-
Exploration of BioMat-X
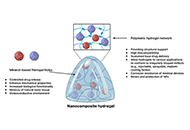
eISSN: 2996-9476EiC: Maryam Tabrizian, CanadaFrequency: Continuous PublicationAPC: No Article Processing Charge before January 31, 2029Publishing Model: Open AccessPeer Review Model: Single BlindPermanent Archive: PorticoIndexing & Archiving: Google Scholar, Dimensions, MyScienceWork, Portico, etc.Articles Mineral nanoparticles and nanocomposite hydrogels with osteoinductive properties for bone regenerationOpen AccessReviewMineral nanoparticles and osteoinductive biomaterials are essential in advancing bone regeneration by addressing skeletal conditions and injuries that compromise structural integrity and functionali [...] Read more.
Mineral nanoparticles and nanocomposite hydrogels with osteoinductive properties for bone regenerationOpen AccessReviewMineral nanoparticles and osteoinductive biomaterials are essential in advancing bone regeneration by addressing skeletal conditions and injuries that compromise structural integrity and functionali [...] Read more.Mineral nanoparticles and osteoinductive biomaterials are essential in advancing bone regeneration by addressing skeletal conditions and injuries that compromise structural integrity and functionality. These biomaterials stimulate the differentiation of precursor cells into osteoblasts, creating biocompatible environments conducive to bone tissue regeneration. Among the most promising innovations, mineral-based nanoparticles and nanocomposite hydrogels have emerged as effective strategies for enhancing osteoinductive potential. This review explores the diverse types of osteoinductive biomaterials, including natural sources, synthetic compounds, and hybrid designs that incorporate mineralized nanoparticles. Emphasis is placed on polymeric hydrogels as delivery platforms for these materials, highlighting their dual role as structural supports and bioactive agents that promote osteogenesis. Challenges such as immune rejection, biodegradability, mechanical stability, and short in vivo residence time are critically discussed, alongside their impact on clinical translation. By presenting a comprehensive analysis of mechanisms, applications, and limitations, this review identifies opportunities for integrating osteoinductive biomaterials with emerging fields like immunology and biomechanics. Ultimately, this work aims to provide actionable insights and advance the development of novel, clinically relevant solutions that improve patient outcomes and address the growing global need for effective bone repair and regeneration.
Cho-E Choi, Arghya PaulPublished: March 17, 2025 Explor BioMat-X. 2025;2:101332
DOI: https://doi.org/10.37349/ebmx.2025.101332Mineral nanoparticles and osteoinductive biomaterials are essential in advancing bone regeneration by addressing skeletal conditions and injuries that compromise structural integrity and functionality. These biomaterials stimulate the differentiation of precursor cells into osteoblasts, creating biocompatible environments conducive to bone tissue regeneration. Among the most promising innovations, mineral-based nanoparticles and nanocomposite hydrogels have emerged as effective strategies for enhancing osteoinductive potential. This review explores the diverse types of osteoinductive biomaterials, including natural sources, synthetic compounds, and hybrid designs that incorporate mineralized nanoparticles. Emphasis is placed on polymeric hydrogels as delivery platforms for these materials, highlighting their dual role as structural supports and bioactive agents that promote osteogenesis. Challenges such as immune rejection, biodegradability, mechanical stability, and short in vivo residence time are critically discussed, alongside their impact on clinical translation. By presenting a comprehensive analysis of mechanisms, applications, and limitations, this review identifies opportunities for integrating osteoinductive biomaterials with emerging fields like immunology and biomechanics. Ultimately, this work aims to provide actionable insights and advance the development of novel, clinically relevant solutions that improve patient outcomes and address the growing global need for effective bone repair and regeneration.
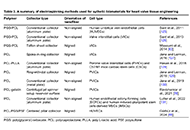
 Electrospun scaffolds for heart valve tissue engineeringOpen AccessReviewA potential solution for prosthetic heart valves is tissue-engineered heart valves. Tissue-engineered heart valves (TEHVs) are designed to replicate the complex properties found in natural tissues, [...] Read more.
Electrospun scaffolds for heart valve tissue engineeringOpen AccessReviewA potential solution for prosthetic heart valves is tissue-engineered heart valves. Tissue-engineered heart valves (TEHVs) are designed to replicate the complex properties found in natural tissues, [...] Read more.A potential solution for prosthetic heart valves is tissue-engineered heart valves. Tissue-engineered heart valves (TEHVs) are designed to replicate the complex properties found in natural tissues, such as stiffness, anisotropy, and composition and organization of cells and extracellular matrix (ECM). Electrospinning is regarded as a highly versatile and innovative approach for fabricating numerous fibrous designs. In this review, we discuss recent developments in electrospun heart valve scaffolds, including scaffold materials, cell types, and electrospinning setups used to prepare aligned nanofibers. Despite the fact that natural biomaterials provided excellent biocompatibility, nanofibers from synthetic materials provided the required mechanical compatibility. Accordingly, most studies highlighted the benefits of designing composite heart valves using biological and synthetic polymers. Various strategies, such as the application of motorized mandrel and micropatterned collector in electrospinning were effective in controlling nanofiber alignment. Studies also showed that aligned nanofiber’s mechanical strength and anisotropic structure promote cell proliferation, and differentiation, and promote attachment. Numerous studies have reported that multiple cell sources are suitable for producing heart valves. Successful results were obtained with human umbilical vein endothelial cells (HUVECs), since they provide a convenient cell source for cellularization of valve leaflets. A higher conductivity of scaffolds was achieved by using biomaterials that conduct electricity, such as polyaniline, polypyrrole, and carbon nanotubes, which resulted in better differentiation of precursor cells to cardiomyocytes and higher cell beating rates. In light of these attributes, nanofibrous scaffolds produced through electrospinning are expected to offer numerous advantages for tissue engineering and medical applications in the near future. However, multiple challenges were identified as cell infiltration and 2D nature of nanofiber mats necessitate further engineering approaches in electrospinning procedure leaflet production.
Betül Gürbüz ... Erkan Türker BaranPublished: February 27, 2025 Explor BioMat-X. 2025;2:101331
DOI: https://doi.org/10.37349/ebmx.2025.101331
This article belongs to the special issue Trends in Biomaterials Research for Cardiovascular ApplicationsA potential solution for prosthetic heart valves is tissue-engineered heart valves. Tissue-engineered heart valves (TEHVs) are designed to replicate the complex properties found in natural tissues, such as stiffness, anisotropy, and composition and organization of cells and extracellular matrix (ECM). Electrospinning is regarded as a highly versatile and innovative approach for fabricating numerous fibrous designs. In this review, we discuss recent developments in electrospun heart valve scaffolds, including scaffold materials, cell types, and electrospinning setups used to prepare aligned nanofibers. Despite the fact that natural biomaterials provided excellent biocompatibility, nanofibers from synthetic materials provided the required mechanical compatibility. Accordingly, most studies highlighted the benefits of designing composite heart valves using biological and synthetic polymers. Various strategies, such as the application of motorized mandrel and micropatterned collector in electrospinning were effective in controlling nanofiber alignment. Studies also showed that aligned nanofiber’s mechanical strength and anisotropic structure promote cell proliferation, and differentiation, and promote attachment. Numerous studies have reported that multiple cell sources are suitable for producing heart valves. Successful results were obtained with human umbilical vein endothelial cells (HUVECs), since they provide a convenient cell source for cellularization of valve leaflets. A higher conductivity of scaffolds was achieved by using biomaterials that conduct electricity, such as polyaniline, polypyrrole, and carbon nanotubes, which resulted in better differentiation of precursor cells to cardiomyocytes and higher cell beating rates. In light of these attributes, nanofibrous scaffolds produced through electrospinning are expected to offer numerous advantages for tissue engineering and medical applications in the near future. However, multiple challenges were identified as cell infiltration and 2D nature of nanofiber mats necessitate further engineering approaches in electrospinning procedure leaflet production.
 Preparation and characterization of stearyl glycyrrhetinate/cyclodextrin complex using co-grindingOpen AccessOriginal ArticleAim: In this study, the physicochemical properties of stearyl glycyrrhetinate/β-cyclodextrin (SG/βCD) and SG/γCD complexes were characterized, and the complexes were prepared using the co-milling [...] Read more.
Preparation and characterization of stearyl glycyrrhetinate/cyclodextrin complex using co-grindingOpen AccessOriginal ArticleAim: In this study, the physicochemical properties of stearyl glycyrrhetinate/β-cyclodextrin (SG/βCD) and SG/γCD complexes were characterized, and the complexes were prepared using the co-milling [...] Read more.Aim:
In this study, the physicochemical properties of stearyl glycyrrhetinate/β-cyclodextrin (SG/βCD) and SG/γCD complexes were characterized, and the complexes were prepared using the co-milling method. The molecular interactions within the SG/CD complexes were also investigated using nuclear magnetic resonance (NMR) measurements to determine the mode of interaction.
Methods:
Here, we evaluated the physicochemical properties of SG complexes with CDs prepared by ground mixtures (GM SG/βCD or γCD = 1/1, 1/2).
Results:
Powder X-ray diffraction (PXRD) showed that the characteristic SG and CD peaks disappeared after co-grinding with GM (SG/CD = molar ratio of 1/2), indicating a halo pattern. Differential scanning calorimetry (DSC) measurements showed that after co-grinding, the endothermic peak due to SG melting, as well as the dehydration peak and the endothermic peak due to SG melting, disappeared. Near-infrared (NIR) spectroscopy results showed that the peaks of SG-derived –CH moieties and CD-derived –OH and –CH moieties broadened in GM, suggesting their involvement in complex formation through SG/CDs intermolecular interactions. In GM (SG/CDs), NMR measurements showed broadened H-A and H-F peaks of the steroid skeleton derived from SG. In GM (SG/βCD = 1/2), the doublet peak, especially OH-3 at the wide edge of CD, shifted to a singlet peak. In GM (SG/γCD = 1/2), the H-3 peak, which is the wide edge of γCD, and the H-6 peak, which is the narrow edge, shifted to broad peaks, suggesting that γCD is deeply encapsulated in the steroidal structure.
Conclusions:
These findings suggest that complex formation occurred in SG/CDs and that inclusion behavior is different between GM (SG/βCD = 1/2) and GM (SG/γCD = 1/2).
Momoko Ebisawa ... Yutaka InouePublished: February 26, 2025 Explor BioMat-X. 2025;2:101330
DOI: https://doi.org/10.37349/ebmx.2025.101330
This article belongs to the special issue Nature-Based Biomaterials for Biomedical ApplicationsAim:
In this study, the physicochemical properties of stearyl glycyrrhetinate/β-cyclodextrin (SG/βCD) and SG/γCD complexes were characterized, and the complexes were prepared using the co-milling method. The molecular interactions within the SG/CD complexes were also investigated using nuclear magnetic resonance (NMR) measurements to determine the mode of interaction.
Methods:
Here, we evaluated the physicochemical properties of SG complexes with CDs prepared by ground mixtures (GM SG/βCD or γCD = 1/1, 1/2).
Results:
Powder X-ray diffraction (PXRD) showed that the characteristic SG and CD peaks disappeared after co-grinding with GM (SG/CD = molar ratio of 1/2), indicating a halo pattern. Differential scanning calorimetry (DSC) measurements showed that after co-grinding, the endothermic peak due to SG melting, as well as the dehydration peak and the endothermic peak due to SG melting, disappeared. Near-infrared (NIR) spectroscopy results showed that the peaks of SG-derived –CH moieties and CD-derived –OH and –CH moieties broadened in GM, suggesting their involvement in complex formation through SG/CDs intermolecular interactions. In GM (SG/CDs), NMR measurements showed broadened H-A and H-F peaks of the steroid skeleton derived from SG. In GM (SG/βCD = 1/2), the doublet peak, especially OH-3 at the wide edge of CD, shifted to a singlet peak. In GM (SG/γCD = 1/2), the H-3 peak, which is the wide edge of γCD, and the H-6 peak, which is the narrow edge, shifted to broad peaks, suggesting that γCD is deeply encapsulated in the steroidal structure.
Conclusions:
These findings suggest that complex formation occurred in SG/CDs and that inclusion behavior is different between GM (SG/βCD = 1/2) and GM (SG/γCD = 1/2).
 Antifungal effect against wilting disease factor Verticillium dahliae Kleb. by green synthesized silver nanoparticlesOpen AccessOriginal ArticleAim: This study aimed to synthesize, characterize, and evaluate the antifungal efficacy of green-synthesized silver nanoparticles (AgNPs) against Verticillium dahliae Kleb., a soil-borne fungal p [...] Read more.
Antifungal effect against wilting disease factor Verticillium dahliae Kleb. by green synthesized silver nanoparticlesOpen AccessOriginal ArticleAim: This study aimed to synthesize, characterize, and evaluate the antifungal efficacy of green-synthesized silver nanoparticles (AgNPs) against Verticillium dahliae Kleb., a soil-borne fungal p [...] Read more.Aim:
This study aimed to synthesize, characterize, and evaluate the antifungal efficacy of green-synthesized silver nanoparticles (AgNPs) against Verticillium dahliae Kleb., a soil-borne fungal pathogen that affects numerous crops.
Methods:
AgNPs were synthesized using Laurus nobilis L. (laurel) leaf extract. The synthesized AgNPs were characterized using UV-VIS spectroscopy, Fourier-Transform Infrared Spectroscopy (FTIR), zeta potential, particle size analysis (PSA), and scanning electron microscopy (SEM). In vitro antifungal assays were conducted to assess the impact of AgNPs on V. dahliae mycelial growth, and SEM was used to examine the morphological changes in treated mycelium.
Results:
UV-VIS spectroscopy confirmed AgNP synthesis with a characteristic SPR peak between 400–450 nm. FTIR analysis identified the presence of phenolic compounds on the nanoparticle surface. Zeta potential analysis (–27.7 mV) indicated stable dispersion. Zeta size analysis indicated an average diameter of approximately 100 nm and a polydispersity index (PdI) of 0.229. SEM imaging confirmed a predominantly spherical morphology and PSA revealed a size range of 14–34 nm, with an average diameter of 24 nm. In vitro antifungal assays showed significant inhibition of V. dahliae mycelial growth, with radial mycelial growth reduced to 2.75 cm compared to 4.8–6.4 cm in the control group after 14 days. SEM imaging of treated mycelium revealed pronounced morphological damage, including collapse and shrinkage of hyphae and spores.
Conclusions:
Green-synthesized AgNPs using L. nobilis leaf extract demonstrated significant antifungal activity against V. dahliae. The observed inhibition of mycelial growth and morphological damage suggests the potential of these AgNPs as a sustainable and eco-friendly alternative for managing this fungal pathogen. The antifungal mechanism may involve membrane disruption, increased permeability, oxidative stress, and the inactivation of cellular components.
Alp Cokislerel ... Lale Yildiz AktasPublished: February 18, 2025 Explor BioMat-X. 2025;2:101329
DOI: https://doi.org/10.37349/ebmx.2025.101329Aim:
This study aimed to synthesize, characterize, and evaluate the antifungal efficacy of green-synthesized silver nanoparticles (AgNPs) against Verticillium dahliae Kleb., a soil-borne fungal pathogen that affects numerous crops.
Methods:
AgNPs were synthesized using Laurus nobilis L. (laurel) leaf extract. The synthesized AgNPs were characterized using UV-VIS spectroscopy, Fourier-Transform Infrared Spectroscopy (FTIR), zeta potential, particle size analysis (PSA), and scanning electron microscopy (SEM). In vitro antifungal assays were conducted to assess the impact of AgNPs on V. dahliae mycelial growth, and SEM was used to examine the morphological changes in treated mycelium.
Results:
UV-VIS spectroscopy confirmed AgNP synthesis with a characteristic SPR peak between 400–450 nm. FTIR analysis identified the presence of phenolic compounds on the nanoparticle surface. Zeta potential analysis (–27.7 mV) indicated stable dispersion. Zeta size analysis indicated an average diameter of approximately 100 nm and a polydispersity index (PdI) of 0.229. SEM imaging confirmed a predominantly spherical morphology and PSA revealed a size range of 14–34 nm, with an average diameter of 24 nm. In vitro antifungal assays showed significant inhibition of V. dahliae mycelial growth, with radial mycelial growth reduced to 2.75 cm compared to 4.8–6.4 cm in the control group after 14 days. SEM imaging of treated mycelium revealed pronounced morphological damage, including collapse and shrinkage of hyphae and spores.
Conclusions:
Green-synthesized AgNPs using L. nobilis leaf extract demonstrated significant antifungal activity against V. dahliae. The observed inhibition of mycelial growth and morphological damage suggests the potential of these AgNPs as a sustainable and eco-friendly alternative for managing this fungal pathogen. The antifungal mechanism may involve membrane disruption, increased permeability, oxidative stress, and the inactivation of cellular components.
 Silver nanoparticles biosynthesized from Stenocereus queretaroensis with antiproliferative activityOpen AccessOriginal ArticleAim: This work aimed to evaluate the antiproliferative activity of silver nanoparticles (AgNPs) biosynthesized with aqueous extract of Stenocereus queretaroensis peel (SAgNPs) in pancreatic ducta [...] Read more.
Silver nanoparticles biosynthesized from Stenocereus queretaroensis with antiproliferative activityOpen AccessOriginal ArticleAim: This work aimed to evaluate the antiproliferative activity of silver nanoparticles (AgNPs) biosynthesized with aqueous extract of Stenocereus queretaroensis peel (SAgNPs) in pancreatic ducta [...] Read more.Aim:
This work aimed to evaluate the antiproliferative activity of silver nanoparticles (AgNPs) biosynthesized with aqueous extract of Stenocereus queretaroensis peel (SAgNPs) in pancreatic ductal cancer cells PANC-1.
Methods:
Nanoparticles were synthesized using 2 mM silver nitrate (AgNO3) and a 1% aqueous extract of Stenocereus queretaroensis peel. SAgNPs were characterized by ultraviolet-visible spectroscopy (UV-Vis) light spectroscopy, dynamic light scattering analysis, and transmission electron microscopy. The antiproliferative activity was evaluated in the PANC-1 cell line by measuring the viability percentage with the 3'-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method and subsequently the IC50 of SAgNPs.
Results:
The presence of AgNPs was confirmed by silver surface plasmon resonance at 420 nm. The average size obtained by dynamic light scattering analysis was 98.96 nm, with a spherical and uniform shape according to transmission electron microscopy analysis. SAgNPs were tested at concentrations from 10 µg/mL to 0.3125 µg/mL and presented inhibition percentages from 3.76% to 90.09% with an IC50 value of 3.04 µg/mL (p-value of 0.02, 95% confidence level) in PANC-1 cells.
Conclusions:
The biologically synthesized nanoparticles with Stenocereus queretaroensis peel showed antiproliferative activity in PANC-1 pancreatic cancer cells. Therefore, these results suggest their potential use in the prevention and treatment of pancreatic cancer with further investigation.
Ariadna Abigail Villarreal-Amézquita ... Eduardo Padilla-CamberosPublished: February 16, 2025 Explor BioMat-X. 2025;2:101328
DOI: https://doi.org/10.37349/ebmx.2025.101328Aim:
This work aimed to evaluate the antiproliferative activity of silver nanoparticles (AgNPs) biosynthesized with aqueous extract of Stenocereus queretaroensis peel (SAgNPs) in pancreatic ductal cancer cells PANC-1.
Methods:
Nanoparticles were synthesized using 2 mM silver nitrate (AgNO3) and a 1% aqueous extract of Stenocereus queretaroensis peel. SAgNPs were characterized by ultraviolet-visible spectroscopy (UV-Vis) light spectroscopy, dynamic light scattering analysis, and transmission electron microscopy. The antiproliferative activity was evaluated in the PANC-1 cell line by measuring the viability percentage with the 3'-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method and subsequently the IC50 of SAgNPs.
Results:
The presence of AgNPs was confirmed by silver surface plasmon resonance at 420 nm. The average size obtained by dynamic light scattering analysis was 98.96 nm, with a spherical and uniform shape according to transmission electron microscopy analysis. SAgNPs were tested at concentrations from 10 µg/mL to 0.3125 µg/mL and presented inhibition percentages from 3.76% to 90.09% with an IC50 value of 3.04 µg/mL (p-value of 0.02, 95% confidence level) in PANC-1 cells.
Conclusions:
The biologically synthesized nanoparticles with Stenocereus queretaroensis peel showed antiproliferative activity in PANC-1 pancreatic cancer cells. Therefore, these results suggest their potential use in the prevention and treatment of pancreatic cancer with further investigation.
 Advances in bone tissue engineering using biomaterial based scaffolds, purine crosslinking and Wnt signalingOpen AccessReviewThe design of effective treatments for critical size bone defects, which result from various conditions such as trauma, infection, injury, or tumor resection, presents a significant challenge in cli [...] Read more.
Advances in bone tissue engineering using biomaterial based scaffolds, purine crosslinking and Wnt signalingOpen AccessReviewThe design of effective treatments for critical size bone defects, which result from various conditions such as trauma, infection, injury, or tumor resection, presents a significant challenge in cli [...] Read more.The design of effective treatments for critical size bone defects, which result from various conditions such as trauma, infection, injury, or tumor resection, presents a significant challenge in clinical practice. While autologous grafts are commonly regarded as gold standard treatments in these complex healing scenarios, they are often associated with notable limitations, including donor site morbidity and limited graft volume. As a result, recent research trends have shifted towards developing biomaterials that better emulate the inherent complexity of the native bone structure and function through implementation of a “Diamond Concept” polytherapy strategy. Central to this approach is the utilization of biomaterials, increasingly composed of composite materials that integrate bioactive osteoinductive factors and cell sources to enhance healing outcomes. The usage of Wnt signaling specific agonists as osteoinductive mediators has been recently shown to be a promising strategy for promoting healing, as this pathway is well established to have an important role in both osteogenic differentiation and bone formation processes. Implementation of a localized delivery system through scaffold incorporation is necessary in this scenario, however, to minimize any potential off-target effects caused by the Wnt signaling cascade’s non-specificity to bone. Findings in the literature clearly show that this approach holds promise to improve clinical healing outcomes, paving the way for more effective treatment options. In this review, we will generally discuss the design of biomaterials, specifically bulk materials and composites, for the treatment of critical size bone defects. Additionally, we will highlight recent work on the design of chitosan-based scaffolds modified with purine crosslinking, to overcome cytotoxicity issues associated with other chemical crosslinkers. In this context, we focus on optimizing material design for this bone healing application and discuss the benefits of localized Wnt agonist as mediators to improve the scaffold’s osteoinductive behavior.
Celine J. Agnes ... Maryam TabrizianPublished: February 14, 2025 Explor BioMat-X. 2025;2:101327
DOI: https://doi.org/10.37349/ebmx.2025.101327The design of effective treatments for critical size bone defects, which result from various conditions such as trauma, infection, injury, or tumor resection, presents a significant challenge in clinical practice. While autologous grafts are commonly regarded as gold standard treatments in these complex healing scenarios, they are often associated with notable limitations, including donor site morbidity and limited graft volume. As a result, recent research trends have shifted towards developing biomaterials that better emulate the inherent complexity of the native bone structure and function through implementation of a “Diamond Concept” polytherapy strategy. Central to this approach is the utilization of biomaterials, increasingly composed of composite materials that integrate bioactive osteoinductive factors and cell sources to enhance healing outcomes. The usage of Wnt signaling specific agonists as osteoinductive mediators has been recently shown to be a promising strategy for promoting healing, as this pathway is well established to have an important role in both osteogenic differentiation and bone formation processes. Implementation of a localized delivery system through scaffold incorporation is necessary in this scenario, however, to minimize any potential off-target effects caused by the Wnt signaling cascade’s non-specificity to bone. Findings in the literature clearly show that this approach holds promise to improve clinical healing outcomes, paving the way for more effective treatment options. In this review, we will generally discuss the design of biomaterials, specifically bulk materials and composites, for the treatment of critical size bone defects. Additionally, we will highlight recent work on the design of chitosan-based scaffolds modified with purine crosslinking, to overcome cytotoxicity issues associated with other chemical crosslinkers. In this context, we focus on optimizing material design for this bone healing application and discuss the benefits of localized Wnt agonist as mediators to improve the scaffold’s osteoinductive behavior.
 Navigating regulatory challenges in molecularly tailored nanomedicineOpen AccessCommentaryNanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough underst [...] Read more.
Navigating regulatory challenges in molecularly tailored nanomedicineOpen AccessCommentaryNanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough underst [...] Read more.Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
Ajay Vikram Singh ... Paolo ZamboniPublished: April 25, 2024 Explor BioMat-X. 2024;1:124–134
DOI: https://doi.org/10.37349/ebmx.2024.00009Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
 Fabrication and characterization of pHEMA hydrogel conduit containing GelMA-HaMA IPN for peripheral nerve regenerationOpen AccessOriginal ArticleAim: Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, resear [...] Read more.
Fabrication and characterization of pHEMA hydrogel conduit containing GelMA-HaMA IPN for peripheral nerve regenerationOpen AccessOriginal ArticleAim: Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, resear [...] Read more.Aim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
Damla Arslantunali Sahin ... Vasif HasirciPublished: February 26, 2024 Explor BioMat-X. 2024;1:34–57
DOI: https://doi.org/10.37349/ebmx.2024.00005
This article belongs to the special issue Nature-Based Biomaterials for Biomedical ApplicationsAim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
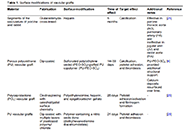
 Advancements in surface modification strategies of vascular grafts to improve biocompatibility and tissue integrationOpen AccessReviewImproving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification o [...] Read more.
Advancements in surface modification strategies of vascular grafts to improve biocompatibility and tissue integrationOpen AccessReviewImproving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification o [...] Read more.Improving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification of the biomaterials from which the vascular grafts are constructed has been used to reduce the risk of complications such as thrombosis and infection. Herein with a focus on vascular tissue engineering, we provided an overview of (a) fundamental hemodynamic considerations for blood-contacting biomaterials, (b) surface modification strategies to attenuate nonspecific adhesion of proteins, improve hemocompatibility, and induce the formation of a confluent endothelial lining, and (c) the guidelines for the clinical development of surface modified biomaterials.
Noor Abu Jarad ... Tohid F. DidarPublished: September 13, 2024 Explor BioMat-X. 2024;1:241–265
DOI: https://doi.org/10.37349/ebmx.2024.00018Improving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification of the biomaterials from which the vascular grafts are constructed has been used to reduce the risk of complications such as thrombosis and infection. Herein with a focus on vascular tissue engineering, we provided an overview of (a) fundamental hemodynamic considerations for blood-contacting biomaterials, (b) surface modification strategies to attenuate nonspecific adhesion of proteins, improve hemocompatibility, and induce the formation of a confluent endothelial lining, and (c) the guidelines for the clinical development of surface modified biomaterials.
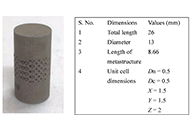
 On 17-4PH stainless steel dental implant for premolar 4 in canine under compressive loading: effect of solid and octet metastructureOpen AccessOriginal ArticleAim: The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical pro [...] Read more.
On 17-4PH stainless steel dental implant for premolar 4 in canine under compressive loading: effect of solid and octet metastructureOpen AccessOriginal ArticleAim: The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical pro [...] Read more.Aim:
The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical properties, the 17-4 precipitate hardened (PH) stainless steel (SS) prototypes were successfully 3D printed by powder bed fusion (PBF) process with solid and octet metastructure to reduce stress shielding.
Methods:
The maxillary PM4 tooth of a male German shepherd dog was selected as the subject for the proposed study. As PM4 loading in canines is analogous to compressive loading conditions, finite element analysis (FEA) under compression was performed to compare simulated results of solid and octet meta-structure specimens. Solid and octet meta structure-based compression samples were prepared per ASTM E9 standard using SolidWorks software. The octet metastructure was designed with node and connector diameters of 0.5 mm each on 3DXpert software. Further FEA analysis of designed compression samples was performed using Ansys Workbench by selecting 17-4PH SS material at loading conditions of 800 N and 5,000 N.
Results:
The FEA results at the loading of 800 N show that maximum Von-Mises stress in the case of the solid and octet meta structure-based compression specimen was 10.029 MPa and 131.61 MPa, respectively. Further, the maximum Von-Mises strain for the solid and octet meta-structure-based specimens was 0.000049163 and 0.00067179, respectively. Similarly, deformation (in mm) for solid and octet truss lattice-based compression samples were 0.00075097 and 0.001451, respectively. The results observed at the loading condition of 5,000 N followed a pattern similar to that of 800 N loading conditions.
Conclusions:
Octet metastructure-based compression sample showed encouraging potential for withstanding maximum compression loading applicable to canine (800 N) while lowering the impacts of stress shielding. The safety factor against failure (N) was 4.33 and 62.31 for the octet meta-structure and solid compression samples, respectively.
Bharat Kalia ... Gurwinder SinghPublished: August 23, 2024 Explor BioMat-X. 2024;1:202–214
DOI: https://doi.org/10.37349/ebmx.2024.00015
This article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and ImplantsAim:
The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical properties, the 17-4 precipitate hardened (PH) stainless steel (SS) prototypes were successfully 3D printed by powder bed fusion (PBF) process with solid and octet metastructure to reduce stress shielding.
Methods:
The maxillary PM4 tooth of a male German shepherd dog was selected as the subject for the proposed study. As PM4 loading in canines is analogous to compressive loading conditions, finite element analysis (FEA) under compression was performed to compare simulated results of solid and octet meta-structure specimens. Solid and octet meta structure-based compression samples were prepared per ASTM E9 standard using SolidWorks software. The octet metastructure was designed with node and connector diameters of 0.5 mm each on 3DXpert software. Further FEA analysis of designed compression samples was performed using Ansys Workbench by selecting 17-4PH SS material at loading conditions of 800 N and 5,000 N.
Results:
The FEA results at the loading of 800 N show that maximum Von-Mises stress in the case of the solid and octet meta structure-based compression specimen was 10.029 MPa and 131.61 MPa, respectively. Further, the maximum Von-Mises strain for the solid and octet meta-structure-based specimens was 0.000049163 and 0.00067179, respectively. Similarly, deformation (in mm) for solid and octet truss lattice-based compression samples were 0.00075097 and 0.001451, respectively. The results observed at the loading condition of 5,000 N followed a pattern similar to that of 800 N loading conditions.
Conclusions:
Octet metastructure-based compression sample showed encouraging potential for withstanding maximum compression loading applicable to canine (800 N) while lowering the impacts of stress shielding. The safety factor against failure (N) was 4.33 and 62.31 for the octet meta-structure and solid compression samples, respectively.
 A tri-layer tissue engineering heart valve scaffold based on atelocollagen, hyaluronic acid, and elastinOpen AccessOriginal ArticleAim: This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart va [...] Read more.
A tri-layer tissue engineering heart valve scaffold based on atelocollagen, hyaluronic acid, and elastinOpen AccessOriginal ArticleAim: This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart va [...] Read more.Aim:
This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart valve leaflets in structure, composition, and mechanical properties, among which, the bending anisotropic behaviour in both the with curvature (WC) and the against curvature (AC) directions, is the most desired. The use of atelocollagen is of significant importance in highlighting the non-antigenic potential of the design.
Methods:
Porous scaffolds were freeze-dried, then crosslinked using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The fibrillogenesis occurrence and the scaffold microstructure were imaged using scanning electron microscopy (SEM). Fourier transform infrared spectroscopy (FITR) investigated the effect of fabrication and crosslinking on the backbone structure. Dynamic mechanical analysis (DMA) characterised the compressive and bending properties of the scaffolds in hydrated and non-hydrated states. Three-point bending and a “self-deflection” test were performed on tri-layer scaffolds in both WC and AC directions.
Results:
Atelocollagen-based scaffolds were successfully produced, rendering this study the first to report a tri-layer structure using atelocollagen, HA, and elastin fibrillar gel. The scaffolds’ porosity was tailored to accommodate potential future biological studies and the transition between layers appeared seamless. FITR unveiled effective crosslinking and the backbone structure preservation. The scaffolds exhibited lightly crosslinked polymer resembling mechanical responses when non-hydrated, and the desired J-curve stress-strain response was observed when hydrated. The tri-layer scaffolds showed anisotropic bending behaviour with a bending modulus of 5.41 ± 1.14 kPa (WC) and 7.98 ± 2.22 kPa (AC).
Conclusions:
The tri-layer scaffolds fabricated resemble the native aortic valve leaflets in structure and composition, and successfully introduced bending anisotropy in physiological conditions. Together with the suitable microstructure and promising mechanical properties, the design is reckoned to be a potential tissue engineering heart valve candidate.
Zhaoying Ma ... Jan T. CzernuszkaPublished: August 29, 2024 Explor BioMat-X. 2024;1:215–230
DOI: https://doi.org/10.37349/ebmx.2024.00016
This article belongs to the special issue Bioinspired Material for Regenerative MedicineAim:
This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart valve leaflets in structure, composition, and mechanical properties, among which, the bending anisotropic behaviour in both the with curvature (WC) and the against curvature (AC) directions, is the most desired. The use of atelocollagen is of significant importance in highlighting the non-antigenic potential of the design.
Methods:
Porous scaffolds were freeze-dried, then crosslinked using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The fibrillogenesis occurrence and the scaffold microstructure were imaged using scanning electron microscopy (SEM). Fourier transform infrared spectroscopy (FITR) investigated the effect of fabrication and crosslinking on the backbone structure. Dynamic mechanical analysis (DMA) characterised the compressive and bending properties of the scaffolds in hydrated and non-hydrated states. Three-point bending and a “self-deflection” test were performed on tri-layer scaffolds in both WC and AC directions.
Results:
Atelocollagen-based scaffolds were successfully produced, rendering this study the first to report a tri-layer structure using atelocollagen, HA, and elastin fibrillar gel. The scaffolds’ porosity was tailored to accommodate potential future biological studies and the transition between layers appeared seamless. FITR unveiled effective crosslinking and the backbone structure preservation. The scaffolds exhibited lightly crosslinked polymer resembling mechanical responses when non-hydrated, and the desired J-curve stress-strain response was observed when hydrated. The tri-layer scaffolds showed anisotropic bending behaviour with a bending modulus of 5.41 ± 1.14 kPa (WC) and 7.98 ± 2.22 kPa (AC).
Conclusions:
The tri-layer scaffolds fabricated resemble the native aortic valve leaflets in structure and composition, and successfully introduced bending anisotropy in physiological conditions. Together with the suitable microstructure and promising mechanical properties, the design is reckoned to be a potential tissue engineering heart valve candidate.
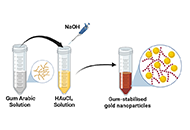
 Gum Arabic-assisted green synthesis of gold nanoparticles as fluorescence modulator for potential analytical applicationsOpen AccessOriginal ArticleAim: To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing age [...] Read more.
Gum Arabic-assisted green synthesis of gold nanoparticles as fluorescence modulator for potential analytical applicationsOpen AccessOriginal ArticleAim: To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing age [...] Read more.Aim:
To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing agent.
Methods:
Green synthesis of nanoparticles, characterization by absorption, infra-red and fluorescence spectroscopy.
Results:
The absorption spectrum (UV-Vis) showed an absorption peak ~522 nm corresponding to the surface plasmon resonance (SPR) absorption peak of AuNPs. Transmission electron microscopy (TEM) images revealed spherical-shaped nanoparticles with an average size of 15 nm. Fourier transform infrared (FTIR) analysis showed that the nanoparticles are coated with organic compounds that are present in GA. The fluorescence quenching properties of the AuNPs were assessed by monitoring their effects on fluorescence intensity of coumarin 153 (C153) dye. The fluorescence of the dye decreased with an increase in concentration of the nanoparticles. Upon addition of the protein bovine serum albumin (BSA) to the mixture the fluorescence increased (recovery) again.
Conclusions:
The fluorescence quenching and recovery (turn-on/off system) is a valuable method for protein detection in solution. By observing the effect of BSA on the quenched fluorescence, this nanoparticle system shows promise in biomedicine, drug delivery and environmental monitoring.
Ahmed T. Algahiny ... Fryad Z. HenariPublished: July 28, 2024 Explor BioMat-X. 2024;1:190–201
DOI: https://doi.org/10.37349/ebmx.2024.00014
This article belongs to the special issue Green Nanoparticles for Biomedical ApplicationsAim:
To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing agent.
Methods:
Green synthesis of nanoparticles, characterization by absorption, infra-red and fluorescence spectroscopy.
Results:
The absorption spectrum (UV-Vis) showed an absorption peak ~522 nm corresponding to the surface plasmon resonance (SPR) absorption peak of AuNPs. Transmission electron microscopy (TEM) images revealed spherical-shaped nanoparticles with an average size of 15 nm. Fourier transform infrared (FTIR) analysis showed that the nanoparticles are coated with organic compounds that are present in GA. The fluorescence quenching properties of the AuNPs were assessed by monitoring their effects on fluorescence intensity of coumarin 153 (C153) dye. The fluorescence of the dye decreased with an increase in concentration of the nanoparticles. Upon addition of the protein bovine serum albumin (BSA) to the mixture the fluorescence increased (recovery) again.
Conclusions:
The fluorescence quenching and recovery (turn-on/off system) is a valuable method for protein detection in solution. By observing the effect of BSA on the quenched fluorescence, this nanoparticle system shows promise in biomedicine, drug delivery and environmental monitoring.
 Navigating regulatory challenges in molecularly tailored nanomedicineOpen AccessCommentaryNanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough underst [...] Read more.
Navigating regulatory challenges in molecularly tailored nanomedicineOpen AccessCommentaryNanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough underst [...] Read more.Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
Ajay Vikram Singh ... Paolo ZamboniPublished: April 25, 2024 Explor BioMat-X. 2024;1:124–134
DOI: https://doi.org/10.37349/ebmx.2024.00009Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
 Fabrication and characterization of pHEMA hydrogel conduit containing GelMA-HaMA IPN for peripheral nerve regenerationOpen AccessOriginal ArticleAim: Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, resear [...] Read more.
Fabrication and characterization of pHEMA hydrogel conduit containing GelMA-HaMA IPN for peripheral nerve regenerationOpen AccessOriginal ArticleAim: Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, resear [...] Read more.Aim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
Damla Arslantunali Sahin ... Vasif HasirciPublished: February 26, 2024 Explor BioMat-X. 2024;1:34–57
DOI: https://doi.org/10.37349/ebmx.2024.00005
This article belongs to the special issue Nature-Based Biomaterials for Biomedical ApplicationsAim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
 On 17-4PH stainless steel dental implant for premolar 4 in canine under compressive loading: effect of solid and octet metastructureOpen AccessOriginal ArticleAim: The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical pro [...] Read more.
On 17-4PH stainless steel dental implant for premolar 4 in canine under compressive loading: effect of solid and octet metastructureOpen AccessOriginal ArticleAim: The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical pro [...] Read more.Aim:
The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical properties, the 17-4 precipitate hardened (PH) stainless steel (SS) prototypes were successfully 3D printed by powder bed fusion (PBF) process with solid and octet metastructure to reduce stress shielding.
Methods:
The maxillary PM4 tooth of a male German shepherd dog was selected as the subject for the proposed study. As PM4 loading in canines is analogous to compressive loading conditions, finite element analysis (FEA) under compression was performed to compare simulated results of solid and octet meta-structure specimens. Solid and octet meta structure-based compression samples were prepared per ASTM E9 standard using SolidWorks software. The octet metastructure was designed with node and connector diameters of 0.5 mm each on 3DXpert software. Further FEA analysis of designed compression samples was performed using Ansys Workbench by selecting 17-4PH SS material at loading conditions of 800 N and 5,000 N.
Results:
The FEA results at the loading of 800 N show that maximum Von-Mises stress in the case of the solid and octet meta structure-based compression specimen was 10.029 MPa and 131.61 MPa, respectively. Further, the maximum Von-Mises strain for the solid and octet meta-structure-based specimens was 0.000049163 and 0.00067179, respectively. Similarly, deformation (in mm) for solid and octet truss lattice-based compression samples were 0.00075097 and 0.001451, respectively. The results observed at the loading condition of 5,000 N followed a pattern similar to that of 800 N loading conditions.
Conclusions:
Octet metastructure-based compression sample showed encouraging potential for withstanding maximum compression loading applicable to canine (800 N) while lowering the impacts of stress shielding. The safety factor against failure (N) was 4.33 and 62.31 for the octet meta-structure and solid compression samples, respectively.
Bharat Kalia ... Gurwinder SinghPublished: August 23, 2024 Explor BioMat-X. 2024;1:202–214
DOI: https://doi.org/10.37349/ebmx.2024.00015
This article belongs to the special issue Metal 3D Printing of Biometals for Prostheses and ImplantsAim:
The study aims to analyze the canine’s implant behaviour under compressive loading [to be installed in a maxilla at a premolar 4 (PM4) location]. After simulation of various mechanical properties, the 17-4 precipitate hardened (PH) stainless steel (SS) prototypes were successfully 3D printed by powder bed fusion (PBF) process with solid and octet metastructure to reduce stress shielding.
Methods:
The maxillary PM4 tooth of a male German shepherd dog was selected as the subject for the proposed study. As PM4 loading in canines is analogous to compressive loading conditions, finite element analysis (FEA) under compression was performed to compare simulated results of solid and octet meta-structure specimens. Solid and octet meta structure-based compression samples were prepared per ASTM E9 standard using SolidWorks software. The octet metastructure was designed with node and connector diameters of 0.5 mm each on 3DXpert software. Further FEA analysis of designed compression samples was performed using Ansys Workbench by selecting 17-4PH SS material at loading conditions of 800 N and 5,000 N.
Results:
The FEA results at the loading of 800 N show that maximum Von-Mises stress in the case of the solid and octet meta structure-based compression specimen was 10.029 MPa and 131.61 MPa, respectively. Further, the maximum Von-Mises strain for the solid and octet meta-structure-based specimens was 0.000049163 and 0.00067179, respectively. Similarly, deformation (in mm) for solid and octet truss lattice-based compression samples were 0.00075097 and 0.001451, respectively. The results observed at the loading condition of 5,000 N followed a pattern similar to that of 800 N loading conditions.
Conclusions:
Octet metastructure-based compression sample showed encouraging potential for withstanding maximum compression loading applicable to canine (800 N) while lowering the impacts of stress shielding. The safety factor against failure (N) was 4.33 and 62.31 for the octet meta-structure and solid compression samples, respectively.
 Advancements in surface modification strategies of vascular grafts to improve biocompatibility and tissue integrationOpen AccessReviewImproving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification o [...] Read more.
Advancements in surface modification strategies of vascular grafts to improve biocompatibility and tissue integrationOpen AccessReviewImproving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification o [...] Read more.Improving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification of the biomaterials from which the vascular grafts are constructed has been used to reduce the risk of complications such as thrombosis and infection. Herein with a focus on vascular tissue engineering, we provided an overview of (a) fundamental hemodynamic considerations for blood-contacting biomaterials, (b) surface modification strategies to attenuate nonspecific adhesion of proteins, improve hemocompatibility, and induce the formation of a confluent endothelial lining, and (c) the guidelines for the clinical development of surface modified biomaterials.
Noor Abu Jarad ... Tohid F. DidarPublished: September 13, 2024 Explor BioMat-X. 2024;1:241–265
DOI: https://doi.org/10.37349/ebmx.2024.00018Improving the performance of blood-contacting medical implants is a global health necessity aimed at reducing mortality and morbidity in patients with cardiovascular diseases. Surface modification of the biomaterials from which the vascular grafts are constructed has been used to reduce the risk of complications such as thrombosis and infection. Herein with a focus on vascular tissue engineering, we provided an overview of (a) fundamental hemodynamic considerations for blood-contacting biomaterials, (b) surface modification strategies to attenuate nonspecific adhesion of proteins, improve hemocompatibility, and induce the formation of a confluent endothelial lining, and (c) the guidelines for the clinical development of surface modified biomaterials.
 Gum Arabic-assisted green synthesis of gold nanoparticles as fluorescence modulator for potential analytical applicationsOpen AccessOriginal ArticleAim: To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing age [...] Read more.
Gum Arabic-assisted green synthesis of gold nanoparticles as fluorescence modulator for potential analytical applicationsOpen AccessOriginal ArticleAim: To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing age [...] Read more.Aim:
To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing agent.
Methods:
Green synthesis of nanoparticles, characterization by absorption, infra-red and fluorescence spectroscopy.
Results:
The absorption spectrum (UV-Vis) showed an absorption peak ~522 nm corresponding to the surface plasmon resonance (SPR) absorption peak of AuNPs. Transmission electron microscopy (TEM) images revealed spherical-shaped nanoparticles with an average size of 15 nm. Fourier transform infrared (FTIR) analysis showed that the nanoparticles are coated with organic compounds that are present in GA. The fluorescence quenching properties of the AuNPs were assessed by monitoring their effects on fluorescence intensity of coumarin 153 (C153) dye. The fluorescence of the dye decreased with an increase in concentration of the nanoparticles. Upon addition of the protein bovine serum albumin (BSA) to the mixture the fluorescence increased (recovery) again.
Conclusions:
The fluorescence quenching and recovery (turn-on/off system) is a valuable method for protein detection in solution. By observing the effect of BSA on the quenched fluorescence, this nanoparticle system shows promise in biomedicine, drug delivery and environmental monitoring.
Ahmed T. Algahiny ... Fryad Z. HenariPublished: July 28, 2024 Explor BioMat-X. 2024;1:190–201
DOI: https://doi.org/10.37349/ebmx.2024.00014
This article belongs to the special issue Green Nanoparticles for Biomedical ApplicationsAim:
To demonstrate a simple, eco-friendly, and cost-effective green method to synthesize gold nanoparticles (AuNPs) using the aqueous extract of gum Arabic (GA) as a reducing and stabilizing agent.
Methods:
Green synthesis of nanoparticles, characterization by absorption, infra-red and fluorescence spectroscopy.
Results:
The absorption spectrum (UV-Vis) showed an absorption peak ~522 nm corresponding to the surface plasmon resonance (SPR) absorption peak of AuNPs. Transmission electron microscopy (TEM) images revealed spherical-shaped nanoparticles with an average size of 15 nm. Fourier transform infrared (FTIR) analysis showed that the nanoparticles are coated with organic compounds that are present in GA. The fluorescence quenching properties of the AuNPs were assessed by monitoring their effects on fluorescence intensity of coumarin 153 (C153) dye. The fluorescence of the dye decreased with an increase in concentration of the nanoparticles. Upon addition of the protein bovine serum albumin (BSA) to the mixture the fluorescence increased (recovery) again.
Conclusions:
The fluorescence quenching and recovery (turn-on/off system) is a valuable method for protein detection in solution. By observing the effect of BSA on the quenched fluorescence, this nanoparticle system shows promise in biomedicine, drug delivery and environmental monitoring.
 A tri-layer tissue engineering heart valve scaffold based on atelocollagen, hyaluronic acid, and elastinOpen AccessOriginal ArticleAim: This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart va [...] Read more.
A tri-layer tissue engineering heart valve scaffold based on atelocollagen, hyaluronic acid, and elastinOpen AccessOriginal ArticleAim: This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart va [...] Read more.Aim:
This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart valve leaflets in structure, composition, and mechanical properties, among which, the bending anisotropic behaviour in both the with curvature (WC) and the against curvature (AC) directions, is the most desired. The use of atelocollagen is of significant importance in highlighting the non-antigenic potential of the design.
Methods:
Porous scaffolds were freeze-dried, then crosslinked using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The fibrillogenesis occurrence and the scaffold microstructure were imaged using scanning electron microscopy (SEM). Fourier transform infrared spectroscopy (FITR) investigated the effect of fabrication and crosslinking on the backbone structure. Dynamic mechanical analysis (DMA) characterised the compressive and bending properties of the scaffolds in hydrated and non-hydrated states. Three-point bending and a “self-deflection” test were performed on tri-layer scaffolds in both WC and AC directions.
Results:
Atelocollagen-based scaffolds were successfully produced, rendering this study the first to report a tri-layer structure using atelocollagen, HA, and elastin fibrillar gel. The scaffolds’ porosity was tailored to accommodate potential future biological studies and the transition between layers appeared seamless. FITR unveiled effective crosslinking and the backbone structure preservation. The scaffolds exhibited lightly crosslinked polymer resembling mechanical responses when non-hydrated, and the desired J-curve stress-strain response was observed when hydrated. The tri-layer scaffolds showed anisotropic bending behaviour with a bending modulus of 5.41 ± 1.14 kPa (WC) and 7.98 ± 2.22 kPa (AC).
Conclusions:
The tri-layer scaffolds fabricated resemble the native aortic valve leaflets in structure and composition, and successfully introduced bending anisotropy in physiological conditions. Together with the suitable microstructure and promising mechanical properties, the design is reckoned to be a potential tissue engineering heart valve candidate.
Zhaoying Ma ... Jan T. CzernuszkaPublished: August 29, 2024 Explor BioMat-X. 2024;1:215–230
DOI: https://doi.org/10.37349/ebmx.2024.00016
This article belongs to the special issue Bioinspired Material for Regenerative MedicineAim:
This study aims to fabricate and characterise a novel tri-layer scaffold based on type I atelocollagen, hyaluronic acid (HA), and a novel fibrillar elastin gel, mimicking the native heart valve leaflets in structure, composition, and mechanical properties, among which, the bending anisotropic behaviour in both the with curvature (WC) and the against curvature (AC) directions, is the most desired. The use of atelocollagen is of significant importance in highlighting the non-antigenic potential of the design.
Methods:
Porous scaffolds were freeze-dried, then crosslinked using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The fibrillogenesis occurrence and the scaffold microstructure were imaged using scanning electron microscopy (SEM). Fourier transform infrared spectroscopy (FITR) investigated the effect of fabrication and crosslinking on the backbone structure. Dynamic mechanical analysis (DMA) characterised the compressive and bending properties of the scaffolds in hydrated and non-hydrated states. Three-point bending and a “self-deflection” test were performed on tri-layer scaffolds in both WC and AC directions.
Results:
Atelocollagen-based scaffolds were successfully produced, rendering this study the first to report a tri-layer structure using atelocollagen, HA, and elastin fibrillar gel. The scaffolds’ porosity was tailored to accommodate potential future biological studies and the transition between layers appeared seamless. FITR unveiled effective crosslinking and the backbone structure preservation. The scaffolds exhibited lightly crosslinked polymer resembling mechanical responses when non-hydrated, and the desired J-curve stress-strain response was observed when hydrated. The tri-layer scaffolds showed anisotropic bending behaviour with a bending modulus of 5.41 ± 1.14 kPa (WC) and 7.98 ± 2.22 kPa (AC).
Conclusions:
The tri-layer scaffolds fabricated resemble the native aortic valve leaflets in structure and composition, and successfully introduced bending anisotropy in physiological conditions. Together with the suitable microstructure and promising mechanical properties, the design is reckoned to be a potential tissue engineering heart valve candidate.
 Navigating regulatory challenges in molecularly tailored nanomedicineOpen AccessCommentaryNanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough underst [...] Read more.
Navigating regulatory challenges in molecularly tailored nanomedicineOpen AccessCommentaryNanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough underst [...] Read more.Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
Ajay Vikram Singh ... Paolo ZamboniPublished: April 25, 2024 Explor BioMat-X. 2024;1:124–134
DOI: https://doi.org/10.37349/ebmx.2024.00009Nanomedicine, a convergence of nanotechnology and medical sciences, has unleashed transformative potential in healthcare. However, harnessing the benefits of nanomedicine requires a thorough understanding of its regulatory landscape. An in-depth discussion of regulatory considerations, including molecular safety assessment, harmonization of the regulatory landscape, and shaping the future of innovation, is presented in this discourse. The molecular safety assessment entails evaluating interactions between nanoparticles and biomolecules, ensuring compatibility at the molecular level. Harmonization involves developing international standards and guidelines for a consistent regulatory approach, while shaping innovations emphasizes integrating molecular safety assessments into early stages of development. Challenges encompass the need for standardized assessment methods, balancing innovation with safety, and addressing unique features of novel molecular designs. As the nanomedicine landscape evolves, effective regulatory strategies must navigate the intricate interplay of molecules and technologies, ensuring both patient access and product safety.
 Unlocking the potential of circulating small extracellular vesicles in neurodegenerative disease through targeted biomarkers and advancements in biosensingOpen AccessReviewNeurodegenerative diseases (NDDs) gradually affect neurons impacting both their function and structure, and they afflict millions worldwide. Detecting these conditions before symptoms arise is cruci [...] Read more.
Unlocking the potential of circulating small extracellular vesicles in neurodegenerative disease through targeted biomarkers and advancements in biosensingOpen AccessReviewNeurodegenerative diseases (NDDs) gradually affect neurons impacting both their function and structure, and they afflict millions worldwide. Detecting these conditions before symptoms arise is cruci [...] Read more.Neurodegenerative diseases (NDDs) gradually affect neurons impacting both their function and structure, and they afflict millions worldwide. Detecting these conditions before symptoms arise is crucial for better prognosis and duality of life, given that the disease processes often begin years earlier. Yet, reliable and affordable methods to diagnose NDDs in these stages are currently lacking. There’s a growing interest in using circulating extracellular vesicles (EVs), like small EVs (sEVs) also known as exosomes, as potential sources of markers for screening, diagnosing, and monitoring NDDs. This interest stems from evidence showing that these EVs can carry brain pathological proteins implicated in NDD pathology, and they can even traverse the blood-brain barrier. This review focuses on the creation of EVs, particularly sEVs with a size of less than 200 nanometers, methods for isolating sEVs, and recent advancements in biosensor development to detect NDD-related markers found in sEVs. Furthermore, it explores the potential of sEVs in diagnosing four major NDDs: Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and multiple system atrophy (MSA).
Saqer Al Abdullah ... Kristen DellingerPublished: April 24, 2024 Explor BioMat-X. 2024;1:100–123
DOI: https://doi.org/10.37349/ebmx.2024.00008Neurodegenerative diseases (NDDs) gradually affect neurons impacting both their function and structure, and they afflict millions worldwide. Detecting these conditions before symptoms arise is crucial for better prognosis and duality of life, given that the disease processes often begin years earlier. Yet, reliable and affordable methods to diagnose NDDs in these stages are currently lacking. There’s a growing interest in using circulating extracellular vesicles (EVs), like small EVs (sEVs) also known as exosomes, as potential sources of markers for screening, diagnosing, and monitoring NDDs. This interest stems from evidence showing that these EVs can carry brain pathological proteins implicated in NDD pathology, and they can even traverse the blood-brain barrier. This review focuses on the creation of EVs, particularly sEVs with a size of less than 200 nanometers, methods for isolating sEVs, and recent advancements in biosensor development to detect NDD-related markers found in sEVs. Furthermore, it explores the potential of sEVs in diagnosing four major NDDs: Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and multiple system atrophy (MSA).
 Exploration of biomaterials: a multidisciplinary ventureOpen AccessEditorialMaryam TabrizianPublished: January 01, 2024 Explor BioMat-X. 2024;1:1–4
Exploration of biomaterials: a multidisciplinary ventureOpen AccessEditorialMaryam TabrizianPublished: January 01, 2024 Explor BioMat-X. 2024;1:1–4
DOI: https://doi.org/10.37349/ebmx.2023.00001 Fabrication and characterization of pHEMA hydrogel conduit containing GelMA-HaMA IPN for peripheral nerve regenerationOpen AccessOriginal ArticleAim: Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, resear [...] Read more.
Fabrication and characterization of pHEMA hydrogel conduit containing GelMA-HaMA IPN for peripheral nerve regenerationOpen AccessOriginal ArticleAim: Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, resear [...] Read more.Aim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
Damla Arslantunali Sahin ... Vasif HasirciPublished: February 26, 2024 Explor BioMat-X. 2024;1:34–57
DOI: https://doi.org/10.37349/ebmx.2024.00005
This article belongs to the special issue Nature-Based Biomaterials for Biomedical ApplicationsAim:
Small defects after any injury to the periperal nerves results in self-regeneration. However, for larger defects, suturing or grafting are necessary, which may have limitations. Thus, research on nerve guidence conduits is needed without drawbacks. The aim of the study was to develop hydrogel-based conduits containing interpenetrating network (IPN).
Methods:
Methacrylated gelatin (GelMA)-methacrylated hyaluronic acid (HaMA) IPN was filled the poly(2-hydroxyethylmethacrylate) (pHEMA) the outer conduit. Schwann cells (SCs) were used on the pHEMA and the distal end of the tube was injected with netrin-1 to support model SH-SY5Y cells.
Results:
1H-nuclear magnetic resonance (1H-NMR) showed that methacrylation degrees were 94% ± 2% for GelMA and 60% ± 7% for HaMA. The fraction of HaMA increased the degradation rate; pure HaMA degraded in 3 weeks, while pure GelMA in more than 5 weeks. An increase in the fraction of 2-hydroxyethylmethacrylate (HEMA) from 20% to 56% decreased the porosity and the pore size, significantly. SH-SY5Y cells migrated along the conduit in the presence of netrin-1. NeuN expression was increased in 2 weeks indicating neuronal activity.
Conclusions:
SH-SY5Y cells produced neurites in the IPN. pHEMA conduit including GelMA-HaMA IPN is a good candidate for peripheral nerve regeneration applications. As future studies, the conduit will be tested in vivo for nerve regeneration.
 A biomimetic approach to modulating the sustained release of fibroblast growth factor 2 from fibrin microthread scaffoldsOpen AccessOriginal ArticleAim: The pleiotropic effect of fibroblast growth factor 2 (FGF2) on promoting myogenesis, angiogenesis, and innervation makes it an ideal growth factor for treating volumetric muscle loss (VML) i [...] Read more.
A biomimetic approach to modulating the sustained release of fibroblast growth factor 2 from fibrin microthread scaffoldsOpen AccessOriginal ArticleAim: The pleiotropic effect of fibroblast growth factor 2 (FGF2) on promoting myogenesis, angiogenesis, and innervation makes it an ideal growth factor for treating volumetric muscle loss (VML) i [...] Read more.Aim:
The pleiotropic effect of fibroblast growth factor 2 (FGF2) on promoting myogenesis, angiogenesis, and innervation makes it an ideal growth factor for treating volumetric muscle loss (VML) injuries. While an initial delivery of FGF2 has demonstrated enhanced regenerative potential, the sustained delivery of FGF2 from scaffolds with robust structural properties as well as biophysical and biochemical signaling cues has yet to be explored for treating VML. The goal of this study is to develop an instructive fibrin microthread scaffold with intrinsic topographic alignment cues as well as regenerative signaling cues and a physiologically relevant, sustained release of FGF2 to direct myogenesis and ultimately enhance functional muscle regeneration.
Methods:
Heparin was passively adsorbed or carbodiimide-conjugated to microthreads, creating a biomimetic binding strategy, mimicking FGF2 sequestration in the extracellular matrix (ECM). It was also evaluated whether FGF2 incorporated into fibrin microthreads would yield sustained release. It was hypothesized that heparin-conjugated and co-incorporated (co-inc) fibrin microthreads would facilitate sustained release of FGF2 from the scaffold and enhance in vitro myoblast proliferation and outgrowth.
Results:
Toluidine blue staining and Fourier transform infrared spectroscopy confirmed that carbodiimide-conjugated heparin bound to fibrin microthreads in a dose-dependent manner. Release kinetics revealed that heparin-conjugated fibrin microthreads exhibited sustained release of FGF2 over a period of one week. An in vitro assay demonstrated that FGF2 released from microthreads remained bioactive, stimulating myoblast proliferation over four days. Finally, a cellular outgrowth assay suggests that FGF2 promotes increased outgrowth onto microthreads.
Conclusions:
It was anticipated that the combined effects of fibrin microthread structural properties, topographic alignment cues, and FGF2 release profiles will facilitate the fabrication of a biomimetic scaffold that enhances the regeneration of functional muscle tissue for the treatment of VML injuries.
Meagan E. Carnes ... George D. PinsPublished: April 23, 2024 Explor BioMat-X. 2024;1:58–83
DOI: https://doi.org/10.37349/ebmx.2024.00006Aim:
The pleiotropic effect of fibroblast growth factor 2 (FGF2) on promoting myogenesis, angiogenesis, and innervation makes it an ideal growth factor for treating volumetric muscle loss (VML) injuries. While an initial delivery of FGF2 has demonstrated enhanced regenerative potential, the sustained delivery of FGF2 from scaffolds with robust structural properties as well as biophysical and biochemical signaling cues has yet to be explored for treating VML. The goal of this study is to develop an instructive fibrin microthread scaffold with intrinsic topographic alignment cues as well as regenerative signaling cues and a physiologically relevant, sustained release of FGF2 to direct myogenesis and ultimately enhance functional muscle regeneration.
Methods:
Heparin was passively adsorbed or carbodiimide-conjugated to microthreads, creating a biomimetic binding strategy, mimicking FGF2 sequestration in the extracellular matrix (ECM). It was also evaluated whether FGF2 incorporated into fibrin microthreads would yield sustained release. It was hypothesized that heparin-conjugated and co-incorporated (co-inc) fibrin microthreads would facilitate sustained release of FGF2 from the scaffold and enhance in vitro myoblast proliferation and outgrowth.
Results:
Toluidine blue staining and Fourier transform infrared spectroscopy confirmed that carbodiimide-conjugated heparin bound to fibrin microthreads in a dose-dependent manner. Release kinetics revealed that heparin-conjugated fibrin microthreads exhibited sustained release of FGF2 over a period of one week. An in vitro assay demonstrated that FGF2 released from microthreads remained bioactive, stimulating myoblast proliferation over four days. Finally, a cellular outgrowth assay suggests that FGF2 promotes increased outgrowth onto microthreads.
Conclusions:
It was anticipated that the combined effects of fibrin microthread structural properties, topographic alignment cues, and FGF2 release profiles will facilitate the fabrication of a biomimetic scaffold that enhances the regeneration of functional muscle tissue for the treatment of VML injuries.
 Effects of cold plasma treatment on the biological performances of decellularized bovine pericardium extracellular matrix-based films for biomedical applicationsOpen AccessOriginal ArticleAim: Since decades, decellularized extracellular matrix (dECM)-derived materials have received worldwide attention as promising biomaterials for tissue engineering and biomedical applications. So [...] Read more.
Effects of cold plasma treatment on the biological performances of decellularized bovine pericardium extracellular matrix-based films for biomedical applicationsOpen AccessOriginal ArticleAim: Since decades, decellularized extracellular matrix (dECM)-derived materials have received worldwide attention as promising biomaterials for tissue engineering and biomedical applications. So [...] Read more.Aim:
Since decades, decellularized extracellular matrix (dECM)-derived materials have received worldwide attention as promising biomaterials for tissue engineering and biomedical applications. Soluble dECM is a versatile raw material that can be easily engineered into the desired shapes and structures. However, there are still some limitations restricting its use, including low hydrophilicity and smooth surfaces, which negatively influence cell adhesion/spreading. The objective of the present study was to investigate surface modification by nitrogen/hydrogen (N2/H2) low-pressure cold plasma treatment as a potential technique to improve the biological response of bovine pericardium dECM films.
Methods:
Bovine pericardium dECM was enzymatically digested and lyophilized prior to the preparation of thin films via solvent-casting method. Changes in surface properties after plasma treatment were investigated using water contact angle (WCA) and X-ray photoelectron spectroscopy (XPS) measurements. Immunofluorescence staining and resazurin assay for human dermal fibroblasts (HDFs) cultured on the dECM films were used to assess the bioactivity of dECM films. Finally, the hemocompatibility of the films was investigated via clotting time and hemolysis assay.
Results:
WCA and XPS results revealed that oxygen (O)- and N-containing functional groups were incorporated onto the film surface and an increase in hydrophilicity was observed after plasma treatment. In vitro experiments showed that cell adhesion in plasma-treated dECM films is much faster if compared to the untreated controls. Moreover, the fibroblast proliferation increased after plasma surface modifications. Finally, the hemocompatibility analysis results indicated a delayed blood clotting and no hemolytic effects for all the tested samples.
Conclusions:
These findings confirmed the potential of dECM as raw material for biocompatible thin films fabrication. Additionally, plasma surface treatment emerged as an eco-friendly and cost-effective strategy to enhance in vitro cell attachment and proliferation on dECM films, expanding their applications in biomedicine.
Maria Elena Lombardo ... Diego MantovaniPublished: April 23, 2024 Explor BioMat-X. 2024;1:84–99
DOI: https://doi.org/10.37349/ebmx.2024.00007Aim:
Since decades, decellularized extracellular matrix (dECM)-derived materials have received worldwide attention as promising biomaterials for tissue engineering and biomedical applications. Soluble dECM is a versatile raw material that can be easily engineered into the desired shapes and structures. However, there are still some limitations restricting its use, including low hydrophilicity and smooth surfaces, which negatively influence cell adhesion/spreading. The objective of the present study was to investigate surface modification by nitrogen/hydrogen (N2/H2) low-pressure cold plasma treatment as a potential technique to improve the biological response of bovine pericardium dECM films.
Methods:
Bovine pericardium dECM was enzymatically digested and lyophilized prior to the preparation of thin films via solvent-casting method. Changes in surface properties after plasma treatment were investigated using water contact angle (WCA) and X-ray photoelectron spectroscopy (XPS) measurements. Immunofluorescence staining and resazurin assay for human dermal fibroblasts (HDFs) cultured on the dECM films were used to assess the bioactivity of dECM films. Finally, the hemocompatibility of the films was investigated via clotting time and hemolysis assay.
Results:
WCA and XPS results revealed that oxygen (O)- and N-containing functional groups were incorporated onto the film surface and an increase in hydrophilicity was observed after plasma treatment. In vitro experiments showed that cell adhesion in plasma-treated dECM films is much faster if compared to the untreated controls. Moreover, the fibroblast proliferation increased after plasma surface modifications. Finally, the hemocompatibility analysis results indicated a delayed blood clotting and no hemolytic effects for all the tested samples.
Conclusions:
These findings confirmed the potential of dECM as raw material for biocompatible thin films fabrication. Additionally, plasma surface treatment emerged as an eco-friendly and cost-effective strategy to enhance in vitro cell attachment and proliferation on dECM films, expanding their applications in biomedicine.
Special Issues Bioprinted Hydrogels for Engineering Tissues and Organs
Bioprinted Hydrogels for Engineering Tissues and OrgansVahid Serpooshan Liqun Ning
Submission Deadline: September 30, 2025
Published Articles: 1
 Green Nanoparticles for Biomedical Applications
Green Nanoparticles for Biomedical ApplicationsPriyanka Singh
Submission Deadline: September 30, 2025
Published Articles: 1
 Innovations in Biomaterials for Dentistry and Oral Surgery
Innovations in Biomaterials for Dentistry and Oral SurgeryLuca Fiorillo
Submission Deadline: September 30, 2025
Published Articles: 2
 Metal 3D Printing of Biometals for Prostheses and Implants
Metal 3D Printing of Biometals for Prostheses and ImplantsRupinder Singh J. Paulo Davim
Submission Deadline: September 30, 2025
Published Articles: 3
 Applications of Biomaterials in Wearable Medical Devices and Bioelectronics
Applications of Biomaterials in Wearable Medical Devices and BioelectronicsAmirsalar Khandan
Submission Deadline: June 30, 2025
Published Articles: 0
 Magnetic Materials and Their Biomedical Applications
Magnetic Materials and Their Biomedical ApplicationsKhalid Batoo
Submission Deadline: June 30, 2025
Published Articles: 0
 The Use of 2D Materials in Bio-tribology
The Use of 2D Materials in Bio-tribologyAndreas Rosenkranz
Submission Deadline: June 30, 2025
Published Articles: 0
 Plasmonic Nanostructures for Designing Optical Biosensors
Plasmonic Nanostructures for Designing Optical BiosensorsMohammad Tavakkoli Yaraki
Submission Deadline: June 30, 2025
Published Articles: 2
 Trends in Biomaterials Research for Cardiovascular Applications
Trends in Biomaterials Research for Cardiovascular ApplicationsFeng Chen
Submission Deadline: June 30, 2025
Published Articles: 1
 Nature-Based Biomaterials for Biomedical Applications
Nature-Based Biomaterials for Biomedical ApplicationsJayachandran Venkatesan
Submission Deadline: June 30, 2025
Published Articles: 2
 Bioinspired Material for Regenerative Medicine
Bioinspired Material for Regenerative MedicineAjay Vikram Singh
Submission Deadline: June 30, 2025
Published Articles: 2
 Lignin-based Hydrogels for Energy, Sensing and Medical Applications
Lignin-based Hydrogels for Energy, Sensing and Medical ApplicationsFarzad Seidi
Submission Deadline: June 30, 2025
Published Articles: 0
From the Editor-in-Chief I am very proud to announce launching Exploration of BioMat-X an open access journal Published by Open Exploration Publisher in the field of biomaterials. With a global vision of democratization of science and innovating in publishing high-quality research for global health, the aim is to attract renowned researcher from all horizons, nationalities, and cultures and allow them to disseminate the best of their research related to the biomaterials for free of charge. Exploration of BioMat-X is a multifaceted and multidisciplinary journal for a wide readership who are interested in various aspects of research in biomaterials. With this mind, the authors will be invited to self-criticizing their research and include the limitations of their study in their article. A rigorous peer-review process involving an international Editorial Board has been established to ensure that the high-quality requirement for publication in Exploration of BioMat-X will be met.Maryam Tabrizian
I am very proud to announce launching Exploration of BioMat-X an open access journal Published by Open Exploration Publisher in the field of biomaterials. With a global vision of democratization of science and innovating in publishing high-quality research for global health, the aim is to attract renowned researcher from all horizons, nationalities, and cultures and allow them to disseminate the best of their research related to the biomaterials for free of charge. Exploration of BioMat-X is a multifaceted and multidisciplinary journal for a wide readership who are interested in various aspects of research in biomaterials. With this mind, the authors will be invited to self-criticizing their research and include the limitations of their study in their article. A rigorous peer-review process involving an international Editorial Board has been established to ensure that the high-quality requirement for publication in Exploration of BioMat-X will be met.Maryam Tabrizian
Editor-in-Chief of Exploration of BioMat-X -
-
NewsletterWinners of Highly Cited Paper Award 2024
 Jan. 23, 2025MoreEBMX in the 8th National Conference on Advances in Manufacturing Technology
Jan. 23, 2025MoreEBMX in the 8th National Conference on Advances in Manufacturing Technology Apr. 15, 2024
Apr. 15, 2024 -
Selected Special IssuesTopic: Bioprinted Hydrogels for Engineering Tissues and Organs Topic: Green Nanoparticles for Biomedical Applications Topic: Innovations in Biomaterials for Dentistry and Oral Surgery Topic: Metal 3D Printing of Biometals for Prostheses and Implants Topic: Applications of Biomaterials in Wearable Medical Devices and Bioelectronics More