Abstract
Background:
Breast cancer is a leading cause of mortality in women. Hormone therapy plays a crucial role in treatment of hormone receptor-positive metastatic breast cancer. Elacestrant is a selective estrogen receptor degrader (SERD) that has shown promise in early-phase clinical trials. This post-hoc analysis systematically evaluates elacestrant’s effectiveness in hormone receptor-positive metastatic breast cancer patients, providing insights into its efficacy, safety, and potential advantages over existing treatments.
Methods:
We adhered to the PRISMA Statement 2020 guidelines and systematically searched the databases PubMed/MEDLINE, ClinicalTrials.gov, Web of Science, and Embase. We conducted the post-hoc analysis using R software (V 4.3.3), applying the inverse variance method and the DerSimonian-Laird estimator to pool effect estimates with a random-effects model. We assessed heterogeneity using the Cochran’s Q test and the I2 statistic.
Results:
Our post-hoc analysis encompassed 3 clinical trials and a total of 835 participants. The mean age of all 835 participants across the three trials was 59.5 years (95% CI: 58.7–60.3). The pooled progression-free survival (PFS)—was estimated at 4.38 (95% CI: –7.58–16.35, P = 0.47), and the pooled objective response rate (ORR) was 7% (95% CI: 2–18%, P = 0.04), with significant heterogeneity observed among the studies.
Discussion:
Elacestrant shows promise for improving outcomes in hormone receptor-positive metastatic breast cancer, but further research is needed to confirm its effectiveness. Future studies should include larger sample sizes, comprehensive phase II and III trials, and investigation of elacestrant in combination with other drugs or in preoperative settings.
Keywords
Elacestrant, selective estrogen receptor degraders, breast cancer, antineoplastic agents, hormone receptor-positiveIntroduction
Breast cancer is the second leading cause of death among women worldwide, impacting approximately 260,000 individuals and resulting in 40,000 deaths annually [1]. Over two-thirds of these cases are classified as hormone receptor-positive breast cancer [2]. Affecting about one in eight women during their lifetime, breast cancer is one of the most prevalent cancers diagnosed in women. Although rarer in men, the incidence of breast cancer in males is increasing, with contributing factors including Klinefelter syndrome, high body mass index, testicular and liver diseases, radiation exposure, and alcohol consumption [3]. Additionally, about 10–15% of breast cancer patients develop brain metastases, typically appearing 2–3 years after the initial diagnosis [1].
In patients with estrogen receptor-positive (ER+) metastatic breast cancer, hormone therapy remains the primary treatment option to delay the need for chemotherapy [4]. The current standard of care (SOC) for ER+ metastatic breast cancer involves a combination of hormone therapy and CDK4/6 inhibitors [5]. However, resistance often arises due to mutations in the ESR1 gene, which encodes the estrogen receptor [6]. For patients who progress despite hormone therapy and CDK4/6 inhibitors, sequential endocrine monotherapy is typically recommended. Fulvestrant, approved by the U.S. Food and Drug Administration (FDA) in 2002, is administered via intramuscular injection and has shown better efficacy than tamoxifen and aromatase inhibitors (AIs). It is commonly used as a second- or third-line treatment [7]. Despite its effectiveness, fulvestrant is associated with a relatively low median progression-free survival (PFS) of just 2 months. Additionally, most patients eventually develop resistance to the drug, although the precise mechanisms behind this resistance are not yet fully understood [8]. Furthermore, fulvestrant’s limited bioavailability has prompted the development of oral selective estrogen receptor degraders (SERDs), which offer the potential for improved bioavailability and effectiveness [9].
SERDs are antiestrogens designed to destabilize the H12 region of the estrogen receptor, working by binding to the receptor and promoting the degradation of the ER signaling pathway [10]. Several SERDs are currently in clinical development [7], with elacestrant (RAD-1901) being one of the notable non-steroidal small molecules that acts as an estrogen receptor antagonist [11]. On January 27, 2023, the U.S. FDA approved elacestrant for the treatment of advanced or metastatic breast cancer in patients with ER+, ESR1-mutated, and HER2-negative (HER2–) profiles, following progression after at least one line of endocrine therapy [12]. This approval marked elacestrant as the first oral estrogen receptor antagonist approved for patients with ESR1 mutations [13]. Two phase I clinical trials (NCT02650817, NCT02338349) evaluated elacestrant in patients with hormone receptor-positive breast cancer that had metastasized to the brain [1]. With elacestrant now approved, patients with PIK3CA mutations and metastatic ER+ breast cancer may need to consider the benefits and risks of combining alpelisib and fulvestrant vs. using elacestrant as a single agent [7]. As elacestrant is integrated into standard care, molecular profiling will become increasingly important in treatment decisions, underscoring the critical role of precision medicine in managing breast cancer [14].
This post-hoc analysis aims to systematically evaluate the effectiveness of elacestrant as a therapeutic option in the management of breast cancer. By aggregating and analyzing data from clinical trials, we intend to provide a comprehensive understanding of the drug’s efficacy, safety, and potential advantages over existing treatments.
Materials and methods
Search strategy
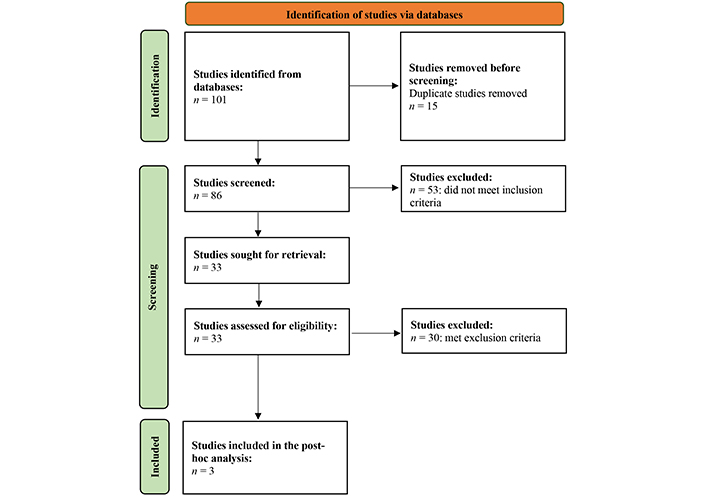
A comprehensive literature search was conducted following the PRISMA Statement 2020 guidelines [15] in the following databases: PubMed/MEDLINE, ClinicalTrials.gov, Web of Science, and Embase. In PubMed/MEDLINE, 28 published studies were obtained using specific keywords related to elacestrant and breast cancer. Similarly, 10 records were identified in ClinicalTrials.gov using relevant keywords. In Web of Science, 39 studies were retrieved, and in Embase, 24 results were found. The PRISMA flowchart is appended in Figure 1.
Keyword combinations for all the databases are as follows:
PubMed/MEDLINE: (“elacestrant”[Supplementary Concept] OR “elacestrant”[All Fields]) AND (“breast neoplasms”[MeSH Terms] OR (“breast”[All Fields] AND “neoplasms”[All Fields]) OR “breast neoplasms”[All Fields] OR (“breast”[All Fields] AND “cancer”[All Fields]) OR “breast cancer”[All Fields]).
ClinicalTrials.Gov: elacestrant and breast cancer.
Web of Science: (elacestrant OR RAD1901) AND [breast AND (cancer OR neoplasm* OR carcinoma OR tumor OR tumour)].
Embase: (elacestrant OR RAD1901) AND [breast AND (cancer OR neoplasm* OR carcinoma OR tumor OR tumour)].
The final search was conducted on 31st March, 2023 with no language restrictions. The population of interest consisted of patients with hormone receptor-positive breast cancer. The intervention under investigation was elacestrant treatment, while the comparator group included SOC or other treatments for hormone receptor-positive breast cancer. The outcomes assessed in the included studies were PFS, objective response rate (ORR), overall survival (OS), and adverse events (AE).
Data extraction and synthesis
Data from eligible studies were extracted and tabulated according to the following variables: Author, Year, Study Design, Inclusion Criteria, Participant Count, Intervention Given, Previous Treatment, Primary Endpoints. For the datasheet extraction, the following information was included: Author, Year, Age, Previous treatment, PFS (%), ORR, OS (%), and AE (any).
Statistical analysis
The post-hoc analysis was performed using R software. For PFS, the inverse variance method was employed to pool the effect estimates from individual studies, accounting for differences in sample sizes and variances. The DerSimonian-Laird estimator was used to estimate between-study variance (τ2) which represents the heterogeneity in the true effect sizes across studies. A random-effects model was applied to account for potential clinical and methodological diversity among the included studies. For ORR, the inverse variance method was used for pooling the effect estimates, similar to the PFS analysis. The DerSimonian-Laird estimator was applied to estimate τ2, and a random-effects model was used to account for heterogeneity. Additionally, the logit transformation was employed to stabilize the variances of the proportions, making the data more suitable for pooling. Clopper-Pearson confidence intervals were calculated for individual studies to account for the uncertainty in the estimated proportions. Heterogeneity among the included studies was assessed using the Cochran’s Q test and quantified using the I2 statistic.
Results
Overview of the included trials
Three clinical trials were included in this study exploring elacestrant as a therapeutic option for hormone receptor-positive breast cancer [16–18]. Although the design, goals, and outcomes of each study varied, all three aimed to assess the effectiveness and safety of elacestrant. The mean age of all 835 participants across the three trials was 59.5 years (95% CI: 58.7–60.3) years. AE (any) were reported in 282 of 303 participants (93%) in the elacestrant group [16–18], whereas in the standard care group of the phase III trial, 197 of 229 participants reported any AE [16]. The characteristics of the included trials are enlisted in Table 1.
Characteristics of the included trials
| Author, year | Study design | Inclusion criteria | Participant count | Intervention given | Previous treatment | Primary endpoints |
|---|---|---|---|---|---|---|
| Bidard et al. [16], 2022 | Randomized, open-label, phase III trial (NCT03778931) | - Postmenopausal women or men- Age: 18 years or older- Histologically or cytologically proven ER+/HER2– breast adenocarcinoma- Either locoregionally recurrent or metastatic disease | 477; elacestrant (n = 239), SOC (n = 238) | Elacestrant dosing:- 400 mg orally once daily- Reductions to 300 mg or 200 mg daily permitted for toxicityStandard of care (SOC) treatment:- Per investigator’s choice (fulvestrant, anastrozole, letrozole, or exemestane monotherapy)- Dosed according to the labeling | - CDK4/6 inhibitors (progression on previous treatment was required) in combination with fulvestrant or an AI- One chemotherapy regimen in the advanced/metastatic setting was permitted | - PFS in all patients, assessed by BICR using standard RECIST v1.1 criteria—PFS in patients with detectable ESR1 mutation, assessed by BICR using standard RECIST v1.1 criteria |
| Bardia et al. [17], 2021 | Multicenter, open-label, four-part, dose-escalation study (NCT02338349) | - Postmenopausal women, age ≥ 18 years- ER+ (≥ 1% staining by immunohistochemistry)- HER2− locally advanced, inoperable, and/or metastatic breast adenocarcinoma- ECOG performance status 0–1 | 50 | The RP2D was 400 mg of elacestrant once daily | Part A–C:- Required ≤ 2 prior lines of chemotherapy for advanced/metastatic breast cancer- Required ≥ 6 months of prior ET (no limit) in any settingPart D:- Required ≤ 1 prior line of chemotherapy for advanced/metastatic breast cancer- Required ≥ 2 prior lines of ET for advanced/metastatic breast cancer (single agent/in combination)- Required one prior line of treatment with fulvestrant with documented progression and prior CDK4/6i | Frequency of DLTs during the first 28 days of treatment |
| Jager et al. [18], 2020 | Phase 1b, open-label, non-randomized (NCT02650817) | - Postmenopausal women, prior bilateral ovariectomy- Histologically proven ER+ (defined as ≥ 1% staining by immunohistochemistry), HER2− ABC (either inoperable primary breast cancer or mBC)- ECOG performance status: 0–2 | 16 | - Initially enrolled and treated with 400 mg elacestrant capsule once daily (QD)- Second cohort of 8 patients enrolled and treated with 200 mg elacestrant capsule QD for 14 days to assess target engagement of a lower dose-After 14 days, the dose was escalated to 400 mg QD | - 6 or more months of 1–3 lines of systemic endocrine treatment for mBC- Prior CDK4/6 inhibitor therapy were allowed | Percentage difference in FES uptake in tumor lesions (up to a maximum of 20 lesions) after 14 days of treatment with elacestrant compared to baseline |
AI: aromatase inhibitor; BICR: blinded independent central review; DLTs: dose-limiting toxicities; ER+: estrogen receptor-positive; FES: fluoroestradiol; PFS: progression-free survival; RP2D: recommended phase 2 dose; HER2–: HER2-negative
In their 2022 phase III trial (NCT03778931), Bidard et al. [16] enrolled 477 participants with ER+/HER2– breast adenocarcinoma. The subjects were divided into two groups: one received elacestrant (400 mg daily), while the other received SOC treatment (fulvestrant, anastrozole, letrozole, or exemestane monotherapy). The study’s primary endpoints were PFS for all participants and for those with a detectable ESR1 mutation, as assessed by a blinded independent central review (BICR) using RECIST v1.1 criteria.
In a 2021 multicenter, open-label, four-part, dose-escalation study (NCT02338349), Bardia et al. [17] included 50 postmenopausal women with ER+, HER2– breast cancer. The subjects received 400 mg of elacestrant once daily. The study aimed to establish the recommended phase 2 dose (RP2D) of elacestrant and evaluate the frequency of dose-limiting toxicities (DLTs) during the initial 28 days of treatment.
Jager et al.’s [18] 2020 phase 1b, open-label, non-randomized trial (NCT02650817) involved 16 participants with ER+, HER2– advanced breast cancer. Initially, patients were treated with 400 mg of elacestrant daily, while a second cohort received a lower dose of 200 mg daily for 14 days before increasing to 400 mg daily. The study’s primary endpoint was the percentage difference in fluoroestradiol (FES) uptake in tumor lesions after 14 days of elacestrant treatment compared to baseline.
Overall, all three studies focused on elacestrant as a potential treatment for hormone receptor-positive breast cancer. Despite their differing designs and objectives, they collectively aimed to assess the efficacy and safety of elacestrant across various patient populations and treatment settings. These studies provided crucial insights into elacestrant as a novel SERD and its possible role in treating hormone receptor-positive advanced breast cancer.
Post-hoc findings
We analyzed PFS outcomes from three combined trials. The pooled PFS based on the random-effects model was estimated at 4.38 (95% CI: –7.58 to 16.35; Figure 2). However, the results were not statistically significant (z = 0.72, P = 0.47), indicating that the intervention did not show a significant effect on PFS. The heterogeneity analysis demonstrated no τ2, with a τ2 value of 0, and the test for heterogeneity showed a Q-value of 0.11 with 2 degrees of freedom (P = 0.94), suggesting no heterogeneity among the studies.
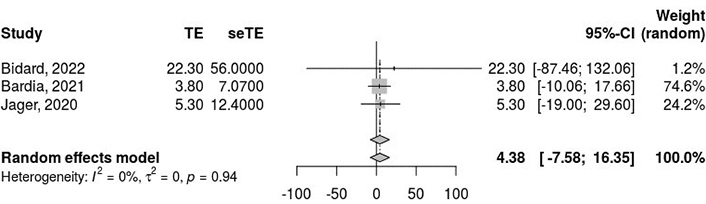
Pooled progression-free survival (PFS) outcomes from the three combined studies using a random-effects model, revealing no significant difference in PFS outcomes and no heterogeneity among the included studies. I2: inconsistency index; τ2: between-study variance; CI: confidence interval; TE: treatment effect/effect estimate; seTE: standard error of the treatment effect
The pooled ORR was 7% (95% CI: 2% to 18%; Figure 3), based on the random-effects model. Heterogeneity analysis revealed considerable variability among the studies, with a τ2 value of 0.5749, an H-value of 1.78 (95% CI: 1.00 to 3.30), and an I2 statistic of 68% (95% CI: 0.0% to 90.8%). The Rb value was 63.6% (95% CI: 4.7% to 100.0%). The test for heterogeneity yielded a Q-value of 6.33 with 2 degrees of freedom (P = 0.04), confirming significant heterogeneity among the included studies.
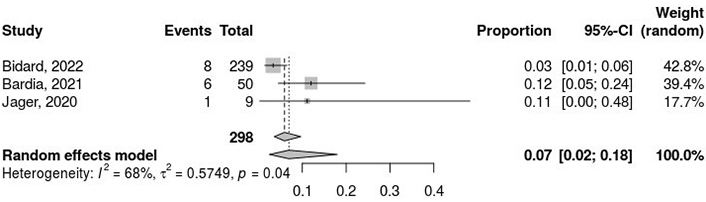
Pooled proportion analysis of objective response rate (ORR) outcomes. The meta-analytical method applied consists of the inverse variance method, DerSimonian-Laird estimator for τ2, logit transformation, and Clopper-Pearson confidence intervals for individual studies. I2: inconsistency index; τ2: between-study variance; CI: confidence interval
Ongoing clinical trials
Table 2 summarizes ongoing/unreported trials related to elacestrant, including their identifiers, interventions, outcome measures, participant counts, completion dates, and locations. Seven trials with various aims are outlined, including studies focused on CDK4/6 inhibitor-naive ER+, HER2– metastatic breast cancer, safety, and efficacy of elacestrant in combination with other drugs, and preoperative settings. These trials are in different stages, such as not yet recruiting, recruiting, active but not recruiting, and completed. They involve diverse interventions and outcome measures, such as PFS, ORR, duration of response, clinical benefit rate, and AE. The number of participants varies across the trials, ranging from 23 to 322, and the completion dates span from February 2022 to May 2030. The trials are being conducted in the United States, Belgium, Greece, and Spain.
Identifier, interventions, outcome measures, participants, completion date and locations of ongoing/unreported trials
| No. | NCT | Title | Status | Conditions | Interventions | Outcome measures | Phase | N | Study Design | Completion Date | Collaborators | Locations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NCT05596409 | ELACESTRANT in Women and Men With CDK4/6 Inhibitor-Naive Estrogen Receptor Positive, HER-2 Negative Metastatic Breast Cancer Study (ELCIN) | Not yet recruiting | Metastatic breast cancer | Elacestrant | PFS; ORR; DOR; clinical benefit rate; PFS; OS | 2 | 80 | Single group, open label, interventional | August, 2025 | Stemline Therapeutics, Inc. | United States |
| 2 | NCT05563220 | Open-Label Umbrella Study To Evaluate Safety And Efficacy Of Elacestrant In Various Combination In Patients With Metastatic Breast Cancer (ELEVATE) | Recruiting | Breast cancer; metastatic breast cancer | Elacestrant; alpelisib; everolimus; ribociclib; palbociclib | RP2D; safety; pharmacokinetic assessment profile; ORR; DOR; clinical benefit rate; PFS; OS | 1 & 2 | 322 | Non-randomized, parallel assignment, open label, interventional | August, 2026 | Stemline Therapeutics, Inc. | United States |
| 3 | NCT05618613 | Study of Elacestrant in Combination With Onapristone in Patients With Advanced or Metastatic Breast Cancer (ELONA) | Active, not recruiting | Breast cancer | Elacestrant; onapristone | RP2D; ORR per RECIST version 1.1.; AE, SAE, changes in clinical laboratory values; vital sign measurements; changes in ECG parameters; area under the plasma concentration-time curve over the dosing interval; Cmax, Tmax; trough concentration; clinical benefit rate; PFS; OS | 1 & 2 | 67 | Single group, open label, interventional | April, 2026 | Context Therapeutics Inc. | United States |
| 4 | NCT04791384 | Phase Ib/II Trial of Abemaciclib and Elacestrant in Patients With Brain Metastasis Due to HR+/Her2- Breast Cancer | Recruiting | Breast cancer | Abemaciclib; elacestrant | AE; efficacy of the combination abemaciclib and elacestrant; tumor response rates; duration of tumor response rates; completion rate | 1 & 2 | 44 | Sequential assignment, open label, interventional | June, 2023 | Criterium, Inc. | United States |
| 5 | NCT05512364 | TREAT ctDNA Elacestrant | Not yet recruiting | ER+ breast cancer; HER2– breast cancer; stage IIB breast cancer; stage III breast cancer | Elacestrant; tamoxifen; letrozole 2.5 mg; anastrozole 1 mg; exemestane 25 mg | DMFS; ctDNA elimination rate at month 1 | 3 | 220 | Randomized, parallel assignment, open label, interventional | May, 2030 | European Organisation for Research and Treatment of Cancer - EORTC; Breast International Group; Menarini Group | Belgium, Greece |
| 6 | NCT05386108 | Study of Abemaciclib and Elacestrant in Patients With Brain Metastasis Due to HR+/HER2- Breast Cancer (ELECTRA) | Recruiting | Breast neoplasms; breast diseases | Elacestrant; abemaciclib | RP2D; ORR; intracranial response rate per RECIST; intracranial response rate per RANO; duration of tumor response; clinical benefit rate; duration of PFS | 1 & 2 | 106 | Non-randomized, sequential assignment, open label, interventional | December, 2025 | Stemline Therapeutics, Inc. | United States, Korea, Spain |
| 7 | NCT04797728 | Elacestrant in Preoperative Setting, a Window of Opportunity Study (ELIPSE) | Completed | Breast cancer; hormone receptor positive breast carcinoma | Elacestrant | Complete cell cycle arrest; PAM50 (prediction analysis of microarray 50) subtype change; gene expression change; AE; global gene expression changes; gene expression-based signature of response; CelTIL score; mean change in Ki67; changes in the distribution of somatic mutations; ctDNA | Early, 1 | 23 | Single group, open label, interventional | February, 2022 | SOLTI Breast Cancer Research Group; Radius Health, Inc. | Spain |
AE: adverse events; OS: overall survival; PFS: progression-free survival; RP2D: recommended phase 2 dose; ORR: objective response rate; HER2–: HER2-negative; ER+: estrogen receptor-positive
Discussion
The current evidence supporting the use of elacestrant in patients with advanced breast cancer has led to the initiation of several other clinical trials. These trials are exploring the use of elacestrant in early-stage breast cancer and its combination with other therapies for metastatic cases, including drugs like alpelisib, everolimus, and CDK4/6 inhibitors [19]. The research includes two phase I trials and one phase III trial (EMERALD), which revealed significant insights into disease progression and patient survival, though no conclusive results were achieved. The phase I trials specifically targeted ER+, HER2– breast cancer, with the objective of evaluating elacestrant’s safety, tolerability, and preliminary efficacy. However, the small sample sizes may limit the broader applicability of the findings. A post-hoc analysis of PFS outcomes across these trials showed no statistically significant benefit for the intervention group. While the uniformity across the studies suggests methodological consistency and similar patient populations, this does not confirm the efficacy of elacestrant. The ORR was estimated at 0.07, with a 95% confidence interval ranging from 0.02 to 0.18, pointing to a relatively low response rate. Nevertheless, due to the variability and limited scope of the available data, it remains difficult to draw a definitive conclusion regarding the effectiveness of elacestrant in treating breast cancer.
The phase III EMERALD trial highlighted encouraging outcomes for elacestrant in the management of ER+/HER2– metastatic breast cancer [20]. The study showed a significantly extended PFS for patients receiving oral elacestrant compared to those on standard care, particularly in individuals whose disease had progressed after prior endocrine therapy and CDK4/6 inhibitor treatment [21]. However, its efficacy in patients who have not received fulvestrant remains unclear, emphasizing the need for further research. Elacestrant’s pharmacological profile is complex, exhibiting dose-dependent ER antagonist and agonist effects, along with the ability to penetrate the blood-brain barrier (BBB) [20]. This feature could be significant in treating breast cancer patients with CNS involvement.
In the phase III EMERALD trial, patients with mESR1-mutant tumors experienced a 45% reduction in the risk of disease progression or death when treated with elacestrant compared to standard endocrine therapy. In this group, the median PFS was 3.79 months with elacestrant, compared to 1.87 months for those receiving standard treatment (HR, 0.54; P < 0.001) [22]. Elacestrant also showed higher PFS rates at both 6 months (34.3% vs. 20.4%) and 12 months (22.3% vs. 9.4%), indicating sustained benefits from this oral SERD therapy. The improved PFS was notably consistent among patients with mESR1 mutations. Specifically, in this subgroup, the PFS rates at 6 months were 40.8% for elacestrant compared to 19.1% for standard therapy, and at 12 months, they were 26.8% vs. 8.2% [22]. Additionally, subgroup analysis from the EMERALD trial revealed that elacestrant provided clinical benefits for patients who had previously received fulvestrant, regardless of their ESR1 mutational status, highlighting its potential as a treatment option for refractory HR+ breast cancer [23].
Elacestrant, a nonsteroidal SERD, exhibits dose-dependent ER antagonist and agonist activities, positioning it as a leading candidate for treating HR-positive breast cancer [24]. Studies have shown that elacestrant can act as an ER agonist within the CNS and readily crosses the BBB [25]. Laboratory research with doses ranging from 0.3 mg/kg to 100 mg/kg has indicated no significant effects on uterine wet weight or epithelial thickness [26]. However, at a low dose of 0.3 mg/kg, a notable increase in uterine weight was observed, whereas no such effect occurred at doses of 1 mg/kg or higher [25]. This suggests that elacestrant’s activity may shift towards antagonism at higher doses [25]. In both single-ascending dose and multiple-ascending dose trials, doses up to 1,000 mg daily were found to be safe and well tolerated, with no maximum tolerated dose identified. Elacestrant’s oral bioavailability was approximately 10%, with a half-life ranging from 27 h to 47 h, reaching steady-state levels after 5–6 days of administration. After seven days of treatment, the mean ER occupancy in the uterus was 83% at 200 mg and 92% at 500 mg daily. The median ratio of elacestrant in cerebrospinal fluid compared to plasma was 0.126% for the 500 mg dose and 0.205% for the 200 mg dose [27]. These complex pharmacological properties may influence how elacestrant is used in the treatment of breast cancer in the future.
Reported side effects of elacestrant include hypercholesterolemia and hypertriglyceridemia, occurring in 30% and 27% of patients, respectively. The rates of severe (Grade 3 and 4) hypercholesterolemia and hypertriglyceridemia were relatively low, at 0.9% and 2.2%, respectively [28]. Other more frequently observed side effects include musculoskeletal pain, nausea, and various gastrointestinal issues [29]. Patients taking elacestrant are also advised against breastfeeding due to potential risks [29]. However, due to the limited ability of elacestrant and other SERDs to effectively cross the BBB, breast cancer patients with brain metastases are still unable to fully benefit from SERD treatments [30].
The findings from this post-hoc analysis suggest a relationship between the effectiveness of ER-targeting therapies and the levels of ER and PR expression in tumor cells. High ER expression enhances the interaction with SERDs, while PR expression indicates a reliance on ER signaling for tumor growth and survival [11]. This is significant because current oncology guidelines recommend endocrine therapy for ER+ tumors, even when ER is expressed in as few as 1% of tumor cells [31]. Upcoming clinical trials that will compare elacestrant with fulvestrant as the foundational endocrine therapy, in combination with treatments like CDK4/6 inhibitors, alpelisib, and everolimus, are expected to provide valuable insights into the best SERD for second-line treatment [32]. Based on this information, the effectiveness of endocrine therapies in breast cancer treatment appears to be closely tied to the expression of ER and PR within tumors [33]. However, to determine the most suitable endocrine therapy, whether SERD or another type, further research and clinical trials are required to assess their efficacy and safety fully [34]. Ultimately, the selection of treatment should be individualized, taking into consideration the tumor’s characteristics and the patient’s overall health, with input from a multidisciplinary team of healthcare professionals [35].
Conclusion
Although elacestrant has been approved for use in patients who have undergone at least one round of endocrine therapy, our study highlights the need for additional clinical research to explore its potential in various contexts, such as treating endocrine-naive patients or those who have received extensive treatment for ER+ breast cancer. While phase I trials and post-hoc analyses show promise for elacestrant as a treatment for ER+, HER2– breast cancer, the current evidence is not yet strong enough to confirm its effectiveness. Studies with larger sample sizes and more comprehensive phase II and III trials are needed to establish elacestrant's definitive role in treatment of patients with breast cancer.
Abbreviations
| AE: | adverse events |
| BBB: | blood-brain barrier |
| BICR: | blinded independent central review |
| ER+: | estrogen receptor-positive |
| HER2–: | HER2-negative |
| ORR: | objective response rate |
| OS: | overall survival |
| PFS: | progression-free survival |
| RP2D: | recommended phase 2 dose |
| SERD: | selective estrogen receptor degrader |
| SOC: | standard of care |
| τ2: | between-study variance |
Declarations
Author contributions
AS: Conceptualization, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing. MS: Conceptualization, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing. FJ: Validation, Writing—original draft, Writing—review & editing. MK: Data curation, Investigation, Writing—original draft, Writing—review & editing. BS: Writing—original draft, Writing—review & editing. AG: Writing—original draft, Writing—review & editing. MAG: Formal analysis, Software, Writing—original draft, Writing—review & editing. AI: Writing—original draft, Writing—review & editing. KC: Supervision, Visualization, Writing—original draft, Writing—review & editing.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
All data utilized for the purpose of this study are available publicly and online.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.