Affiliation:
1Departamento de Psicología Biológica y de la Salud, Facultad de Psicología, Universidad Autónoma de Madrid, 28049 Madrid, Spain
Email: m.avilavillanueva@gmail.com
ORCID: https://orcid.org/0000-0001-8537-2046
Affiliation:
2Centro de Biología Molecular Severo Ochoa (UAM-CSIC), 28049 Madrid, Spain
3Center for Networked Biomedical Research on Neurodegenerative Diseases (CIBERNED), 28029 Madrid, Spain
Email: javila@cbm.csic.es
ORCID: https://orcid.org/0000-0002-6288-0571
Explor Neuroprot Ther. 2024;4:392–400 DOI: https://doi.org/10.37349/ent.2024.00090
Received: June 14, 2024 Accepted: September 02, 2024 Published: September 27, 2024
Academic Editor: Thomas Müller, St. Joseph Hospital Berlin-Weissensee, Germany
Alzheimer’s disease continuum has been described as the progressive stages of the disease over a long period. This progression can be categorized into three main stages: preclinical, mild cognitive impairment (MCI), and dementia. It has been suggested that there is a bidirectional relationship between the preclinical stage and MCI, but not between dementia and the earlier stages. The stage of MCI should be further analyzed, especially in cases where there is a reversion from MCI to a normal cognitive condition. The mechanisms behind this reversion deserve further investigation to differentiate true reversion from compensatory mechanisms. Analyzing reversion in greater detail could help identify potential therapies aimed at preventing or delaying the onset of dementia. As indicated, the primary focus has been on research indicating that MCI can revert to normal cognition. This reversion can occur by addressing risk factors through lifestyle changes, although a novel mechanism involving a transient functional compensation process in response to cognitive impairment should be also taken into account.
Alzheimer’s disease (AD) is the most prevalent form of human dementia and a significant health concern, alongside cancers, cardiovascular disorders, and microbial infections. In contrast to these health issues, the rise in AD cases over the last five years has been particularly notable [1–3]. This increase is likely due to a lack of effective disease modifiers. By the time clinical diagnosis of dementia occurs, it may be too late for pharmacological treatments or other types of interventions to be effective. Therefore, it is advisable to prevent the development of the disease before it reaches the dementia stage, such as during the mild cognitive impairment (MCI) stage.
Indeed, alterations in the brain can take place up to 20 years before a clinical diagnosis, as evidenced, for instance, in familial Alzheimer’s disease (FAD) [4]. A similar process can occur in sporadic AD (SAD) [5]. However, these initial brain changes are typically unnoticed by the individual affected, especially when they are not actively seeking treatment during that period. Essentially, AD manifests as a silent disease.
Nevertheless, prior to a clinical diagnosis, various stages preceding dementia have been identified. These stages encompass three distinct phases: preclinical, MCI, and dementia [6], with the potential for these stages to form a continuum over time [7]. The duration of each phase can vary and may be impacted by factors such as age, genetics, lifestyle choices [8], as well as early-life environmental influences [2].
Recent findings from the Framingham Heart Study Cohort indicate that individuals born between 1930 and 1970 exhibit increased brain volumes, suggesting improved brain development in younger generations (born in later decades) [2]. These results may be attributed to early life environmental factors or to an increased cognitive reserve [9], which could also be contributing to a decline in the incidence of dementia [3]. Modifying these environmental factors may retard or even reverse cognitive decline to a normal cognitive condition. In this way, a proper diagnosis of stages like MCI is important.
A long time ago, Khachaturian [10] highlighted the challenges in accurately diagnosing AD, as seen also [11, 12], and diagnosing MCI is even more complex [13–17]. MCI can be classified into four stages: a) non-cognitive impairment, b) very mild cognitive decline, c) mild cognitive decline, and d) moderate cognitive decline. However, the distinctions between these stages are not well defined. For instance, moderate cognitive decline can overlap with the early stages of AD [18]. Diagnosis primarily relies on psychological tests, which can be challenging.
There is an ongoing debate about whether digital clock and recall tests or Montreal Cognitive Assessment (MoCA) are superior to the Mini-Mental State Examination (MMSE) for detecting MCI [19–22]. Digital trail-making tests have also been suggested as an alternative to paper-and-pencil tests to reduce the subjectivity associated with traditional cognitive assessments when tests like MMSE or the MoCA are not administered properly [13]. Additionally, deep learning methods have been proposed to aid in the classification and prediction of MCI and AD [23].
For a more accurate diagnosis of MCI, complementary analyses have been recommended. Sometimes, cerebrospinal fluid (CSF) analysis and electroencephalograms are used to supplement test results [24, 25]. Moreover, imaging techniques such as NMR (nuclear magnetic resonance) or PET (positron emission tomography) scans can provide insights into brain changes that may correlate with the onset of MCI [26, 27].
On the other hand, variations in testing methods and analyses across different laboratories can lead to discrepancies in results. To standardize these results, the U.S. Food and Drug Administration (FDA) issued a Final Rule on April 29, 2024, to oversee laboratory tests, including those for AD. In Europe, efforts to harmonize neuropsychological assessments for MCI were previously documented in an article [28]. In essence, reproducible measurements using standardized parameters are essential for the development of potential future treatments for AD.
Progression from MCI to dementia can occur in about 15% of cases within two years [29], and this progression may increase to one-third of MCI patients after five years [30]. The conversion rate from MCI to AD varies widely, with reports ranging from 15% to 45% [31]. Consequently, not all MCI patients progress to dementia; a proportion may even revert to normal cognitive function. For instance, the Nun’s study suggested that 15% of MCI cases returned to normal cognition (NC) after an 11-year follow-up [32].
Several studies have reported that MCI can revert to normal cognitive function, though the rate of reversion varies widely among different reports, ranging from 15% to 45% [31, 33–37].
Understanding the true rate of reversion from MCI to NC is crucial for developing potential treatments for MCI patients (see below). Reversion can occur spontaneously without pharmacological intervention [38], although lifestyle changes may also contribute. Avoiding modifiable risk factors can reduce dementia cases and potentially induce reversion from MCI to NC [39]. Modifiable risk factors include conditions related to other disorders [40], such as hypertension [41] or diabetes [42], which can be managed with medication, as well as factors like loneliness [43], which can be alleviated through increased social activity [44].
The reported differences in the progression to dementia or reversion to NC may result from the existence of different MCI subtypes. Primarily, two types have been identified: amnestic MCI (aMCI) and non-amnestic MCI (naMCI) [45]. Additionally, a third subtype, multidomain MCI (mdMCI), has also been indicated [46]. Recent studies have identified additional criteria for recognizing MCI subtypes, utilizing more sophisticated diagnostic tools [47–49].
Little is known about the possible transition between MCI subtypes. However, several studies describe the progression of these subtypes to dementia or reversion to normal cognitive function. MCI is often considered a prodromal stage of AD [45]. Notably, aMCI has been reported to have a higher progression rate to dementia compared to other MCI subtypes [50, 51].
Structural differences between aMCI and naMCI have been observed, aMCI cases show a decreased size of the hippocampus, entorhinal cortex, and amygdala compared to naMCI cases [52]. However, about a quarter of aMCI may revert to NC.
Interestingly, a comprehensive individual study has shown a 5% progression rate from MCI to dementia within one year, which escalates to 42% within a five-year period [53]. Regarding the reversion to NC, it is noteworthy that 38% of individuals reverted to NC. However, of that 38%, 65% subsequently reverted to MCI or progressed to dementia [53] (Figure 1).
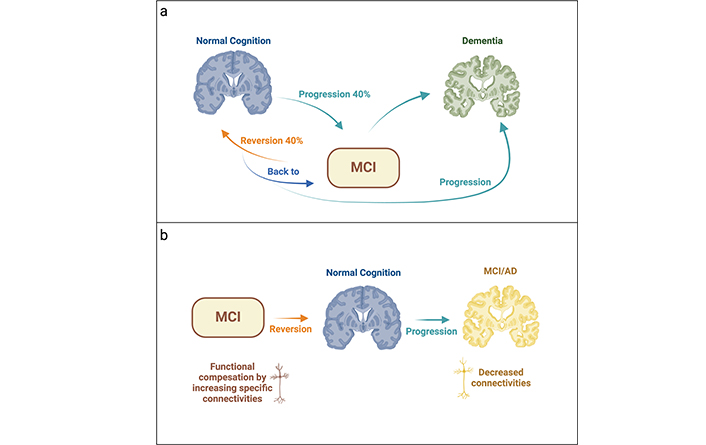
Transition from MCI to dementia or normal cognition. (a) In cases of MCI, up to 40% may progress to dementia, while another 40% may revert to a normal cognitive state. A portion of cases remains stable without significant changes, maintaining the MCI status. Over time, some individuals (around 2/3) who had previously reverted to normal cognition function may experience a regression back to MCI or dementia. (b) A potential explanation for the transition from MCI to normal cognition and vice versa, oscillating between normal cognition and MCI/AD, is proposed. MCI: mild cognitive impairment; AD: Alzheimer’s disease. Created in BioRender. Avila, J. (2024) BioRender.com/q47b111
One plausible explanation for the previous findings might involve a transient functional compensation mechanism in response to cognitive impairment, wherein alternative cognitive pathways are employed. This compensatory process could be elucidated by examining alternative patterns of connectivity through structural analysis. Longitudinal studies employing image analysis techniques have revealed notable changes in connectivity patterns. For instance, increased anterior insula connectivity, correlated with cognitive preservation (linked to overall cognition), is observed to enhance functional connectivity (FC) with the anterior cingulate cortex. Conversely, in individuals with aMCI compared to those with normal cognitive function, there is a decrease in FC between the left posterior insula and the right precuneus [54].
The heightened connectivity observed in the right insula may serve as a temporary compensatory mechanism for cognitive deficits, potentially aiding in the transition from MCI to NC. However, as the disease advances, this cognitive enhancement is likely to diminish over time. Nevertheless, some individuals may remain in a reverted NC state without returning to MCI.
As mentioned earlier, MCI presents an opportune stage for intervening in the AD continuum, ideally before the onset of dementia.
Potential interventions encompass both pharmacological and non-pharmacological approaches. Among non-pharmacological methods, lifestyle adjustments and addressing other diseases acting as risk factors could be pivotal in facilitating the reversal of MCI to normal cognitive function. However, to substantiate the efficacy of such reversals, it is advisable to not only rely on cognitive assessments but also incorporate complementary markers derived from CSF [55] or other parameters like imaging analyses [47–49]. These additional measures provide a more comprehensive understanding and validation of the transition to a normal cognitive state.
Exploring the potential mechanisms underlying the “spontaneous” reversions from MCI to normal cognitive states involves examining factors such as age, gender, social interaction, and the overall health status of MCI patients.
Recently, there has been a discussion on how the behavioral environment can influence a person’s physiology, leading to the introduction of a new concept called ‘vitaction’ [56]. This concept, along with its synonym ‘vitactin’, suggests that positive environmental behaviors may offer benefits to the brain similar to how vitamins benefit overall bodily health [57].
Regarding pharmacological interventions, patients typically selected for treatment are those in the late stage of MCI or early AD, characterized by the presence of amyloid deposits. In addition to those drugs described previously [58], new compounds like aducanumab or lecanemab are administered to assess their effects, primarily aimed at moderating the rate of cognitive decline rather than achieving clear reversals. The use of these compounds for AD has been approved by the U.S. FDA but not by the corresponding European Agency. However, studies have shown a greater efficacy in the removal of amyloid aggregates compared to cognitive improvement [59]. Unfortunately, adverse effects such as headaches, serious allergic reactions, and amyloid-related abnormalities (ARIA) have been observed under these pharmacological treatments [60].
In summary, it is established that brain changes leading to AD dementia can commence up to 20 years prior to clinical diagnosis, rendering it too early to pursue treatment at that stage. However, the phase known as MCI, which occurs in the few years preceding dementia onset, presents a promising window for intervention. Notably, reversions from MCI to normal cognitive states have been documented during this phase, suggesting it is an opportune time for treatment initiation. Further research into the mechanisms underlying these reversions is warranted, as it could shed light on their stability and inform more effective interventions [53].
AD: Alzheimer’s disease
aMCI: amnestic mild cognitive impairment
MCI: mild cognitive impairment
naMCI: non-amnestic mild cognitive impairment
NC: normal cognition
We thank Ms. Nuria de la Torre Alonso for technical and editorial assistance. Figure 1 was created with Biorender.com.
JA and MAV: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. Both authors read and approved the submitted version.
Jesús Avila, who is an Editorial Board Member of Exploration of Neuroprotective Therapy, had no involvement in the decision-making or the review process of this manuscript. The other author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work has been supported by a grant from the Spanish Ministry of Science and Innovation [PID2021-123859OB-100] from MCIN/AEI/10.13039/501100011033/FEDER, UE (J.A.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
