Affiliation:
1Department of Histology and Embryology, Faculty of Medicine, Ondokuz Mayıs University, Samsun 55139, Türkiye
2Faculty of Medicine and Health Sciences, Simad University, Mogadishu 999128, Somalia
ORCID: https://orcid.org/0000-0003-1213-9846
Affiliation:
3Department of Histology and Embryology, Faculty of Medicine, İstanbul Okan University, İstanbul 34959, Türkiye
ORCID: https://orcid.org/0000-0001-7513-0094
Affiliation:
1Department of Histology and Embryology, Faculty of Medicine, Ondokuz Mayıs University, Samsun 55139, Türkiye
4Nelson Mandela-African Institution of Science and Technology, Arusha 23311, Tanzania
Email: skaplan@omu.edu.tr; skaplanomu@yahoo.com
ORCID: https://orcid.org/0000-0003-1477-5002
Explor Neuroprot Ther. 2025;5:100496 DOI: https://doi.org/10.37349/ent.2025.100496
Received: August 26, 2024 Accepted: January 10, 2025 Published: January 26, 2025
Academic Editor: Antonio Ibarra, Anahuac University, Mexico
Aim: This study aims to investigate the effects of administering coenzyme Q10 (CoQ10) after both short-term and long-term sciatic nerve damage.
Methods: Six groups of adult male Wistar albino rats were used. Sciatic nerve injury was performed on the rats in the short-term injury (STI) and long-term injury (LTI) groups for 15 and 60 s. For 21 days, the rats in the CoQ10, STI + CoQ10, and LTI + CoQ10 groups were also administered CoQ10 orally at a dose of 10 mg/kg of body weight; the control (Cont) group received no treatment. The nerve samples were evaluated by electrophysiology, the sciatic functional index (SFI), stereological investigations, and light and electron microscopic methods.
Results: The number of myelinated axons was higher in the LTI group according to the Cont and the sham groups. The numbers of axons in the LTI and LTI + CoQ10 groups were higher than that in the STI and STI + CoQ10 groups. Latency and amplitude levels were significantly changed following STI and LTI treatment and CoQ10 treatment significantly improved the results following the injuries. SFI results showed highly significant differences between the Cont and STI, Cont and LTI, Cont and STI + CoQ10, STI + CoQ10 and LTI + CoQ10, and Cont and LTI + CoQ10 groups. Microscopic examinations indicated that LTI produced a significant change in the nerve structure than STI. CoQ10 ameliorated the degree of injury.
Conclusions: Treatment with CoQ10 following sciatic nerve damage was more successful in the LTI than the STI group, and it may, therefore, effectively improve peripheral nerve regeneration, especially following LTI.
Peripheral nerve injuries (PNIs), which are primarily caused by auto accidents and are more common in young men, cause extremely serious health issues [1]. Peripheral nerves that sustain damage from trauma or deliberate surgical resection deteriorate and change in function [2]. An increasing frequency of these injuries brings with it the necessity of treatment. Because poorly treated PNI is an important cause of nerve functional disability later in life, it is imperative that PNI be treated promptly and effectively [3]. With its bioenergetic and anti-inflammatory properties, coenzyme Q10 (CoQ10) is an antioxidant that keeps nerve cells from dying. Antioxidants inactivate redox components and directly neutralize free radicals, preventing or delaying the oxidation of physiologically active substances [4]. The human body produces free radicals, which antioxidants help to break down to prevent cellular damage [5]. CoQ10 acts like a vitamin in the body’s cells and can be used to treat a variety of ailments, such as heart disease, high blood pressure, headaches, high blood sugar levels, fertility problems, malignancies, muscular disorders, neurological disorders like Parkinson’s illness (PD), and many more. Administration of CoQ10 can raise mitochondrial concentrations in the brain, which boosts its potential utility in treating neurodegenerative illnesses [6]. Additionally, CoQ10 is a vital preventive measure against the onset of neuropathic pain [7]. As a result, research has been done on how CoQ10 affects different oxidative processes and its antioxidant qualities have been established. Additionally, research has assessed its neuroprotective potential against loss and damage to neurons [8].
Investigating the possible therapeutic effectiveness of oral CoQ10 treatment against experimentally generated acute sciatic nerve injury in an adult rat model was the main goal of this investigation. By assessing the study groups’ electrophysiological testing and sciatic functional index (SFI) values, another goal was to demonstrate the degree of functional recovery. One useful sign of possible functional improvement at the cellular level will be tracking the functional and morphological changes between the groups.
A thorough analysis of the short- and long-injury and therapy groups at the cellular level may also produce ultrastructural and histopathological results that support the mechanisms via which CoQ10 enhances peripheral nerve regeneration.
Ondokuz Mayıs University’s Animal Experiments Ethics Committee gave the project approval on March 31, 2017, with decision number 2017/13. The experiment was conducted in accordance with Ondokuz Mayıs University’s Animal Research Ethics Committee rules. The National Institutes of Health guide for the care and use of Laboratory animals (NIH publications No. 8023, revised 1978), the EU Directive 2010/63/EU for animal experiments, or the U.K. Animals (Scientific Procedures) Act, 1986 and related guidelines followed for all animal experiments by the authors. All animal experiments also adhered to the ARRIVE guidelines 2.0 (Animal Research: Reporting of In Vivo Experiments).
Thirty-six adult male Wistar albino rats (aged 10 ± 2 weeks and weighing 250 ± 30 g) were used in the study. All the rats were obtained from the Ondokuz Mayıs University Animal and Experimental Research Centre. The animals were housed in plastic cages under normal conditions (at approximately 24°C in a 12/12 hour dark/light cycle) with ad libitum access to food and water.
The experimental groups of the study are summarized as follows and Figure 1.
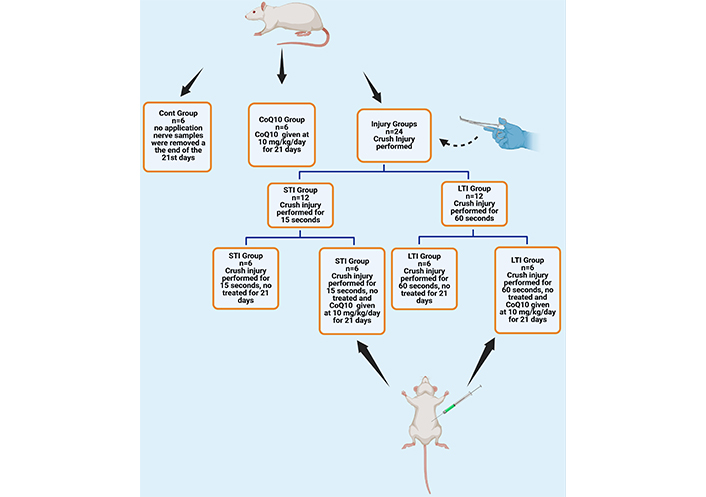
An illustration of the animal groups was used in the experiment. Cont: control; CoQ10: coenzyme Q10; STI: short-term injury; LTI: long-term injury groups. Created in BioRender. Altunkaynak, B. (2025). BioRender.com/e83s272
Groups:
Control (Cont) group: No experimental procedure was applied to this group over the 21-day experimental period. This group was established solely to obtain basal values.
Coenzyme Q10 (CoQ10) group: CoQ10 (Sigma-Aldrich cat no: C9538; Labor Lab Tech; İstanbul- Türkiye) was administered by gavage to this group at a fixed dose of 10 mg/kg rat body weight for 21 consecutive days. CoQ10 powder was dissolved in water [8].
Short-term injury (STI) group: The sciatic nerves of the rats in this group were subjected to crush injury for 15 s [9].
Long-term injury (LTI) group: The sciatic nerves of the rats in this group were subjected to crush injury for 60 s [8].
STI + CoQ10 Group: The sciatic nerves of the rats in this group were treated with CoQ10 at a fixed dose of 10 mg/kg of rat body weight administered by gavage for 21 days consecutively after exposure to crush injury for 15 s.
LTI + CoQ10 Group: The sciatic nerves of the rats in this group were treated with CoQ10 at a fixed dose of 10 mg/kg of rat body weight administered by gavage for 21 days consecutively after exposure to crush injury for 60 s.
The right sciatic nerves of all rats were surgically exposed following anaesthetization by the intraperitoneal administration of 100 mg/kg ketamine hydrochloride (Ketalar, Parke-Davis, Eczacıbaşı) and 10 mg/kg of xylazine hydrochloride (Rompun) solutions, respectively [10]. The skin over the right sciatic nerve was shaved and cleaned with iodine, and an incision was made on the mid-thigh muscle of the right leg. In the short and LTI groups, the injury area and boundaries are signed by a silk suture (Figure 2B). Then, the right sciatic nerve was injured for 15 s and 60 s, respectively, by applying 50 N of force via forceps (Figure 2C). After the injury model had been created, the muscle was sutured with 3-0 silk, and the skin over the wound was sutured with 2-0 silk. At the end of the recovery period (21st day), nerve samples (1 cm) were removed from the 5 mm distal part of the injury site (Figure 2D) [8].
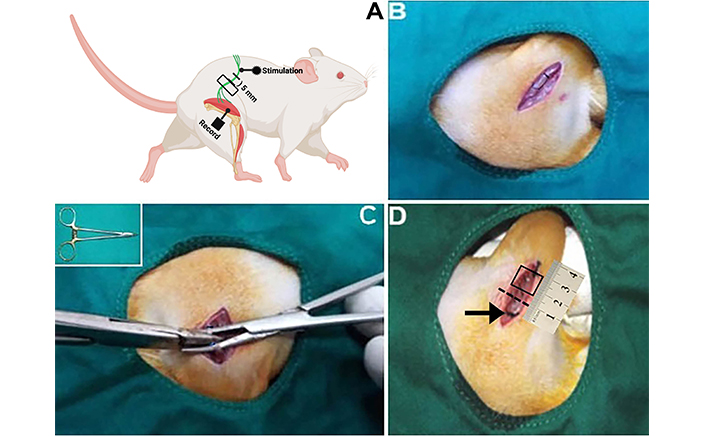
A diagram for electrophysiological record site and pictures of surgery steps. (A) An example of how to perform surgery and where to place the electromyography (EMG) stimulation and recording probes for electrophysical analysis. (B) The rat’s right leg was shaved after it had been positioned and cleaned. The skin is incised in a parallel line, 3–4 mm above the femur. The location was cautiously entered, the muscle was pulled, and the sciatic nerve was reached. A 3-0 silk suture was used to knot the tiny ring around the sciatic nerve, securing it in place and preventing it from sliding along the nerve and obstructing blood flow to the nerve’s outer membrane. (C) The sciatic nerve was injured with 50 N of force via forceps (showed inset in C). (D) At the end of the recovery, nerve samples (1 cm) were removed from the 5 mm distal position of the injury site. The arrow and dashed line show the proximal and distal ends of the injury site. Box in D shows a sample area of removed nerve pieces. The dashed line shows the distal border of the injury site. Figure A was created in BioRender. Altunkaynak, B. (2025). BioRender.com/b69r934
A Power-Lab4SP (AD Instruments, Sydney, Australia) device and Scope software (version 3.7.2, AD Instruments) were used to record electromyography (EMG). A stimulating electrode was attached to the proximal end of the right sciatic nerve trunk in order to electrically stimulate the sciatic nerve after an incision was performed to reveal it. Two monopole electrodes were positioned 2.5 cm from the stimulation electrode to record the medial and lateral gastrocnemius muscles (Figure 2A). A stimulation voltage between 0.01 mV and 10 mV was used to measure the gastrocnemius muscle’s compound muscle action potential (CMAP). Data on latency and amplitude were recorded for each group. During these tests, anesthesia was administered to the rats.
The waveform was measured from the baseline (I) to the negative peak (II) and from the negative peak (II) to the positive peak (III) in order to determine the baseline-to-peak (B-P) and peak-to-peak (P-P) CMAP amplitudes. Each animal was measured three times, and the average results were then statistically examined.
The walking footprints of the animals were measured after their feet had been immersed in methylene blue prior to testing. The following numerical parameters were computed following the test:
PL (print length): [normal print length (NPL) – experimental print length]/NPL is the distance between the heel and the third toe.
The distance between the first and fifth toes is known as TS (toe spread): [experimental toe spread – normal toe spread (NTS)]/NTS. The distance between the second and fourth toes is known as ITS (intermediary toe spread): [normal intermediate toe spread (NITS) – experimental intermediate toe spread/NITS]. A standardized test called the SFI is used to assess the functional nerve recovery following sciatic nerve damage in rats. This test uses the many relationships between the toes of the body to identify sciatic nerve repair. A standardized test called the SFI is used to assess the functional nerve recovery following sciatic nerve damage in rats. In this test, the various interactions between the hind limbs’ toes are used to identify sciatic nerve repair. The following formula is used to determine this functional relationship from the footprints found along a consistent walking route:
where SFI is the sciatic function index, EPL, NPL, and ETS, NTS, EITS, and NITS stand for experimental print length, normal print length, experimental toe spread, normal toe spread, experimental intermediate toe spread, normal intermediate toe spread, respectively. A normal or ordinary function is indicated by an SFI value of zero (0), whereas a total loss of function is indicated by an SFI value of minus one hundred (–100) [11]. The footprints of the healthy (left) and injured (right) individuals were manually measured in millimeters.
On the 21st day of the experiment, following the electrophysiological analyses, sciatic nerve samples were excised from just the distal position of the injury sites while the rats were under anesthesia (by the intraperitoneal administration of 1 mg/kg and 5 mg/kg ketamine and xylazine solutions, respectively). After taking nerve samples animals were decapitated by personnel with a certificate for the use of laboratory animals. Nerve samples immediately were placed into a 5% glutaraldehyde solution (Sigma-Aldrich cat no: G6257; Labor Lab Tech; İstanbul-Türkiye) for fixation. Following, the preparation of polymerized resin blocks [12]; nerve sections were taken at the transverse plan by using an ultramicrotome (Leica EM UC7, Vienna, Austria). Semi-thin sections (at 500 nm thickness) were taken on glass slides and stained by toluidine blue (Sigma-Aldrich cat no: 198161; Labor Lab Tech; İstanbul-Türkiye), while thin sections (at 70 nm thickness) were placed on grid meshes and stained by uranyl acetate (Sisco-Lab cat no: 6159-44-0; Analitik Kimya Ltd. Sti; İstanbul-Türkiye) and lead citrate (Sigma-Aldrich cat no: 15326; Labor Lab Tech; İstanbul-Türkiye). Semi-thin sections were examined under the light microscope (Olympus BX-43, Tokyo, Japan) and stereologically analyzed. Thin sections were examined under an electron microscope (JEOL JSM-7001F, JEOL Ltd., Tokyo, Japan), and micrographs were captured by the camera attachment of the microscope.
The stereological approach was used to compare the sciatic nerve’s area and axon count between the groups. Sections were analyzed using a light microscope (Olympus BX-43, Tokyo, Japan) on a manual workstation with a digital camera for stereological analysis. A systematic random sampling (SRS) method is used to obtain quantitative and objective information [13, 14]. We estimated the overall myelinated axon number using the two-dimensional fractionator technique. A SRS method was used to choose the sampled area, and an unbiased counting frame was fixed on the monitor screen [12, 15]. Axons that touch the dashed lines and are totally inside the unbiased counting frame were counted; axons that touch the continuous lines were not counted [16]. A plan objective (100×) was used to count axons in the sciatic nerve (objective NA = 1.25 oil). The nucleator method was used in the same sections to estimate myelinated axon cross-section areas and myelin sheath thicknesses [17]. The quantity of error resulting from sampling assessment procedures in stereological research can be defined as the coefficient of error (CE). The CE for appropriate sampling was regarded as ≤ 0.05, and the coefficient of variation (CV) was suitable for determining variability in the estimation of a group [18, 19].
The groups’ mean ± SD values were compared during the statistical analysis, which was performed on SPSS for Windows software version 20.0 (IBM Corp., Armonk, NY). Differences between groups were determined based on a 0.05 significance level (p). One-way ANOVA (post hoc, Tukey’s test) was used for group comparisons. p values < 0.05 were considered statistically significant.
Myelinated axon numbers differed significantly between the Cont and LTI (p < 0.05), STI and STI + CoQ10 (p < 0.01), LTI and LTI + CoQ10 (p < 0.01), STI and CoQ10 (p < 0.01), and STI and LTI groups (p < 0.01) (Figure 3A).
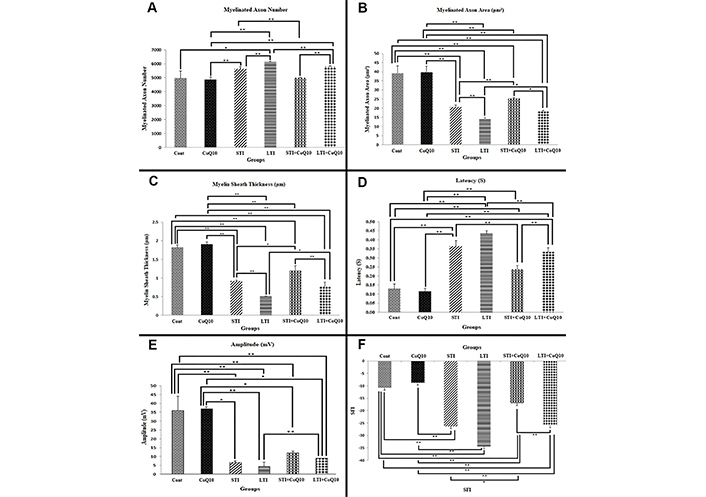
Stereological, electrophysiological analyses, and behavioral tests. (A) A comparison of the numbers of myelinated axons in the different groups. (B) A comparison of the areas of myelinated cross-sectional axons in the different groups. (C) A comparison of the thicknesses of the myelin sheath between the groups. (D) A comparison of group latency values. (E) A comparison of group amplitude values. (F) SFI value comparison between the groups. (*) indicates statistically significant differences at p < 0.05, whereas (**) indicates significant differences at p < 0.01. All data are expressed as mean ± standard deviation
Examination of myelinated axon cross-sectional areas revealed highly significant differences between the Cont and STI (p < 0.01), Cont and LTI (p < 0.01), Cont and STI + CoQ10 (p < 0.01), Cont and LTI + CoQ10 (p < 0.01), STI and STI + CoQ10 (p < 0.01), LTI and LTI + CoQ10 (p < 0.05), and STI and LTI groups (p < 0.01). Myelinated axon cross-sectional areas in the Cont and CoQ10 groups were significantly greater than those in the STI, LTI, STI + CoQ10, and LTI + CoQ10 groups (Figure 3B).
Myelin sheath thickness differed significantly between the Cont and STI (p < 0.01), Cont and LTI (p < 0.01), Cont and STI + CoQ10 (p < 0.01), Cont and LTI + CoQ10 (p < 0.01), and STI and STI + CoQ10 groups (p < 0.05). Similarly, significant differences were also observed between the LTI and LTI + CoQ10 (p < 0.05), STI + CoQ10 and LTI + CoQ10 (p < 0.01), and STI and LTI (p < 0.01) groups (Figure 3C).
CE and CV belonging to the stereological analysis are given in Table 1.
Data on the coefficient of error (CE) and coefficient of variation (CV) for the mean myelinated axon number estimation; the mean cross-sectional area of myelinated axon estimation and the mean myelin sheath thickness estimation, respectively
| Group | Myelinated axon number estimation | Myelinated axon area estimation | Myelin sheath thickness estimation | |||
|---|---|---|---|---|---|---|
| CE | CV | CE | CV | CE | CV | |
| Cont (n = 6) | 0.02 | 0.09 | 0.01 | 0.09 | 0.03 | 0.02 |
| CoQ10 (n = 6) | 0.01 | 0.03 | 0.02 | 0.07 | 0.02 | 0.03 |
| STI (n = 6) | 0.03 | 0.02 | 0.04 | 0.04 | 0.03 | 0.01 |
| LTI (n = 6) | 0.04 | 0.01 | 0.05 | 0.03 | 0.05 | 0.02 |
| STI + CoQ10 (n = 6) | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 0.09 |
| LTI + CoQ10 (n = 6) | 0.04 | 0.01 | 0.04 | 0.03 | 0.03 | 0.12 |
Cont: control; CoQ10: coenzyme Q10; STI: short-term injury; LTI: long-term injury
Figure 3D displays the results of comparing the group data in terms of latency values. Between the Cont and STI (p < 0.01), Cont and LTI (p < 0.01), Cont and STI + CoQ10 (p < 0.01), and Cont and LTI + CoQ10 (p < 0.01) groups, there were highly significant differences. The STI and STI + CoQ10 groups likewise showed a significant difference (p < 0.01), as did the LTI and LTI + CoQ10 groups (p < 0.01) and the STI + CoQ10 and LTI + CoQ10 groups (p < 0.01). The STI and LTI groups did not, however, vary statistically significantly (p > 0.05).
Figure 3E displays the pertinent data from the comparison of the groups’ amplitude values. The amplitudes of the Cont and CoQ10 groups did not differ significantly (p > 0.05). The Cont and STI groups, the Cont and LTI groups, the Cont and STI + CoQ10 groups, and the Cont and LTI + CoQ10 groups, on the other hand, all showed a highly significant difference (p < 0.01). The STI and STI + CoQ10 groups did not differ statistically (p > 0.05). However, there was no significant difference between the STI + CoQ10 and LTI + CoQ10 groups (p > 0.05) or between the STI and LTI groups (Figure 3E), but there was a highly significant difference between the LTI and LTI + CoQ10 groups (p < 0.01).
SFI values were also compared between the groups, the values being shown in Figure 3F. Although there was no significant difference between the Cont and CoQ10 groups (p > 0.05), highly significant differences were observed between the Cont and STI (p < 0.01), Cont and LTI (p < 0.01), Cont and STI + CoQ10 (p < 0.01), STI + CoQ10 and LTI + CoQ10 (p < 0.01), and Cont and LTI + CoQ10 (p < 0.01) groups. A substantial but not highly significant difference was observed between the STI and STI + CoQ10 groups (p < 0.05), but none between the LTI and LTI + CoQ10 (p > 0.05), or STI and LTI (p > 0.05) groups.
The sciatic nerve tissues stained with toluidine blue in the Cont and CoQ10 groups showed normal nerve fiber structure. Except for a few minor myelin sheath abnormalities and small separation regions (Figures 4A–D), the histological characteristics of the axons, myelin sheath, Schwann cells, and epineurium all seemed normal. The STI group’s evaluation demonstrated minimal impairment in nerve fiber architecture as a result of nerve fiber damage. New axons were generated, with smaller diameters than conventional axons. Some nerve sections showed disorganized and separated myelin sheaths. Mast cells and blood vessels were also detected (Figures 4E and 4F). The histological properties of nerve fibers were improved in the STI + CoQ10 group, including enhanced myelin sheath thickness and axon diameters. Several axons seemed disordered, with the myelin sheath split (Figure 4G). Schwan cells were abundant in various regions (Figures 4G and 4H). The architecture of nerve fibers in the LTI group was severely damaged. This group’s nerve cross-sections revealed several freshly regenerated tiny axons with a very thin myelin sheath and small diameter. The loss of several axons in this group caused an increase in the distance between nerve fibers. Following damage, all areas showed an increase in the number of Schwan cells, which play an important role in nerve regeneration. High levels of vascularization and connective tissue were seen (Figures 4J and 4K). The nerve fiber architecture remained intact in the LTI + CoQ10 group. Except for a few minor myelin separations in nerve fibers, myelin sheath thickness and axon diameter increased. This group had an increased number of Schwan cells (Figures 4L and 4M).
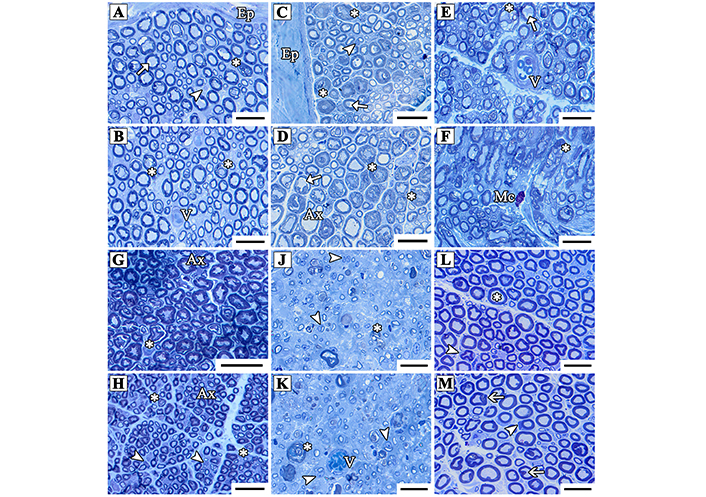
Light micrographs of the cross-sectioned sciatic nerve from the Cont (A, B); CoQ10 (C, D); STI (E, F); STI + CoQ10 (G, H); LTI (J, K); and LTI + CoQ10 (L, M) groups. The arrows show vacuolization in the myelin sheath, the arrowheads indicate Schwann cells, and asterisks indicate myelin sheath disarrangement in the nerve fibers. Ax: healthy axons; Ep: Epineurium; V: blood vessels; Mc: mast cell. The resin sections were taken at a 0.5 µm thickness and stained with toluidine blue. Magnification bars: 10 µm
Electron microscopy showed normal nerve fiber structure in the Cont and CoQ10 groups. Axon diameter and myelin sheaths were typical, with the exception of a little separation of the myelin sheath from the axon. Some portions of the Cont group had unmyelinated nerve fibers and Schwann cells (Figures 5A–D). The majority of nerve fibers in the STI group appeared to have normal structure. Many axons had normal sizes and were myelinated. Only a few nerve fibers have substantially damaged myelin sheaths. Most sections revealed unmyelinated nerve fibers and Schwann cells, with blood vessels visible in a few places from the STI group (Figures 5E and 5F).
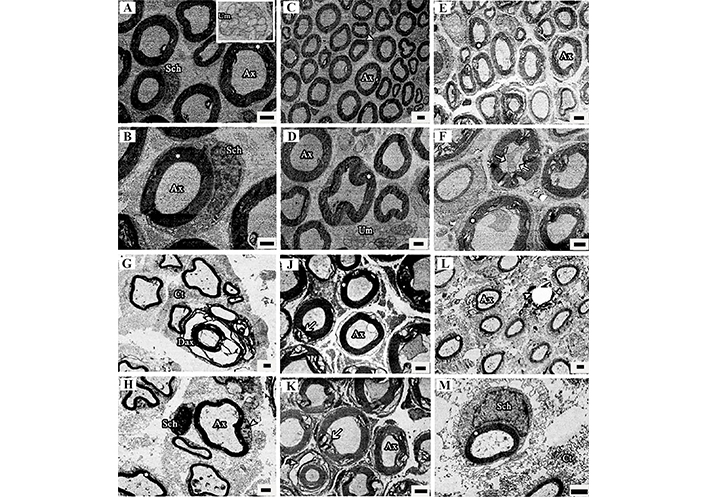
The electron microscopic images of sciatic nerves from the Cont (A, B); CoQ10 (C, D); STI (E, F); LTI (G, H); STI + CoQ10 (J, K); and LTI + CoQ10 (L, M) groups. Asterisks show a healthy myelin sheath in the nerve fibers. The arrows show degenerations in the myelin sheath, and the arrowheads indicate regenerated immature axons; Ax: healthy axons; Sch: Schwann cell; Um: unmyelinated nerve; Dax: degenerated axon; Ct: connective tissue. The resin sections were 70 nm in thickness and stained with uranyl acetate and lead citrate. Magnification bars are 2 μm
LTI significantly destroyed the sciatic nerve’s normal histology and many damaged nerve fibers were discovered, and several sciatic nerves in this group showed severe nerve fiber degeneration. In most parts, the myelin sheath and axon diameter were exceedingly thin and tiny, respectively, however, the myelin sheath thicknesses and axon diameter were normal in other locations. Following LTI, the normal microstructure of the sciatic nerve was significantly altered. Many deteriorated nerve fibers were discovered, and certain sciatic nerves in this group showed severe nerve fiber degeneration. In most portions, the myelin sheath and diameter of axons were extremely thin and tiny, respectively, however, the myelin sheath thicknesses and axon diameter were normal in certain locations. After an injury, debris from the nerve fibers and other components must be eliminated, necessitating the presence of a significant number of macrophages. A high density of macrophages, which eliminate nerve fiber and other detritus, was seen in nerve slices from the LTI group (Figures 5G and 5H).
When the sections of the STI + CoQ10 group were evaluated, in certain portions, nerve fibers, axons, and myelin sheaths were restored to their original forms, but in others, structures degraded and myelin separated. Many Schwann cells and unmyelinated nerve fibers were also seen in several sections of the STI + CoQ10 group (Figures 5J and 5K). In the LTI + CoQ10 group, several structural changes reverted to normal state compared to the LTI group. The myelin sheath and axon size increased, although certain myelin sheaths were very thin and the axon diameter was quite tiny. A few deteriorated axons were seen in certain nerve sections, together with macrophages, connective tissues, Schwann cells, and unmyelinated axons (Figures 5L and 5M).
Peripheral nerves are susceptible to a variety of injuries and diseases [20, 21]. These are typically connected with traumatic experiences, which have a severe influence on quality of life [22]. However, these nerves can recover following damage [23]. Post-PNI repair is determined by the nature and severity of the injury. Nerve compression preserves the structural components of the nerve tissue, allowing for non-surgical repair [24]. Much study has focused on specific healing pathways to improve peripheral nerve regeneration [25]. Research on peripheral nerve repair has also led to the discovery of more effective nerve regeneration technologies [23].
In the inner mitochondrial membrane, CoQ10 exchanges electrons and is secreted endogenously [26]. Reactive oxygen species (ROS) are produced by mitochondria during the electron transport chain. Additionally, they aid in the regulation of oxidative stress, cell death, and preservation [27]. Oxidative damage happens as a result of increased free radical production, which triggers oxidative stress systems in the cells. Here, CoQ10 scavenges free radicals and stops proteins and lipids from peroxiding. One essential property of an antioxidant is the ability to donate and accept electrons [28]. CoQ10 can lessen oxidative stress and regulate cellular oxidation capability [29]. Moreover, CoQ10 comes in a variety of forms and is offered. The Food and Drug Administration (FDA) is aware of the inactive basic compounds present in these formulations [26].
It is widely known that CoQ10 has anti-inflammatory properties [28]. Studies conducted at the cellular level and in animal models have documented the anti-inflammatory properties of CoQ10. Antioxidant substances like CoQ10, which work to stop inflammation and oxidative stress, can stop the development of many illnesses and may even be used as a treatment [30]. By lowering inflammation, it seems to be a potent antioxidant that plays a crucial part in preventing damage to neural tissue and neuropathic pain [31]. CoQ10 is a powerful scavenger of free radicals that can lessen oxidative stress and lipid oxidation in DNA damage [32]. Functional recovery is one of the primary objectives of treatment options for PNI [33]. The possible therapeutic benefits of CoQ10 in the various groups’ functional recovery and sciatic nerve repair were compared in this study. Compared to the Cont and CoQ10 groups, sciatic nerve function was noticeably worse in the LTI and STI groups. In comparison to the LTI + CoQ10 group, the STI + CoQ10 group had a more robust functional recovery. SFI levels were measured following 21 days of CoQ10 treatment in order to assess recovery. After being exposed to CoQ10, the SFI results demonstrated a good functional improvement. These findings led us to the conclusion that CoQ10 promoted and improved functional recovery. The STI + CoQ10 and LTI + CoQ10 groups showed nearly no sciatic nerve healing. When it came to SFI values, CoQ10 worked better in the STI + CoQ10 group than in the LTI + CoQ10 group.
In comparison to the STI and LTI groups, our results showed that CoQ10 had a favorable impact on the number of myelinated axons in the STI + CoQ10 and LTI + CoQ10 groups. Additionally, because nerve fibers spread, there were significantly more axons in the STI and LTI groups than in the Cont and CoQ10 groups. The number of myelinated axons was found to differ significantly between the STI and LTI groups. In contrast to the Cont and CoQ10 groups, these were greater in the STI, LTI, STI + CoQ10, and LTI + CoQ10 groups. Due to nerve fiber spreading following damage, the LTI group had the greatest number of axons. This is because the axons produce several branches so that one of them can reach the distal end when the Schwann cell numbers continue to rise following PNI. Following any damage, the affected nerve’s myelinated axon count significantly rises. But compared to the Cont group, they are smaller and have weaker myelin sheaths. In comparison to the Cont and CoQ10 groups, the stereological data showed a reduced mean axon area in the STI, LTI, STI + CoQ10, and LTI + CoQ10 groups. The LTI group had the smallest mean axon area. This result was in line with that of Ramli et al. [9], who found that the mean axon area in their damage group was much less than that of the Cont group. Stereological evaluations comparing the groups showed that the mean axonal area was negatively correlated in the wounded groups relative to the Cont group, while the number of axons was positive. As the number of myelinated axons increased, the mean axon area decreased. The LTI group, of course, had the greatest number of axons and the lowest mean axon area. Comparing the STI, LTI, STI + CoQ10, and LTI + CoQ10 groups to the Cont and CoQ10 groups, a significant reduction in myelin sheath thickness was noted. The LTI group had the smallest myelin sheath, whereas the Cont and CoQ10 groups had the thickest. This study’s stereological findings aligned with those of the earlier investigation [34].
The myelinated axon area and myelin sheath thickness of the STI and STI + CoQ10 groups were significantly different from those of the LTI and LTI + CoQ10 groups, according to our research. There was a significant difference between the LTI and STI groups. The myelinated axon area and myelin sheath thickness in the STI and LTI groups were significantly reduced in comparison to the STI + CoQ10 and LTI + CoQ10 groups. These results showed that CoQ10 positively affected the axon area and myelin sheath thickness of the regenerated nerve fibers. In the STI + CoQ10 group, CoQ10 had a greater effect on the myelinated axon area and myelin sheath thickness than in the LTI + CoQ10 group. This implies that over time, the sciatic nerve in the LTI + CoQ10 group would experience further damage.
Rat sciatic nerves from the damage groups showed significant nerve tissue deterioration based on histological evaluation, but sciatic nerve structure improved after CoQ10 therapy. A previous study demonstrated that CoQ10 improved the sciatic nerve’s histology in comparison to the damage groups, which is consistent with our findings [35]. According to our electron microscopy analysis of sciatic nerve regeneration, CoQ10 is useful in restoring the damaged sciatic nerve’s morphology.
In order to start the breakdown and removal of myelin and to activate Schwann cells’ capacity for tissue regeneration, mast cells, and endoneurial macrophages provide a number of cytokines and neurotrophic factors [36]. Peripheral nerve axon myelination depends on Schwann cells and this process takes place when Schwann cells coil over the axons, creating a myelin sheath made up of many layers of thick cytoplasmic membrane [10]. Because more Schwann cells are required to form tubular structures and swiftly eliminate the damaged nerve fiber debris so that new, regenerated nerve fibers may develop, more macrophages and Schwann cells were observed in the injured site in the current investigation. The Schwann cells will construct this tubule, which will direct the newly generated axon to its final location. To summarize, increased numbers of nerve fibers and macrophages will be observed at the site following nerve injury.
The muscle’s CMAP amplitude was significantly high in the Cont and CoQ10 groups in the current study. While CMAP latency highlighting myelin sheath thickness reduced in the STI + CoQ10 and LTI + CoQ10 groups relative to the STI and LTI groups, the amplitude increased in the STI + CoQ10 and LTI + CoQ10 groups. There was no discernible change in latency or amplitude between the STI and LTI groups. According to our results, the CoQ10-treated groups—specifically, the STI + CoQ10 and LTI + CoQ10 groups—show improvements in CMAP amplitude and latency. In both amplitude and latency, CoQ10 performed better in the STI + CoQ10 group than in the LTI + CoQ10 group. These findings align with prior research conducted by Sadraie et al. [37]. The antioxidant of CoQ10 significantly decreased deficits in nerve conduction, according to another research that observed the same result [5].
While the LTI + CoQ10 group did show sciatic nerve repair as a result of CoQ10, the STI + CoQ10 group showed the most improvement. According to our research, taking CoQ10 orally may help to speed up neural regeneration and stop degeneration. A previous research found the same results [34]. Additionally, those scientists noted that CoQ10 had a protective impact on nerve regeneration following damage. The authors came to the conclusion that post-PNI nerve regeneration is enhanced by CoQ10 therapy. They assessed the impact of CoQ10 on damaged nerve regeneration using the same methods, including electrophysiological and walking tracks. In recent years, CoQ10 has been utilized to treat a variety of neurological diseases. Many clinical diseases are also treated with it. Studies highlight the advantages of CoQ10 [38]. Even at the stated maximum dosages, CoQ10 treatment is safe, and several previous studies have found no significant negative effects of CoQ10 usage [39]. CoQ10’s efficacy and safety in treating human illnesses have also been confirmed [40]. After PNI, endogenous neural progenitors contribute to the promotion of nerve regeneration. The regulatory impact of epigenetic changes, including those caused by non-coding RNAs (ncRNAs), covalent histone modification, and DNA methylation, on the expression of genes linked to neural regeneration following PNI has attracted a lot of attention. These results may open up new possibilities for axonal regeneration research.
Nevertheless, further molecular research is required to fully light on the mechanism and route at play. The study’s findings demonstrated that CoQ10 had a protective impact on the damaged nerves. Treatment with CoQ10 for an extended period of time is safe and well tolerated [41]. In terms of CoQ10 therapy safety, this study found no adverse effects. The CoQ10, a safe natural antioxidant, is supported by the lack of adverse effects. In order to properly assess the peripheral nerve using electrophysiology, stereology, and walking track approaches for the sciatic nerve study, we merged these methods.
In summary, CoQ10 contributes to the molecular control of regeneration, which may help reduce the morphological damage and function loss of the damaged sciatic nerve. Therefore, it can be said that the antioxidant CoQ10 prevents nerve weakening and promotes the sciatic nerve’s ability to regenerate after damage.
CMAP: compound muscle action potential
Cont: control
CoQ10: coenzyme Q10
EITS: experimental intermediate toe spread
EPL: experimental print length
ETS: experimental toe spread
ITS: intermediary toe spread
LTI: long-term injury
NITS: normal intermediate toe spread
NPL: normal print length
NTS: normal toe spread
PL: print length
PNIs: peripheral nerve injuries
SFI: sciatic functional index
SRS: systematic random sampling
STI: short-term injury
TS: toe spread
This study is a part of Ahmed Omer Mead’s PhD thesis, “Effects of the Coenzyme Q10 on the peripheral nerve injury” from the Ondokuz Mayıs University. In addition, the text underwent revision by Carl Nino Rossini, a native speaker. We appreciate his excellent language revision.
AOM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft. BZA: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Validation. SK: Conceptualization, Project administration, Supervision, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that there are no conflicts of interest.
The Animal Experiments Ethics Committee of Ondokuz Mayıs University accepted this work under decision no. 2017/13 dated 31.03.2017, which complies with the National Guidelines for Animal Use in Research (Türkiye). The experiment was conducted in compliance with the regulations set out by Ondokuz Mayıs University’s Animal Research Ethics Committee. This experiment was conducted in strict accordance with the ARRIVE standards 2.0 (Animal Research: Reporting of In Vivo Experiments).
Not applicable.
Not applicable.
Upon reasonable request, the corresponding author will provide all datasets used and/or analyzed in the current work.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3551
Download: 44
Times Cited: 0
