| 2nd degree | Axonotmesis | Despite the axon being severed, the endoneurium is protected |
| 3rd degree | Axonotmesis | There is axon and endoneurium discontinuity, but there is no impact on perineurium |
| 4th degree | Axonotmesis | Discontinuity of axons, the endoneurium, and perineurium |
| 5th degree | Neurotmesis | Absence of continuity throughout the nerve, encompassing the epineurium |
Note. Adapted from “Peripheral nerve injury: A review article” by Seddighi A, Nikouei A, Seddighi AS, Zali AR, Tabatabaei SM, Sheykhi AR, et al. Int Clin Neurosci J. 2016;3:1–6 (https://journals.sbmu.ac.ir/Neuroscience/article/view/12016). CC BY.
After PNI, the functional recovery can be successful thanks to newly sprouting axons. These newly generated axons are able to replace myelin and eventually innervate its motor endplates or sensory receptors [16, 17]. The severity and nature of the nerve injury determine each of these procedures. Proliferation of SC is necessary for the regeneration of axons and nerves. Remyelination and axonal development are crucial for successful nerve regeneration. Regular axonal development occurs at about 1–3 mm/day [18].
Managing PNI continues to be a significant therapeutic challenge. Results were unexpected and depressing following the restoration of the nerves despite advancements in minimally invasive surgical techniques. Understanding the peripheral nerve’s anatomy is necessary to manage various crush injuries. Modern PNI care is predicated on the ability to modify the pathophysiological processes triggered by both injured and healed nerves. Applying a nerve autograft is the best course of action (gold standard) if there is a significant nerve gap. Nerve allografts showed promising results for nerve injuries when nerve autografts are not available [19]. The most recent advancements in peripheral nerve restoration have centered on growth factors and stem cells since nerve grafting remains the gold standard of treatment for severely damaged nerves. The requirements for functional nerve regeneration are intricate. Fibroblast growth factors (FGFs) are released from the ends of injured neurons and are crucial for cell division and regeneration. Studies that combine FGF with structural components have been carried out since then. Additionally, neuronal growth factors (NGF) are vital for the physiological repair and regeneration of the neurons [20].
It is common for the injured peripheral nerve to fail to complete regeneration [21]. Neural regeneration and repair are complex processes that can be affected by multiple factors after PNI [22]. Numerous investigations are still working on immune system modulators, pharmaceutical treatments, and enhancing factors. The therapeutic studies will continue to improve by clinically significant findings from this research [23]. Therefore, the potential positive effects of coenzyme Q10 (CoQ10) on peripheral nerve regeneration following injury are covered in this review.
CoQ10 is present in foods in considerable amounts. Food products such as fish, chicken, nuts, animal meat, and vegetable oils are rich sources of CoQ10 [24]. The different foods have varying amounts of CoQ10 in them [25]. The possible sources of CoQ10 would be via either dietary consumption or endogenous production in the mitochondria [26]. In both plant and animal cells, CoQ10 is a naturally occurring coenzyme. The number of isoprenoids’ side chains can be used to distinguish between each family of coenzymes. Among all coenzymes, CoQ10 is the most popular one that is naturally produced in human mitochondria. The number 10 represents how many times isoprene is repeated. The chemical structure of CoQ10 is 2,3-dimethoxy-5-methyl-6-decaprenyl-1,4-benzoquinone which can be seen in Figure 1 [27, 28].
Animal research has advanced in recent years to uncover contemporary pharmacological therapies [29]. Luckily, in 1957, Dr. Fred Crane discovered the essence of CoQ10 [30]. Over the past 50 years, CoQ10 has played a significant role in transforming mitochondrial bioenergy. Its enzymatic mechanisms facilitate electron transport during adenosine triphosphate (ATP) synthesis. Complexes I and II generally provide electrons [31, 32]. CoQ10 can accept and give electrons through a total of three oxidation pathways. This plays a crucial role in the movement of electrons throughout the mitochondrial membranes to produce ATP energy (Figure 2) [33]. CoQ10 is found in the inner mitochondrial membrane, the site of ATP synthesis. Therefore, the energy required for all essential cellular functions is provided by the ATP that CoQ10 produces [34]. By assisting in the correct reproduction of other antioxidants, CoQ10 promotes cell growth and prevents cell death [35]. It reduces the generation of reactive oxygen species (ROS) in the mitochondria and protects the mitochondrial membrane from oxidative stress [35].
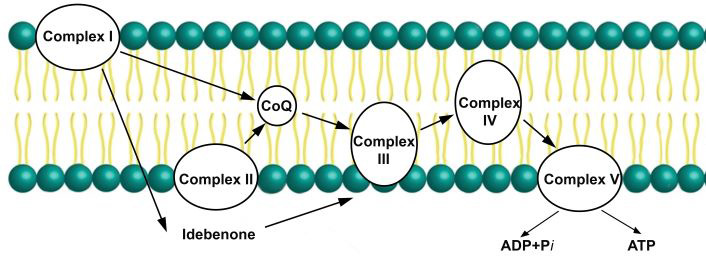
CoQ10 in the mitochondrial electron transport chain. It is essential for the transfer of electrons, required for oxidative phosphorylation to produce ATP. ATP: adenosine triphosphate; ADP: adenosine diphosphate; CoQ: coenzyme Q10
Different body tissues have variable concentrations of CoQ10, which may be related to their metabolic needs. The liver, kidneys, skeletal muscles, and heart have large amounts of it, while the blood plasma contain the least [25]. Oxidative stress and the loss of mitochondrial function may result from CoQ10 depletion inside the mitochondria [33]. Because CoQ10 is essential to all the body’s physiological processes, a decrease in CoQ10 production can harm tissues throughout the body, particularly those that require more energy, such as the kidneys, liver, and heart muscles [25, 36, 37]. The concentration of CoQ10 in plasma is used to determine its levels in humans. A variety of medical conditions can cause tissue deficiencies in CoQ10, such as primary deficiencies in CoQ10, diseases of the mitochondria, dietary deficiencies that impair its synthesis, errors in the formation or utilization of it that are heritable or acquired, and diseases that result in excessive tissue demand [38].
CoQ10 is an antioxidant that can replenish some other antioxidants, like ascorbate and tocopherol. In the human body, it functions similarly to a vitamin. It has a beneficial effect on mitochondrial and neurodegenerative diseases [39]. The presence of CoQ10 in eukaryotic cell membranes raises the idea that it can act as an antioxidant to scavenge free radicals and protect cells from inflammatory processes [40]. One of the fat-soluble molecules, CoQ10, can stimulate cell metabolism and repair cell damage caused by free radicals. The potential benefit of CoQ10 in preventing and treating different clinical diseases associated with oxidative stress has been demonstrated by previous research [41–43]. CoQ10 has been shown in studies to improve peripheral nerve regeneration in patients with diabetic peripheral neuropathy, and the results showed favorable neuroprotective properties in diabetic neuropathy conditions [44]. It can prevent excess fat and improve metabolic abnormalities and insulin resistance brought on by obesity [45]. In histological and immunohistochemical investigations, it was found that CoQ10 reduces interstitial infection, congestion, and cell death [46] and it diminishes interstitial oedema, degeneration of the myofibers, and infiltration of the mast cells [47].
CoQ10 protects lipoproteins from oxidative damage [48], interestingly, it is the only lipid-soluble antioxidant generated endogenously and is regarded as the most crucial lipid antioxidant [28, 49]. Serum or plasma CoQ10 can be utilized to measure intracellular CoQ10 levels, which are influenced by food intake and lipoprotein composition [50]. Research in diverse species has demonstrated the crucial role that CoQ10 plays in cellular energy transfer and its medicinal significance in clinical practice [51]. Overall, the ability of CoQ10 to provide a more active therapeutic level of care to treat certain medical conditions is evident [52].
Some researchers have estimated the daily intake of CoQ10 to be between 3 and 5 mg/day. In contrast, other researchers found that the daily intake of CoQ10 is 5.4 mg/day and 3.8 mg/day for males and females, respectively [53]. In animal models, 10 mg/kg of CoQ10 displayed neuroprotective efficacy [54]. Different doses of CoQ10 as a supplement are found in single capsule of 30, 60, 100, 200, 300, 400, and 600 mg. As seen that although there is no certain minimum or maximum effective dose of CoQ10, but for gaining a therapeutic blood level of > 2.5 μg/mL, a 200 mg taken twice daily was suggested [55].
CoQ10 dosage guidelines, which are safe and well-tolerated, were suggested to be up to 1,200 mg/day for adults and 10 mg/kg per day for children [49]. High doses of 30 and 600 mg/kg in children and adults are suggested to patients as they are free from side effects and bring more advantages to the metabolism of cells [56]. The clinical use of 2,400 mg of CoQ10 daily in humans is possible [57].
Monitoring CoQ10 plasma concentrations can be done after 3–4 weeks of continuous dosing. Intestinal absorption is three times faster if CoQ10 is taken with food. The hydrophobicity displayed by the CoQ10 substance refers to findings of poor absorption, so delivery modes are a rapidly improving therapeutic objective [58]. Animal data indicate that almost all tissues, such as the heart and brain mitochondria, take CoQ10 in high doses. Under the increased availability of fats (lipids), the absorption or uptake of CoQ10 is increased. The absorption often depends on the origin of the formulation, and the solubility of CoQ10 formulations is more bioavailable. Secretions of the pancreas and bile in the small intestine promote the emulsification necessary to absorb lipids [59]. CoQ10 is absorbed slowly from the small intestine because of its high molecular weight and insolubility in water. CoQ10 absorption and bioavailability research shows differences depending on the type of CoQ10 preparation studied. In general, slightly better absorption is achieved with oil-based forms of it. New formulations have been developed to increase the absorption of CoQ10 and its distribution in tissues [49].
There are no known contraindications for CoQ10. Its treatment was found to be safe since side effects are rare. Gastric discomfort was reported in less than 1% of patients and had an excellent safety record [60].
Studies have identified that CoQ10 is well-tolerated and safe in humans, even at higher doses [52]. No side effects are seen even in persons having CoQ10 dosages up to 3,600 mg/day [57]. In previous studies, side effects of it were found low [61]. It appears to be safe and well-tolerated [35]. Due to the considerable lack of adverse effects and possible treatment benefits, it can be assumed to be a safe contribution to standard treatments [62].
Although peripheral nerves can regenerate after injury, attaining an adequate functional recovery is frequently challenging [63, 64]. Despite advancements in microsurgery and our understanding of nerve regeneration, delayed and improper reinnervation remains challenging for functional recovery [65]. Currently, the positive effect of antioxidants on the enhancement of nerve regeneration is the focus of attention [66]. As stated previously, CoQ10 is a well-known antioxidant with bioenergetic, anti-inflammatory, and neuroprotective properties [67]. In the acute inflammatory phase of sciatic nerve injury, intraperitoneal injection of 10 mg/kg per day was observed to enhance nerve regeneration [68].
According to an experiment that examined the cell loss in the dorsal horn of the spinal cord after nerve injury in a rat model, injection of CoQ10 intraperitoneally improved the histological features in rats with a pre-existing sciatic nerve injury. As a result, CoQ10’s ability to protect cells has also been demonstrated [69]. Its antioxidant and free radical scavenger properties in the peripheral nervous system (PNS) may reduce oxidative stress [70]. It was found that CoQ10 dramatically enhanced oxidative stress and inflammation biomarkers. The histopathological results confirmed the biochemical and molecular results. According to the research, it could be a preventative measure for peripheral nerve damage [69].
The administration of CoQ10 has been shown to lower the pro-inflammatory factors in the PNS. It triggers an antioxidant response, reduces peripheral nerve lipid peroxidation, and protects neural tissue from oxidative stress [71]. In many experiments on animal models, CoQ10 treatment has been extensively hypothesized to have neuroprotective effects due to its neuroprotective characteristics [72]. According to reports, it has anti-apoptotic and neuroprotective properties [73]. Neuroprotection by CoQ10 has been linked to neuronal apoptosis prevention [71]. Since it prevents the apoptosis of neurons and cells from oxidative stress in vivo through bioenergetics and anti-inflammatory actions, its long-term treatment was also found effective by enhancing peripheral nerve oxygenation, providing further prevention from apoptosis [74].
CoQ10 has been investigated in preclinical and clinical trials, where it has been proven to affect diabetes positively. The impact of CoQ10 on diabetes complications is the subject of many investigations. It is useful in repairing and enhancing nerve conduction velocity in diabetic PNIs in animal models of diabetic neuropathy. Based on these, it would be suggested that CoQ10 helps PNIs regeneration, especially those linked to diabetic neuropathy. Administering CoQ10 may have a positive impact on treating or preventing diabetic neuropathy [71]. On the other hand, according to some non-randomized, non-blinded trials, CoQ10 is beneficial in improving Charcot-Marie-Tooth disease, the most prevalent inherited neuropathy and one of the most frequent genetic disorders in humans [75].
By increasing antioxidant levels and reducing oxidative stress and neuropathic pain more effectively, CoQ10 treatments are beneficial [76]. It has a scientific basis and can significantly improve patient outcomes if recommended as an adjuvant in patients with diabetic neuropathy [77]. A CoQ10 pretreatment is a powerful approach for preventing the onset of neuropathic pain. Since it quickly neutralizes free radicals and inhibits mitochondrial dysfunction. As a result, CoQ10 treatment prevents nerve injury and reduces severe diabetic neuropathy [78]. A study that investigated the protective effect of CoQ10 on induced neuropathy in rats found that rats with peripheral neuropathy responded better to CoQ10 therapy [79]. Another study examined how CoQ10 affected rats with induced diabetic neuropathy and discovered that it reduced peripheral neuropathy [80]. In an experimental study on diabetic rabbits with polyneuropathy, CoQ10 showed a significant neuroprotective effect against harmful alterations in the sciatic nerve [81]. Another study examined the neuroprotective effect of liposomal CoQ10 on propionic acid-induced oxidative stress, inflammation, and apoptosis in a rat model further supporting these neuroprotective properties. The study showed that CoQ10 reduced oxidative stress, inflammation, apoptosis, and tissue injury [82].
The effect of CoQ10 on the regeneration of peripheral nerves was studied in a work, and the effect of CoQ10 on facial nerve injury regeneration in Sprague-Dawley albino rats was examined. After receiving intraperitoneal CoQ10 at a dose of 10 mg/kg for 30 days, the experimental group was compared to the control group, which received a saline solution of 1 mL/day. The CoQ10 group demonstrated better neurological improvement in comparison to the control group. Furthermore, vascular congestion, macro vacuolization, and myelin thickness between the two groups were noticeably different, according to light microscopy. Similar outcomes were seen with the sciatic nerve crush injury. The intraperitoneal treatment of CoQ10 of 10 mg/kg per day was compared to the control group following compression injuries in 45 male Wistar rats. Myelinated nerve fibres were substantially more numerous and larger in diameter. After treatment with CoQ10, the nerve fibres retained their structure and showed a partly intact myelin sheath [83]. It was found that it is an agent that promotes injured nerve or SC repair and the restoration of mitochondrial function [84].
SCs are affected and stimulated by the initial injury to promote neuroregeneration of the PNS [85]. SCs produce supplies an electrically insulating substance that creates a multilayered sheath that surrounds or wraps the axons of neurons, protecting them from damage, supplying them with nutrients, and speeding up the conduction of action potentials. However, in many cases of nerve repair following PNI, inadequate myelination of the regenerated nerve fibres results in dysfunction of the regenerated nerve. Such inadequate myelination can be visible as small axons with a thin myelin sheath in the light micrographs of an injured peripheral nerve (Figure 3). For that, Hyung et al. [86] investigated the effect of CoQ10 on myelination, and they found that it significantly facilitated and increased myelination, indicating that CoQ10 promoted the myelination of nerve fibres. They also found a positive effect of CoQ10 on myelination due to its role as an electron carrier in ATP synthesis and its antioxidant properties [86].
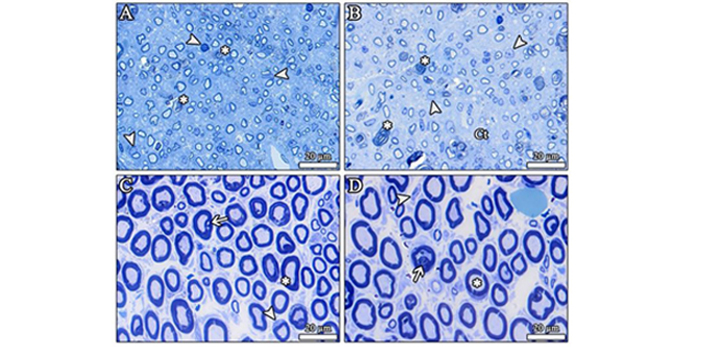
The light micrographs show the toluidine blue staining of the injured sciatic nerve sections. (A, B) After the crush injury, newly formed small-sized axons with thin myelin sheaths are present in response to the nerve injury, as it is seen that the size of axons and myelin sheath thickness were seriously decreased. The number of the SCs were substantially increased after injury. *: destroyed nerve fibres; arrowhead: SC. SC: Schwann cell; Ct: connective tissue. (C, D) The light micrographs show the toluidine blue staining of the injured sciatic nerve treated with CoQ10 at 10 mg/kg orally for 21 days. The nerve fibres have regained their typical structure after treatment with CoQ10. *: nerve fibres with intact myelin sheath; arrow: vacuolization in the myelin sheath; arrowhead: SC. CoQ10: coenzyme Q10. Resin sections: 500 nm [87]
The great importance of PNI treatment has prompted researchers to look for a suitable antioxidant therapy complementary to curing disorders and injuries affecting the peripheral nerves. Due to its antioxidant properties, CoQ10 has received much research about its utility in managing PNI. Previous articles suggest that, according to the pharmacological and antioxidant properties of CoQ10, it might be a helpful agent for PNI treatment and prevention. Previous studies have mentioned that CoQ10 inhibits apoptosis, lowers oxidative stress, and scavenges free radicals. As a result, it has been found that CoQ10 greatly aided and boosted the myelination of peripheral nerve fibres, promoted the regeneration of peripheral nerves, and improved histological characteristics.
ATP: adenosine triphosphate
CoQ10: coenzyme Q10
PNIs: peripheral nerve injuries
PNS: peripheral nervous system
SCs: Schwann cells
WD: Wallerian degeneration
We thank Prof. Berrin Zuhal Altunkaynak for her insightful suggestions and technical help during this study.
AM: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. BD and MEÖ: Supervision, Validation, Writing—review & editing. SK: Supervision, Conceptualization, Investigation, Writing—original draft, Writing—review & editing. All authors granted their final clearance for this version of the work to be published after revising it critically for its intellectual content and gave their final approval for this version of the paper to be published.
Süleyman Kaplan, who is the Guest Editor of Exploration of Neuroprotective Therapy, had no involvement in the decision-making or the review process of this manuscript.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
