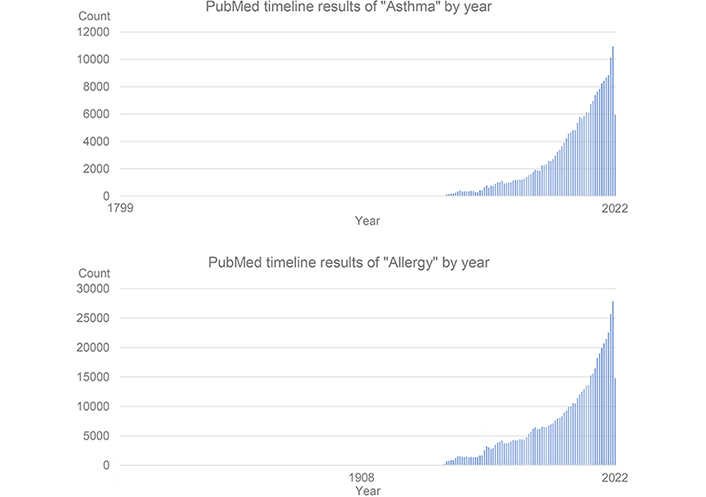
The progress of knowledge in asthma and allergy in the last decades has prompted an improvement in patients’ life. Nonetheless, it’s time to implement the opportunities to evaluate new research to promote new explorative visions and experimental demonstration, to translate basic science to clinical science, and finally to practice. Such a journey is nowadays easier than in the past because, like no other time in history, we have the opportunity to inform our scientific community of the raising evidence coming from laboratories and clinical studies.
The growing interest in our discipline is clearly substantiated in Figure 1, where a PubMed search (July 14th, 2022) having “Allergy” or “Asthma” as the keyword, reports a huge number of published papers, but even more impacting is the “vertical” increase of publications in the last few years.
Such an increase can be related to several aspects of basic research: immunology is finally considered a science implicated in any discipline of medical science and allergy took advantage of this extreme improvement in understanding mechanisms of the disease. Some examples of it are:
(a) allergen immunotherapy (AIT);
(b) component resolved diagnosis for allergic disorders;
(c) novel therapies for rare diseases of allergological interests (e.g., hereditary angioedema, systemic mastocytosis, etc.);
(d) novel diagnostic tools for allergies and anaphylaxis (e.g., basophil activation test, mast-cell activation test, etc.).
Whereas, as asthma is concerned, it is probably sufficient to look at the breakthrough in management due to:
(a) biologics for severe asthma;
(b) biologics for asthma comorbidities;
(c) non-invasive tools for assessing airway inflammation;
(d) the constant look for biomarkers for phenotyping and prognostic purposes.
Exploration of Asthma & Allergy (EAA) is devoted to publishing data coming from experimental approaches in laboratories as from translational research. Clinical trial research papers will be very welcome both as double-blind placebo-controlled randomized clinical trials (DBPCRT) or real-world evidence (RWE). DBPCRT has still been considered the best evidence, but growing attention has to be devoted to RWE when it is properly collected and reviewed methodologically. The relevance of big data nowadays cannot be ignored, but the methodology of collecting such data is indeed crucial, whose scientific credibility can prompt new insights and information in huge populations that are difficult, if not impossible, to be obtained with DBPCRT.
Moreover, EAA will welcome high-standard review articles summarizing the current evidence about the main fields of allergy and asthma, as far as interesting and challenging clinical cases, or any other potentially interesting articles from our field of research.
We therefore are very happy to launch this new and promising journal, having in mind that “research has the final aim of making a happy life for our patients”.
Abbreviations
| DBPCRT: | double-blind placebo-controlled randomized clinical trials |
Declarations
Author contributions
GWC and EH equally contributed in writing the editorial, and read and approved the submitted version of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.