Abstract
Third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have shown impressive results in EGFR mutant lung cancer (LC) patients in terms of disease control rate with a positive impact on overall survival. Nevertheless, after months of treatment with targeted therapy, progression inevitably occurs. Some patients develop oligoprogression and local treatment is required for optimal disease control while maintaining EGFR-TKIs. This work features a clinical case of a patient harboring an EGFR mutant LC undergoing oligoprogression to EGFR-TKIs, first into the brain and afterward to the primary tumor, requiring local ablative strategies, including primary tumor resection three years after the start of osimertinib. Currently, the patient is still alive and continues with a complete response upon EGFR-TKIs maintenance. Hence, oligoprogression, even in driven oncogenic tumors, represents a distinct biological entity and potential curative disease that deserves particular consideration in multidisciplinary tumor boards. In this case, tumor primary resection after three years of the initial diagnosis represents a paradigm shift in the treatment of EGFR mutant patients.
Keywords
Lung adenocarcinoma, epidermal growth factor receptor, oligoprogression, targeted therapy, epidermal growth factor receptor-tyrosine kinase inhibitorsIntroduction
Activating oncogenic mutations in epidermal growth factor receptor (EGFR) are detected in up to 20% of lung adenocarcinomas (LuADCs) and are the most relevant predictor markers of response to EGFR-tyrosine kinase inhibitors (TKIs). Historically, EGFR-TKI treatment prolonged progression-free survival (PFS) in comparison to chemotherapy as an up-front treatment. Osimertinib is an irreversible third (3rd) generation EGFR-TKI that selectively inhibits both EGFR sensitizing mutations and the gatekeeper EGFR T790M, which typically arises after developing resistance to first (1st) generation and second (2nd) generation inhibitors [1]. Results from the FLAURA study showed that osimertinib significantly increases and almost doubles the median PFS in patients with previously untreated EGFR mutant LuADCs compared to 1st generation and 2nd generation EGFR-TKI group-hazard ratio (HR) 0.46 [95% confidence interval (CI), 0.37–0.57] [2]. In addition, osimertinib also has a greater ability to cross the blood-brain barrier and presents a higher intracranial response rate compared to the classic EGFR-TKIs (91% vs. 68%) [3].
However, despite a robust initial response to TKIs, acquired resistance (AR) mechanisms inevitably occur and different mechanisms have been described. This work reported a case of an EGFR mutant patient who underwent oligoprogression to osimertinib and benefited from local ablative strategies during the course of the disease, including resection of the primary lung tumor after three years of initial systemic treatment. The multidisciplinary approach discussed in a multidisciplinary tumor board (MTB) and the study of underlying molecular resistance mechanisms has been essential for the patient’s management.
Case report
Here, a case report of a 53-year-old male patient, Caucasian, ex-smoker (10 packs/year), with no medical-surgical history of interest. His father died of lung cancer (LC) at the age of 67 years, and three nephews were diagnosed with breast cancer (34 years), gynecological cancer (40 years), and colorectal cancer (38 years).
In June 2019, he consulted for right pleuritic pain and the study was started. Chest-abdominal computed tomography (CT) revealed a 45 mm × 39 mm heterogeneous mass in the right upper lobe (RUL) and right lower paratracheal lymphadenopathy. A transbronchial biopsy was performed through bronchoscopy (BS) and pathological anatomy (PA) confirmed the diagnosis of LuADC. The molecular study by next-generation sequencing (NGS, Oncomine Solid Tumour, Life Technologies) identified 18 nucleotides deletion in exon 19 of EGFR (NM_005228.5): c.2240_2257del (p.Leu747_Pro753delinsSer, EGFRdel19). The extension study by positron emission tomography (PET)-CT and brain magnetic resonance imaging (MRI) confirmed: (1) the presence of a hypermetabolic mass in RUL with mediastinal infiltration; (2) a bone lesion in dorsal vertebra 9 (D9) confirmed by spinal MRI; and (3) a single left frontal lesion of 2 cm, compatible with brain metastasis, staging being cT2bN2M1c. In August 2019, the patient with Eastern Cooperative Oncology Group performance status (ECOG PS) 0 started treatment with osimertinib. CT scan and brain MRI for response assessment showed a reduction of the tumor in all locations, assessed as a partial response. In October 2020, cerebral oligoprogression was reported due to an increase in the frontal lesion and single-dose radiosurgery (SRS) up to 20 grays (Gy) was performed maintaining osimertinib afterward, as a systemic treatment (Figure 1). In November 2021, the CT showed an increase in the lung lesion, maintaining a complete response in the rest of the locations, including bone lesion at D9, by PET-CT (Figure 2). A BS was performed but did not obtain enough tumor material to perform a histopathological study. After discussion in the lung MTB, a right upper lobectomy and lymphadenectomy at levels 4R, 7, 9, 10, and 11R were performed in April 2022 obtaining pathological staging of LuADC ypT3N0, with negative surgical margins. Osimertinib was maintained as a systemic treatment. The NGS study by the Oncomine Comprehensive Assay panel (Life Technologies) detected the previously described EGFRdel19, and mesenchymal-epithelial transition (MET) amplification (copy range 9–13; ratio MET/chromosome7: > 5), which was confirmed by fluorescence in situ hybridization (FISH, ZytoLight® SPEC MET/CEN7 Dual Color Probe, ZytoVision) (Figure 3). MET exon 14 skipping was also identified in a low number of reads (2,848). However, a close examination of MET exon 14 skipping read sequences showed two internal deletions at the end of exon 13, which are predicted to originate a non-protein productive frameshift variant. Therefore, this alteration was dismissed as a potential resistance mechanism. In addition, stop-gained mutations in tumor protein p53 (TP53, NM_000546.5) c.430C > T, p.Gln144Ter, variant allele frequency (VAF): 7.97%, and F-Box and WD Repeat Domain Containing 7 (FBXW7, NM_033632.3) c.43_44insACTC, p.Thr15AsnfsTer9, VAF: 54.16%, were also detected but not previously reported since different NGS panels were used in the two analyzed samples. Thus, whether the variants in TP53 and FBXW7 were detected from the beginning could not be confirmed.
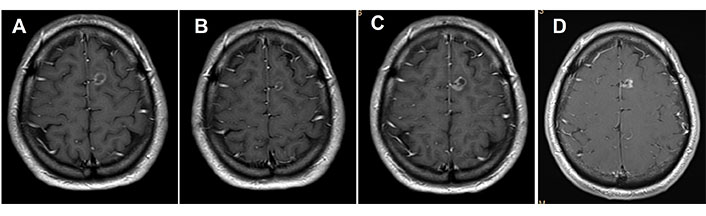
MRI of the brain. (A) Basal brain metastasis; (B) with partial response to osimertinib treatment; (C) progression after 14 cycles of osimertinib; (D) complete response after receiving SRS 20 Gy treatment
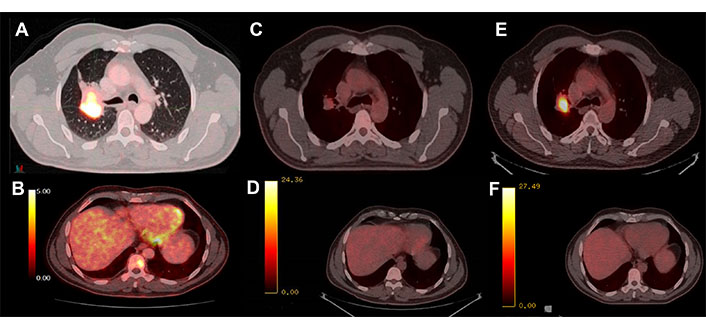
PET-CT with 18-fluorodeoxyglucose (FDG). (A) Hypermetabolism of the basal parahiliar right mass; (B) hypermetabolism of the bone metastasis at D9; (C) initial response to osimertinib in the lung mass; (D) initial response to osimertinib in the bone lesion; (E) progression of the parahiliar mass after 32 cycles of treatment; (F) complete response maintained in the bone
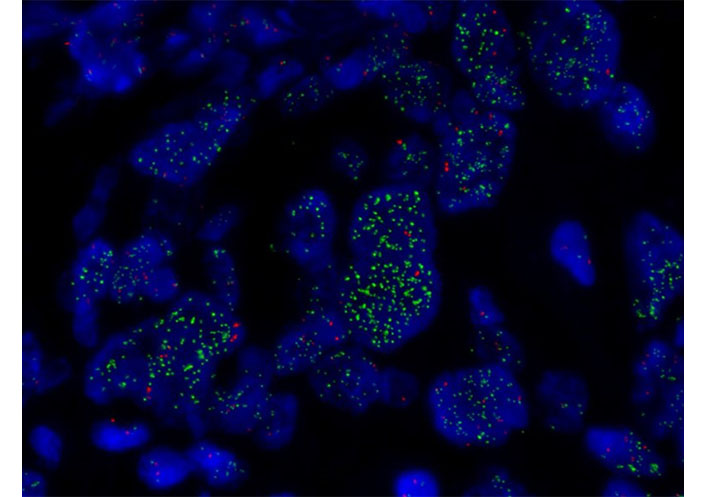
FISH of MET. MET amplification assessed by FISH (×1,000) showing MET high amplification in green (copy range 9–13; ratio MET/chromosome7: > 5)
Currently, the patient continues with a complete response to the primary lesion and improvement of therapeutic control of brain metastasis after almost four years from the start of osimertinib. No therapeutic modification was considered since there is no evidence of measurable active disease and the drug tolerance is excellent.
Discussion
Osimertinib, a 3rd generation EGFR-TKI is the standard first-line treatment for metastatic patients with activating mutations in EGFR, according to clinical guidelines [4]. Despite the impressive results achieving long-lasting responses in a subset of patients, the emergence of AR mechanisms inevitably occurs over time.
Of note, a small proportion of LuADC patients with targetable oncogenic mutations can present oligometastatic disease at diagnosis and/or undergo oligoprogression under TKI treatment. This means that while maintaining a systemic overall response to TKI, a subset of tumor clones becomes resistant and progresses into specific locations. Once the disease is metastatic, the presence of oligoprogression suggests that addiction to the mutated oncogene still exists, although AR has occurred in some other tumor clones. However, the specific biological underlying mechanisms remain unknown [5]. In those cases, local ablative strategies such as surgery, focal radiation therapy (RT), or stereotactic body RT (SBRT), in combination with inhibitors of the altered oncogene, is a reasonable option that has shown a higher disease control rate and survival. Nevertheless, efforts to manage oligometastatic and/or oligoprogressive disease have been hampered by the lack of a uniform definition of oligoprogression, the great heterogeneity in the number and sites of disease progression, and the lack of randomized clinical trials addressing this topic [6, 7]. Therefore, a multidisciplinary approach to patient management remains essential.
In this patient with asymptomatic brain metastasis at the disease onset, it was decided to start with upfront osimertinib since high rates of intracranial response have been described and apply stereotactic radiotherapy upon focal intracranial progression [8]. Afterward, upon disease progression in the lung, surgery on the primary tumor was performed even after three years of the initial diagnosis since there was no evidence of disease in other locations. Complete response while maintaining osimertinib was maintained almost four years after the initial diagnosis. In this other recent work, the authors show a cohort of 121 oligometastatic LC patients harboring different driver mutations that have been treated with local consolidative strategies to all sites of their disease either with surgery or radiotherapy. They concluded that EGFR mutant patients were associated with prolonged overall survival (OS) among oligometastatic patients treated with local ablative strategies in addition to systemic therapy [9].
The molecular and histopathological reassessment of the disease, once it progresses to the EGFR-TKI contributes to a better understanding of the AR mechanisms which contributes to determining further therapeutic strategies. Mechanisms of resistance to osimertinib primarily include secondary EGFR mutations (e.g., C797S), MET and erythroblastic oncogene B-2 (ERBB2) amplification, or histological transformation to small cell carcinoma, among others [5].
In addition, tumor biopsies in LC, which are the most common source of cancer cells for genotyping and categorizing tumors for clinical decisions, are not always feasible. A blood-based test using cell-free circulating tumor DNA (ctDNA), also called “liquid biopsy,” represents a non-invasive, rapid, and cost-effective strategy for obtaining DNA from tumor cells. However, the technique has not yet been fully translated into clinical practice, and the variability in the amount of ctDNA released by the tumors to the bloodstream may prevent the standardization of the procedure [10]. Although no liquid biopsy was performed on our patient, dynamic approaches such as ctDNA clearance or detection of novel arising mutations in ctDNA are gaining interest in our clinical practice.
Finally, the clinical management of LC patients, as for many other cancers, would need to consider genetic predisposition as another variable. Most non-small-cell LCs (NSCLCs) with actionable oncogenic drivers, including EGFR mutations, are found in never-smokers and young patients. To date, the explanation is unclear, and the possibility that these cancers arise within hereditary cancer syndromes, such as the Li-Fraumeni syndrome, should be considered, particularly in EGFR-mutant tumors [11]. Given the family history of our patient, despite there being no major criteria for such hereditary syndrome, the study of different selected members of the family is currently ongoing but did not report any specific finding.
In summary, osimertinib remains the preferred option for patients with EGFR mutant advanced LC, with a significant impact on survival. However, the acquisition of resistance mechanisms is inevitable, and genetic and molecular profiling is key to advancing towards a more personalized medicine. In some cases, oligoprogression occurs and multidisciplinary management remains essential, particularly in those cases where local therapeutic strategies may prolong survival. In this case, tumor primary resection after three years of the initial diagnosis represents a paradigm shift in the treatment of EGFR mutant patients.
Abbreviations
| AR: |
acquired resistance |
| CT: |
computed tomography |
| ctDNA: |
circulating tumor DNA |
| D9: |
dorsal vertebra 9 |
| EGFR: |
epidermal growth factor receptor |
| LC: |
lung cancer |
| LuADCs: |
lung adenocarcinomas |
| MET: |
mesenchymal-epithelial transition |
| MRI: |
magnetic resonance imaging |
| NGS: |
next-generation sequencing |
| PET: |
positron emission tomography |
| TKIs: |
tyrosine kinase inhibitors |
Declaration
Acknowledgments
We thank the Mentoring program at ICO Badalona which logistically supports the realization of the current work. We also thank Iryna Chekhun for the language revision.
Author contributions
SC: Conceptualization, Data curation, Writing—original draft. ALP: Conceptualization, Data curation. AU: Conceptualization, Data curation, Methodology, Validation. TM, AM, MC, CMB, IT, GM, and EC: Data curation, Validation. AMMM: Data curation, Methodology, Validation. MS: Conceptualization, Data curation, Validation, Writing—original draft, Writing—review & editing.
Conflicts of interest
TM reports Consulting/Advisory Role fees by Roche, Bristol Myers, Boeringher, Astra Zeneca. Research Funding Grant by Kyowa Kirin and Janssen, all of them unrelated with the current work. MS reports a sponsored research agreement with Merck Serono and Roche Farma outside the submitted work. The rest of the authors report no conflict of interests of the submitted work.
Ethical approval
The article has approved by Germans Trias i Pujol Institutional Review Board (PI-21-114) and complies with the Declaration of Helsinki.
Consent to participate
Informed consent to participate in the study was obtained from the participant.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author (Maria Saigí, msaigi@iconcologia.net).
Funding
MS is currently supported by a Joan Rodés contract from the ISC-III [JR20/00015]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2023.