Affiliation:
1Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan 48108, Korea
ORCID: https://orcid.org/0000-0003-0905-4649
Affiliation:
2Department of Internal Medicine, Dong-A University College of Medicine, Busan 49201, Korea
Email: yhnam@dau.ac.kr; hspark@ajou.ac.kr
ORCID: https://orcid.org/0000-0001-8759-2982
Affiliation:
3Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon 16499, Korea
Email: yhnam@dau.ac.kr; hspark@ajou.ac.kr
ORCID: https://orcid.org/0000-0003-2614-0303
Explor Asthma Allergy. 2023;1:89–106 DOI: https://doi.org/10.37349/eaa.2023.00011
Received: March 16, 2023 Accepted: June 14, 2023 Published: August 24, 2023
Academic Editor: Giuseppe Guida, University of Torino, Italy
Non-steroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease (NERD) is characterized by adult-onset asthma, chronic rhinosinusitis with nasal polyps (CRSwNPs), and aspirin/NSAID hypersensitivity, presenting recurrent asthma exacerbation and poor clinical outcomes. Patients with NERD have heterogeneous clinical phenotypes/endotypes, and the management of NERD remains challenging. Dysregulation of arachidonic acid (AA) metabolism and persistent eosinophilic airway inflammation are the major pathogenic mechanisms in the upper and lower airways of NERD. To date, increased levels of urinary leukotriene E4 (uLTE4) [a terminal metabolite of the lipoxygenase (LOX) pathway] have been the most relevant biomarker for NERD. It is demonstrated that mast cells, platelets, and epithelial cells can amplify upper and lower airway inflammation in NERD, and several potential biomarkers based on these complicated and heterogeneous mechanisms have been suggested. This review summarizes potential biomarkers for application in the management of NERD.
Non-steroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease (NERD), a distinct phenotype of asthma, is defined by chronic eosinophilic inflammation of the upper and lower airways and is accompanied by asthma, chronic rhinosinusitis with nasal polyps (CRSwNPs), and hypersensitivity to aspirin/NSAID [1]. In a recent population study, the general prevalence of NERD was 1.4%, and the prevalence of NERD among patients with asthma was 6.9% [2]. Considering that more severe asthmatic symptoms and a higher prevalence of chronic rhinosinusitis (CRS) were reported in patients with NERD than in those with aspirin-tolerant asthma (ATA), the prevalence of NERD was reported to be 14.9% in patients with severe asthma [3]. Therefore, optimal management of NERD is required; however, it is still challenging. In addition, the clinical features of NERD are very diverse, and the classification of subgroups (phenotype/endotype) has not been clarified. Much effort has been made to explore relevant biomarkers based on the pathogenic mechanism of NERD; however, there are few validated biomarkers to date. The current management of NERD is not much different from that of ATA. Therefore, the novel and potential biomarkers that may be applicable in clinical practice to achieve better control of NERD are summarized in this review.
Chronic eosinophilic inflammation and dysregulation of arachidonic acid (AA) metabolism are key pathogenic mechanisms of NERD (Figure 1) [1]. AA, a major component of cell membrane lipids, plays a central role in inflammation and the immune system through numerous bioactive metabolites. AA is metabolized by two major pathways, cyclooxygenase (COX) and lipoxygenase (LOX), where leukotriene E4 (LTE4) is the terminal metabolite of the LOX pathway among three cysteinyl LTs (CysLTs) [4]. Dysregulation of the COX and LOX pathways is noted in patients with NERD, and increased production of CysLTs induces upper and lower airway inflammation (nasal congestion, mucus production, and inflammatory cell recruitment) through CysLT receptors (CysLTRs), whereas decreased production of prostaglandin E2 (PGE2; having anti-inflammatory actions) increases the LOX pathway. Additionally, increased PGD2 and thromboxane A2 (TXA2; from the COX pathway) can activate various immune cells, contributing to the development of NERD [5]. Under these conditions, the administration of COX-1 inhibitors (NSAID) amplifies the imbalances of metabolites from the COX to LOX pathways, exacerbating upper and lower respiratory symptoms in patients with NERD [1, 5].
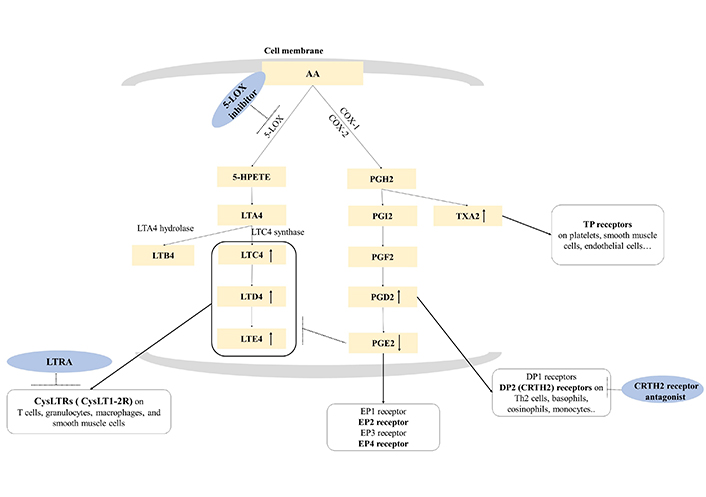
AA metabolism and therapeutic approach to NERD. AA metabolism is divided into two (COX and LOX) pathways. The LOX pathway is initiated by 5-LOX, which generates LTA4. LTA4 is converted to LTC4 by LTC4 synthase, then, metabolized in the order of LTD4 and LTE4. LTC4, LTD4, and LTE4 are bioactive proinflammatory mediators and are known as CysLTs. The COX pathway is initiated by COX-1 and COX-2, and AA is converted to PGH2 and subsequently further metabolized into PGI2, PGF2, PGD2, PGE2, and TXA2 by each specific synthase. Overproduction of CysLTs and increase of PGD2 and TXA2 accompanied by the decrease of PGE2 are major characteristics of NERD. Exposure to COX-1 inhibitor induces a remarkable reduction of PGE2 production and excessive CysLT production, resulting in immediate hypersensitivity reactions to NERD. DP1: D-prostanoid 1; TP: TX prostanoid; HPETE: hydroperoxyeicosatetraenoic acid; LTRA: LT receptor antagonist; EP1: E-prostanoid 1; Th2: T helper 2; CRTH2: chemoattractant receptor-homologous molecule expressed on Th2 cells. ↑: increased production of lipid mediators; ↓: decreased production of lipid mediators; arrow in black dots: inhibition of the action of the target; arrow in black: interaction between lipid mediators and their receptors
Note. Adapted from “Emerging biomarkers beyond leukotrienes for the management of nonsteroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease,” by Rhyou HI, Nam YH, Park HS. Allergy Asthma Immunol Res. 2022;14:153–67 (https://doi.org/10.4168/aair.2022.14.2.153). CC BY-NC 4.0.
Overproduction of CysLTs and PGD2 was associated with chronic eosinophilic inflammation in the upper and lower airway mucosa of patients with NERD (Figure 2). However, more complex and heterogeneous mechanisms of chronic eosinophilic airway inflammation have been reported [1, 5–7]. Interferon γ (IFN-γ), a Th1 cytokine, is increased in patients with NERD, stimulating eosinophils and increasing eosinophilic infiltration [6, 7]. Platelets facilitate the recruitment of granulocytes, which are involved in various inflammatory diseases. Platelet-adherent leukocytes, such as eosinophils, neutrophils, and monocytes, are increased in patients with NERD, increasing the levels of CysLTs [6, 7]. Both CysLTs and platelet-adherent leukocytes activate epithelial cells to release alarming cytokines such as interleukin 25 (IL-25), IL-33, and thymic stromal lymphopoietin (TSLP). These alarming cytokines can reversely activate eosinophils and type 2 innate lymphoid cells (ILC2s) to release IL-4, IL-5, and IL-13 [5, 7]. Therefore, persistent and intense eosinophilic/type 2 inflammation is maintained by signals in several directions in NERD. These complex mechanisms, associated with the heterogeneous clinical features of patients, require variable biomarkers for the management of NERD.
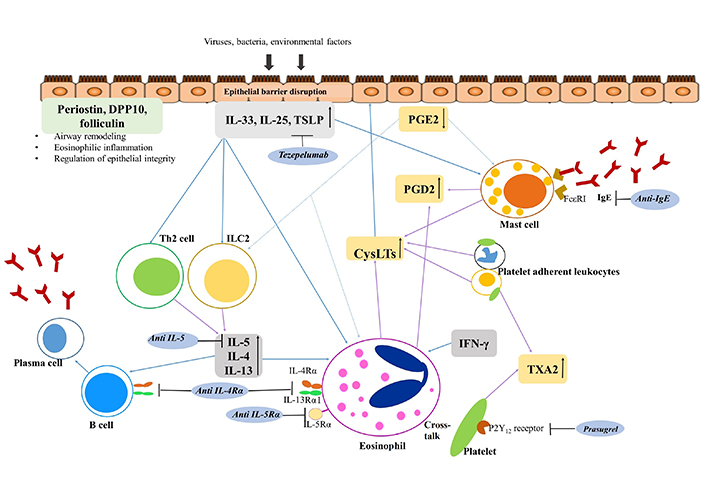
Inflammatory pathway and therapeutic approach to NERD. Epithelial cells are injured by various exogenous factors including viruses, bacteria, and air pollutants, and injured epithelium releases various alarmins and cytokines. CysLTs can also activate epithelial cells, and induce the release of alarmins, IL-33, IL-25, and TSLP in NERD. These elevated levels of alarmins and cytokines from epithelial cells activate ILC2, eosinophils, and mast cells resulting in type 2 inflammation. Activated mast cells and eosinophils release various cytokines and chemokines, and CysLTs that can induce mucosal inflammation and bronchoconstriction. PGE2 is released from epithelial cells, fibroblasts, and leukocytes, and inhibits activation of eosinophils, mast cells, and ILC2. However, PGE2 is downregulated in NERD. Platelet-adherent leukocytes are another potent producer of CysLTs, PGD2, and TXA2, and enhance the inflammation of NERD. Airway epithelial-derived molecules including periostin, DPP10, and folliculin may affect airway remodeling, eosinophilic inflammation, and epithelium integrity and survival. DPP10: dipeptidyl peptidase 10; IgE: immunoglobulin E; P2Y12: purinergic receptor type Y, subtype 12; FcεR1: Fc epsilon receptor 1. ↑: increased production; ↓: decreased production; arrow in black: inhibition of the action of the target; arrow in blue: activation of target cells; arrow in purple: production of lipid mediators or cytokines; arrow in blue dots: inhibitory action to target cells
Note. Adapted from “Emerging biomarkers beyond leukotrienes for the management of nonsteroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease,” by Rhyou HI, Nam YH, Park HS. Allergy Asthma Immunol Res. 2022;14:153–67 (https://doi.org/10.4168/aair.2022.14.2.153). CC BY-NC 4.0.
NERD is generally diagnosed between the ages of 30 and 40 years and is more commonly found in women. Most patients with NERD have reported upper airway symptoms at diagnosis, and after several years, asthma may appear, followed by NSAID hypersensitivity. Patients with NERD have more severe upper airway symptoms, including nasal blockage, stuffiness, and post-nasal drip, associated with nasal polyps (NPs) [8]. Loss of smell is also more frequently reported in patients with NERD than in those with ATA [1, 9]. In addition, patients with NERD reported frequent recurrence of CRS/NPs after surgery and underwent revision surgeries compared with ATA [1, 9, 10].
Although patients with NERD present with a wide range of asthma severities, most of them present with moderate to severe asthma [1, 3]. A meta-analysis reported that patients with NERD had a higher risk of uncontrolled asthma and higher rates of asthma exacerbation, emergency room visits, and hospitalization than those with ATA [11]. However, in a study identifying sub-phenotypes in the NERD cohort, patients with severe upper and lower airway diseases were divided differently [12]. Both shared and individual mechanisms between upper and lower airway inflammation may be present in NERD. A latent class analysis of clinical data from 201 patients with NERD based on a Polish population demonstrated four subtypes of NERD—class 1: moderate asthma, severe CRS, and blood eosinophilia; class 2: mild asthma, relatively well controlled; class 3: severe asthma with severe asthma exacerbations and airway obstruction; and class 4: poorly controlled asthma with frequent asthma exacerbations in female subjects [12]. Blood eosinophilia and increased urinary LTE4 (uLTE4) were characterized as class 1, which was associated with severe upper airway inflammation [12]. Four distinct subtypes were identified in 302 patients with NERD based on a Korean population—class 1: NERD with CRS/atopy and no urticaria; class 2: NERD with CRS and no urticaria; class 3: NERD without CRS/urticaria; and class 4: NERD with urticaria [13]. There were significant differences in the female proportion, asthma severity, sputum/blood eosinophil counts, and serum total IgE levels. Significantly higher levels of uLTE4 were noted in classes 1 and 3, suggesting that blood eosinophilia and uLTE4 are reliable biomarkers of NERD in these two studies [12, 13].
AA is catalyzed to LTA4 by 5-LOX, and unstable LTA4 is converted to LTC4 by LTC4 synthase in mast cells, macrophages, eosinophils, and basophils. The released LTC4 undergoes rapid extracellular metabolism to LTC4, LTD4, and LTE4 [14]. Increased levels of LTE4 in various samples (urine, sputum, saliva, and blood) were noted in patients with NERD compared to ATA [15–18], indicating that LTE4 is a useful biomarker for diagnosis, with proven significance and ease of sampling [18]. The ratio of LTE4 to PGF2α (PGE2 metabolite) was reported to have a more accurate diagnostic value than LTE4 [16], and is expected to be a new diagnostic biomarker for NERD. LTE4 levels in urine were further increased after aspirin challenge in patients with NERD, were associated with more severe asthmatic symptoms during aspirin challenges [15, 17, 19], and were negatively correlated with changes in forced expiratory volume in 1 s (FEV1) during aspirin desensitization (AD) [19]. In addition, uLTE4 has been used as a biomarker for response to zileuton [20], omalizumab [21], mepolizumab [22], dupilumab [23], and prasugrel (inhibiting platelet-leukocyte aggregation by binding to the P2Y12 receptor on the platelet surface) [24] in clinical studies. These findings suggest that uLTE4 may be a useful biomarker for predicting AD tolerance, treatment response, and NERD diagnosis [15–24].
Patients with NERD had 5-fold higher LTC4 synthase-containing cell counts in bronchial biopsies after placebo challenges than those with ATA, and released additional CysLTs after lysin-aspirin challenges, but not in those with ATA [25]. Overexpression of CysLTRs in the airway mucosa has also been observed in NERD [26]. The number of nasal leukocytes expressing the CysLTR1 was higher in patients with NERD and CRS than in those with ATA and was significantly decreased after desensitization with topical lysine. Overexpression of LTC4 synthase and CysLTR1 can explain the enhanced role of CysLTs, which are therapeutic targets for NERD. Taken together, uLTE4 is the most reliable diagnostic and therapeutic marker of NERD.
PGH2, which is metabolized from AA by COX-1 and COX-2, is converted into PGE2 by PGE2 synthase. The physiological action of PGE2 is mediated by four different G protein-coupled receptors (GPRs), EP1 to EP4 [27]. PGE2 deficiency and decreased expression of the EP2 receptor have been reported in NERD. Patients with NERD show decreased production of PGE2 in epithelial cells from NPs [28], bronchial fibroblasts [29], and peripheral blood leukocytes [30]. Hypermethylation of the PGE2 synthase gene in NPs was detected in patients with NERD compared to NPs with aspirin tolerance [31]. Expression of the EP2 receptor was lower in NP fibroblasts in patients with NERD than in those with ATA and healthy controls [32]. Inhaled PGE2 could prevent bronchoconstriction in response to aspirin challenge and excretion of uLTE4 in patients with NERD [33]. PGE2 can suppress CysLT synthesis in mast cells and aspirin/NSAID-induced activation of eosinophils in patients with NERD, but not in those with ATA or healthy controls [34, 35]. An experimental study reported that PGE2 suppressed IL-5 and IL-13 production and proliferation of ILC2 mediated by EP2 and EP4 receptors [36], and a selective EP2 agonist suppressed the release of CysLTs [35]. Taken together, PGE2 can inhibit the activation of mast cells, eosinophils, and ILC2, suppressing CysLT and type 2 cytokine production. Restoration of PGE2 and EP2 receptors is a potential therapeutic target for NERD.
PGD2 induces bronchoconstriction and chemoattraction of eosinophils, basophils, Th2 cells, and ILC2 cells through three receptors: DP1, DP2 (called CRTH2), and TP receptors. Basal levels of PGD2 and its metabolites in the blood, urine, sputum, and NP tissue were higher in patients with NERD than in those with ATA [37–39]. Urinary PGD2 levels were correlated with a decrease in FEV1 during AD [19]. Elevated PGD2 levels can predict AD failure. Considering that increased PGD2 was found in NP tissue of patients with NERD compared to ATA, a recent randomized controlled trial investigated the efficacy of CRTH2 antagonists in 43 patients with CRSwNP, including 27 patients with NERD [40]. However, no clinical efficacy of CRSwNP was observed in the subset of study patients. Taken together, increased urinary PGD2 levels may be a biomarker for the diagnosis or severity of NERD.
Consistently, enhanced eosinophilic inflammation is a prominent feature of NERD, and activated eosinophils are the main producers of CysLTs. Blood eosinophil counts were significantly higher in patients with NERD than in those with ATA [41]. Blood eosinophilia (> 300 cells/µL) could predict NERD with an odds ratio of 1.8, and the combination of blood eosinophilia with high uLTE4 levels had 6-fold higher odds ratio for the diagnosis of NERD. Higher eosinophil counts in bronchial and NP tissues were noted in patients with NERD than in those with ATA [25, 42]; increased eosinophilic influx into nasal tissue after aspirin challenge was found in patients with NERD compared to ATA [43]. Taken together, increased blood eosinophils along with increased uLTE4 can be used as biomarkers for the diagnosis of NERD; however, tissue eosinophilia in the upper and lower airway mucosa is not a suitable biomarker because it is difficult to sample eosinophils from these tissues.
Aspirin had a direct effect on activating eosinophils to secrete eosinophil-derived neurotoxins but did not increase CysLTs in vitro [44]. However, eosinophils derived from CD34+ progenitor cells in the presence of IFN-γ significantly produced CysLTs after the aspirin challenge. When dexmedetomidine (an eosinophil suppressor [45]) was administered to patients with CRSwNP and eosinophilia, there were no significant changes in NP scores or nasal symptoms despite the elimination of blood and tissue eosinophilia [46], questing about the influence of eosinophils in CRSwNP. Taken together, eosinophilic inflammation is known to be a major driver and target for NERD, and IFN-γ may be a novel therapeutic target.
Activated mast cells are considered important players in NERD because they release abundant proinflammatory mediators, including CysLTs, PGD2, cytokines, and histamine [1, 5, 47]. Increased mast cells were noted in bronchial biopsies and nasal fluids of patients with NERD compared to those with ATA [48, 49]. In addition, serum tryptase levels were increased after the aspirin challenge in patients with NERD, indicating mast cell activation after the aspirin challenge [50]. A subset of patients with NERD with mast cell activation exhibited a greater decrease in FEV1 after the aspirin challenge than those without mast cell activation [50]. Higher levels of PGD2 (9α,11β-PGF2) and tryptase in the blood were noted in patients with NERD than in those with ATA, which further increased after the aspirin challenge in approximately 65% of them [38]. Mast cell activation is also initiated by FcεR1 signaling, and higher levels of IgE have been noted in CRS tissues of patients with NERD than in those with ATA [47, 51, 52]. Taken together, activated mast cells and PGD2 may be diagnostic biomarkers for classifying patients with NERD and may be potential therapeutic targets for those presenting with a more severe phenotype.
Platelets release CysLTs after adherence to neutrophils and eosinophils. Patients with NERD had higher proportions of platelet-adherent leukocytes with overexpression of LTC4 synthase in the blood than those with ATA; activated platelet-adherent leukocytes were related to uLTE4 levels and low lung function in patients with NERD [53]. In addition, the expression of p-selectin and GPIIb/IIIa on activated platelets was positively correlated with platelet-adherent leukocytes in NERD; higher levels of plasma soluble p-selectin and soluble CD40 ligand were noted in patients with NERD than in those with ATA [54]. Therefore, activated platelet-adherent leukocytes may predict disease severity and serve as potential therapeutic targets for NERD. However, prasugrel could suppress aspirin-induced nasal symptoms and uLTE4/PGD2 production in a small subgroup of patients with NERD [24], questioning the pathogenic role of platelet-adherent leukocytes. Taken together, further investigations are needed to evaluate the diagnostic or therapeutic value of platelet-adherent leukocytes.
Moreover, activated lung-resident ILC2 by alarmin cytokines and lipid mediators contributed to type-2 inflammation by releasing IL-4, IL-5, and IL-13 [1, 5, 55, 56]. ILC2 numbers in the nasal mucosa increased during aspirin/NSAID challenges, which was associated with nasal symptoms in some patients with NERD [57]. Considering that alarmins could sequentially activate type 2 cells, including mast cells and eosinophils, a therapeutic approach to activated ILC2 will be an effective strategy for NERD.
CysLTs and prostanoids activated airway epithelial cells (AECs) to release alarmin cytokines such as IL-25, IL-33, and TSLP [1, 5]. Plasma IL-25 levels were higher in patients with NERD than in those with ATA, which was positively correlated with a decrease in FEV1 during aspirin challenges [58]. Steroid treatment could suppress IL-33 levels in CRS tissue but did not change eosinophils in CRS tissue and fractional exhaled nitric oxide levels, suggesting that IL-33 may not be associated with type 2/eosinophilic inflammation [59]. TSLP messenger RNA (mRNA) expression in NPs was significantly correlated with the expression of PGD2 synthase, carboxypeptidase (a mast cell-specific marker), and urinary levels of PGD2, but not with uLTE4 levels in patients with NERD [39]. TSLP can induce PGD2 release from mast cells in vitro and activate eosinophils [39]. Recent clinical trials on severe type 2 asthma demonstrated the significant efficacy of tezepelumab [a monoclonal antibody (mAb) to TSLP] on symptom control and lower lung function [60, 61]. Taken together, TSLP may be a potential therapeutic target for patients with NERD with mast cell activation or higher levels of PGD2, although further investigations are needed to evaluate the role of IL-25.
Periostin and transforming growth factor β1 (TGF-β1) released from activated AECs play pivotal roles in type-2 airway inflammation and remodeling in asthma [62, 63]. Higher levels of serum periostin, positively correlated with blood eosinophil counts and CRS severity, could distinguish patients with NERD from those with ATA [64]. In addition, higher levels of serum TGF-β1 were noted in patients with NERD than in those with ATA, with a positive correlation with uLTE4 levels, and TGF-β1 treatment increased eosinophils and LTE4 levels in the lung tissue in vivo [65]. These findings suggest that both periostin and TGF-β1 may be novel therapeutic targets to improve lung function and eosinophilic inflammation and to prevent airway remodeling in NERD [64, 65].
DPP10 is expressed in AECs and is associated with the development and severity of asthma [66–68]. A single nucleotide polymorphism (rs17048175) of DPP10 was significantly associated with the phenotype of NERD and was positively correlated with enhanced expression of DPP10 in patients with NERD than in those with ATA [69]. Increased levels of serum DPP10 were noted in patients with NERD compared to ATA, with a positive correlation with serum levels of TGF-β1 but a negative correlation with FEV1 [70]. These findings suggest that serum DPP10 level is a potential biomarker for the diagnosis and severity of NERD [69, 70].
Surfactant protein D, which is mainly produced from AECs, regulates airway inflammation by inhibiting mast cells and eosinophils [71]. Severe asthma is related to surfactant protein D deficiency in the airway, and it has been suggested as a useful biomarker for predicting severe asthma [72]. Compared to patients with ATA, patients with NERD had lower levels of serum surfactant protein D, and these levels were negatively correlated with a decrease in FEV1 after the lysin-aspirin challenge. In vitro study demonstrated that surfactant protein D could suppress eosinophilic airway remodeling [71], suggesting that this may be a novel therapeutic target to control AEC-mediated type 2 airway inflammation in NERD, although further elucidation is needed.
Folliculin, which is mainly expressed in AECs, is a negative regulator of cell-cell adhesion. Folliculin can affect airway epithelial integrity and survival [73, 74]. Serum folliculin levels were significantly higher in patients with NERD than in those with ATA [75]. Folliculin production increased after LTE4 treatment, followed by disruption of the tight junctions of AECs, suggesting that serum folliculin may be a potential serum biomarker for NERD. Taken together, we suggest that three AEC-derived serum biomarkers (periostin, DPP10, and folliculin) and serum surfactant protein D are potential therapeutic targets for NERD.
Traditional approaches to patients with NERD consist of pharmacological treatment and AD for asthma and CRS (with/without NPs), as well as absolute avoidance of aspirin/NSAID [1, 5]. Most patients with NERD show hypersensitivity to cross-reactive NSAID; alternative drugs must be confirmed by drug provocation tests. Pharmacological treatment of asthma (including inhaled corticosteroids plus long-acting beta-2 agonists with/without additional LT modifiers) and CRS (intranasal corticosteroids) in patients with NERD are similar to those with ATA. Although some patients can be controlled by conventional anti-inflammatory medications, patients with severe or uncontrolled asthma require additional controllers, including biologics, in which biomarkers may be applied considering the heterogeneity in the phenotypes/endotypes of NERD. AD is performed in some patients if indicated.
AD is indicated in patients with NERD who require antiplatelet treatment (e.g., cardiovascular disease and antiphospholipid syndrome), chronic anti-inflammatory treatment (rheumatologic diseases), or severe CRSwNP [76]. AD proceeds with gradually increasing doses of aspirin until reaching a substantial dose range (300–650 mg per day), has been reported to improve asthma and CRS symptoms and reduce the recurrence of NPs, frequency of revision surgery, and requirements of intranasal corticosteroids [1, 5, 77]. Adverse effects, including gastrointestinal tract bleeding, dyspepsia, and urticaria/angioedema, remain the major hurdles to long-term AD [76, 78]. Although the molecular effects of AD are not fully understood, it could reduce sputum PGD2 levels and increase sputum PGE2 levels, suggesting that the reduction of PGD2 may be the major molecular effect of AD [79].
LTRAs, including montelukast and pranlukast, are commonly prescribed for patients with NERD and can improve quality of life, lung function, and symptoms of asthma and CRSwNP [1, 80, 81]. Pretreatment with LTRAs could prevent bronchospasm after the aspirin challenge, which is applied during the AD process [82, 83]. LTRAs are relatively safe, but neuropsychiatric (sleep problems or hyperactivity) and gastrointestinal adverse effects have been reported, especially in children [84, 85]. LTRAs antagonize CysLTR1; however, uLTE4 levels were not associated with the response to LTRAs [82]. A recent study demonstrated higher uLTE4 levels in patients with NERD even while maintaining LTRAs as well as inhaled corticosteroids plus long-acting bronchodilators, which were associated with blood eosinophilia and poor disease control [86]. This suggests that current LTRAs are not sufficient to suppress LTE4-mediated eosinophilic inflammation and additional controllers are required for better control. GPR99 protein, previously identified as an LTE4 receptor, may be an additional therapeutic target to suppress LTE4-mediated airway inflammation in NERD [87].
A cross-sectional questionnaire study reported a higher proportion of effectively treated patients was found in those treated with zileuton (a selective inhibitor of 5-LOX) than in those treated with LTRA [88], which prolonged the time to revision surgery in patients with NERD [89]. Zileuton, after the addition of celecoxib, markedly reduced uLTE4 levels in smokers [20]. The combination of zileuton and LTRA or celecoxib may have a synergistic effect in patients with NERD. However, it is less desirable than LTRA because of concerns regarding side effects, such as hepatotoxicity [90].
Omalizumab can neutralize IgE-mediated responses and has been prescribed to patients with severe allergic asthma, CRSwNP, and chronic spontaneous urticaria. Previous studies have demonstrated that omalizumab improved asthma/CRS symptoms, disease control, and lung function in patients with NERD [91]. Omalizumab is a fairly safe drug, but severe allergic reactions such as anaphylaxis can be occurred [92]. Biomarkers, such as LTE4, PGD2, eosinophil cationic protein (ECP), and total IgE, were used to assess the efficacy of omalizumab in NERD. Omalizumab treatment for 12 months could decrease uLTE4/urinary PGD2 levels and reduce asthma exacerbation and requirements of systemic corticosteroids, as well as symptom scores of asthma and CRS [21], where their symptoms improved within the first week in more than half of the study patients, supporting that omalizumab may stabilize mast cells. Three months of omalizumab treatment reduced uLTE4 levels and increased the tolerable dose of aspirin in AD [93]. Therefore, omalizumab is beneficial for the long-term management of NERD and pre-treatment before AD.
Omalizumab is widely used in chronic urticaria that is refractory to antihistamines [94]. Approximately 20% to 35% of patients with NERD have features of NSAID-induced blended reactions, the coexistence of NERD with NSAID-induced urticaria/angioedema, or NSAID-exacerbated cutaneous disease [95]. Omalizumab was reported to reduce PGD2 production [21] and PGD2 was associated with cutaneous symptoms during aspirin challenge in patients with NERD [19]. Omalizumab may improve both respiratory and cutaneous symptoms in patients with both NERD and NSAID-exacerbated cutaneous disease. A total of 33 patients with NERD treated with omalizumab for 6 months showed improved nasal and asthma-related symptoms regardless of atopic sensitization [96]. The clinical efficacy of omalizumab correlated with elevated levels of serum total IgE, low levels of ECP, and eosinophilia. However, there was a significant decrease in tissue eosinophil count in atopic patients with NERD. These findings suggest that uLTE4/PGD2, blood eosinophils, and serum total IgE and ECP levels are potential biomarkers for predicting responses to omalizumab in NERD [19, 21, 93, 96].
mAb inhibiting IL-5 signaling [anti-IL-5/IL-5 receptor α (IL-5Rα)], such as mepolizumab, reslizumab, and benralizumab, have been used as potent therapeutic options for severe eosinophilic asthma and/or CRSwNP [97–99]. Most common adverse events to anti-IL-5/IL-5Rα such as nasopharyngitis, injection-site reactions, and headache were generally mild [100]. However, exacerbations of asthma were rarely reported, and it should be monitored especially in NERD patients with severe asthma. A retrospective analysis of 22 patients with NERD demonstrated that mepolizumab treatment improved sinonasal symptoms and asthma control [101]. Eighteen patients with NERD receiving mepolizumab showed a significant decrease in blood eosinophils and basophils, accompanied by higher expression of CRTH2 receptors on those cells compared to control groups [22]. PGD2 and LTE4 levels in urine and PGD2, PGF2α, and TX levels in the nasal epithelium were lower in the mepolizumab-treated group than in the control group. In addition, mepolizumab treatment induced the upregulation of tight junction transcripts and cilium organization in the nasal epithelium. IL-5 signaling may be involved in the action of epithelial and immune cells. Therefore, mAbs against IL-5/IL-5Rα can improve epithelial stability and dysregulation of AA metabolism, as well as inhibit eosinophils and basophils in NERD. However, a recent study to elucidate the efficacy of benralizumab revealed that the proportion of patients showing good responses was lower in patients with NERD than in those with ATA [102], suggesting that the mechanism of eosinophilic airway inflammation in NERD is more diverse than that of IL-5 signaling. Further efforts to identify additional targets for controlling eosinophilic inflammation are essential, especially in those showing limited responses to anti-IL-5/IL-5R antibody treatments.
Dupilumab, a mAb to IL-4Rα blocking signals of IL-4 and IL-13, has been approved for the treatment of type 2 asthma, CRSwNP, and atopic dermatitis. Dupilumab is considered as a tolerable drug, but several adverse events such as ophthalmic complications, head and neck dermatitis, and hypereosinophilia have been reported [103]. Blood eosinophil count is commonly elevated in patients with NERD, and should be monitored during dupilumab treatment. Several studies have reported the efficacy of dupilumab in NERD [23, 104, 105]. In a single-blinded trial of dupilumab in NERD, improvement in quality of life, sinonasal symptoms, and computed tomography score were noted during the study period, and decreased levels of nitric oxide, uLTE4, and serum total IgE were also reported [104]. Patients with NERD treated with dupilumab for 3 months showed remarkable improvements in olfactory function, sinonasal symptoms, and lung function [23]. Nasal PGE2 levels increased, while uLTE4 and serum/nasal IgE levels decreased after dupilumab treatment. Transcripts representing epithelial dysfunction and leukocyte chemoattraction were also downregulated in sinonasal tissue. Dupilumab may act on granulocytes, AECs, and B cells, resulting in upregulation of PGE2, downregulation of CysLTs and IgE, and improvement of the epithelial barrier. Dupilumab improved sinonasal and asthmatic symptoms and reduced asthma exacerbations in patients with NERD who failed to respond to anti-IL-5/IL-5Rα treatment [105]. Serum IgE levels were higher in patients with NERD who switched biologics from IL-5/IL-5Rα mAbs to dupilumab than in those who continued with mepolizumab (responders to mepolizumab). The most appropriate biologic treatment should be elucidated considering the heterogeneity of NERD. Elevated serum IgE may be used as a biomarker for predicting patients who have a favorable response to dupilumab treatment than to anti-IL-5/IL-5Rα antibody treatment in NERD.
Tezepelumab is a humanized mAb to TSLP, which is an alarming cytokine of AECs that induces the activation of type 2 inflammation. In 2021, it became the first biologic to be approved for severe asthma by the Food and Drug Administration (FDA) [106], but there have been no studies on the efficacy of tezepelumab in NERD. However, epithelium-immune cell interactions are known to be a pivotal mechanism of NERD, and tezepelumab is expected to be effective in patients with NERD.
Nanoparticles have advantages in controllable size, low cytotoxicity, and good biocompatibility. The use of nanoparticles has therapeutic efficacy by improving pharmacokinetics, reducing drug toxicities, and targeting drug delivery [107]. Therefore, the application of nanoparticles to deliver appropriate medications has been widely explored in various medical fields, particularly in malignancy [108]. Application of nanoparticles such as polymeric nanoparticles, solid lipid nanoparticles, extracellular vesicle nanomaterials, and metal nanoparticles in asthma have been also investigated for overcoming the limitation of classical therapeutic drugs and achieving precise medicine [109, 110]. Ligands targeting AECs such as adhesion molecule-1, anti-epithelial adhesion molecule, and Toll-like receptor 7 (TLR7) are used in the application of airway epithelial-targeted nanoparticles in asthma, and they may have the ability to inhibit proinflammatory response of epithelium [111]. Recently, carbon structures such as graphene and its derivatives are favorable candidates for nanoparticles in biomedicine. Electrochemical graphene-based sensors can detect nitrite in exhaled breathing condensate samples and be expected as portable airway inflammation monitors with high sensitivity and cost-effectiveness compared to traditional nitrite detection technology [112, 113].
Nanobubbles are spherical gas-containing vesicles within liquid having diameters less than 1,000 nm. Nanobubbles are favorable carriers for tissue or cell oxygenation, and oxygen-containing nanobubbles are emphasized as emerging candidates for tissue oxygenation, tissue repair, and anti-inflammatory treatment of various diseases [114, 115]. Especially, intravenous oxygen-containing nanobubbles may be a novel treatment strategy for hypoxic conditions such as acute respiratory failure and corona virus disease 2019 (COVID19) infection [116]. Diverse routes carrying oxygenated nanobubbles are also explored, and inhalation of aqueous dispersion of oxygen nanobubbles can be applied as a novel drug delivery as well as an oxygen supplier in airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) [117]. However, the application of nanotechnology in clinical practice has still challenges and further research is needed. Especially, asthma is a complex and heterogeneous disease, and further clarification of biomarkers is needed to achieve appropriate application of nanotechnology in the management of asthma or NERD.
LTE4 remains the most relevant biomarker for the diagnosis of NERD. Urinary PGD2 has been suggested to be a potential mast cell marker for classifying NERD. Epithelium-derived markers, such as periostin, DPP10, and folliculin have been suggested as potential therapeutic targets and biomarkers for representing the severity of NERD. Anti-inflammatory agents, such as inhaled corticosteroids and/or LTRA, are the mainstay of management in NERD, and biologics are promising agents for controlling NERD. High serum total IgE, eosinophilia, and uLTE4 may be potential biomarkers for predicting the response to omalizumab and dupilumab. Further investigations are needed to validate the role of these biomarkers in longitudinal outcome models of NERD.
AA: arachidonic acid
AD: aspirin desensitization
AECs: airway epithelial cells
ATA: aspirin-tolerant asthma
COX: cyclooxygenase
CRS: chronic rhinosinusitis
CRSwNPs: chronic rhinosinusitis with nasal polyps
CRTH2: chemoattractant receptor-homologous molecule expressed on T helper 2 cells
CysLTRs: cysteinyl leukotriene receptors
CysLTs: cysteinyl leukotrienes
DP1: D-prostanoid 1
DPP10: dipeptidyl peptidase 10
ECP: eosinophil cationic protein
EP1: E-prostanoid 1
FEV1: forced expiratory volume in 1 s
IFN-γ: interferon γ
IgE: immunoglobulin E
IL-25: interleukin 25
IL-5Rα: interleukin 5 receptor α
ILC2s: type 2 innate lymphoid cells
LOX: lipoxygenase
LTE4: leukotriene E4
LTRA: leukotriene receptor antagonist
mAb: monoclonal antibody
NERD: non-steroidal anti-inflammatory drug-exacerbated respiratory disease
NPs: nasal polyps
NSAID: non-steroidal anti-inflammatory drug
PGE2: prostaglandin E2
TGF-β1: transforming growth factor β1
Th2: T helper 2
TSLP: thymic stromal lymphopoietin
TXA2: thromboxane A2
uLTE4: urinary leukotriene E4
HSP: Conceptualization, Writing—review & editing, Supervision. YHN: Writing—original draft, Writing—review & editing. HIR: Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study was supported by the Korea Health Technology R&D Project through a Korea Health Industry Development Institute (KHIDI) grant funded by the Ministry of Health and Welfare, Republic of Korea Grant No. [HR16C0001]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2034
Download: 31
Times Cited: 0
