Abstract
Aim:
This study aims at assessing dupilumab’s response in severe chronic rhinosinusitis with nasal polyps (CRSwNP) and its impact on concurrent mild to moderate asthma.
Methods:
The study involved severe, uncontrolled CRSwNP patients starting dupilumab treatment (300 mg/2 weeks) at the Allergy unit in University General Hospital “Attikon” in Athens, Greece, from May 2020 to July 2022. Assessments were conducted at baseline (week 0) and weeks 2, 4, 16, 24, and 52, covering 22-item Sino-Nasal Outcome Test (SNOT22), blood eosinophil counts, fractional exhaled nitric oxide (FeNO) concentration, Lund-Mackay CT scores (weeks 0, 16, and 52), Asthma Control Test (ACT) scores (weeks 0, 16, and 52), and forced expiratory volume in one second (FEV1) measurements (weeks 0, 16, and 52). Systemic corticosteroid usage, nasal surgeries, and anosmia improvements were also monitored throughout the study.
Results:
Six patients (50% male, mean age 53.1 years) with severe CRSwNP had severe uncontrolled baseline symptoms: complete anosmia, impaired quality of life (mean SNOT22: 71.6 ± 16.2), and Lund-Mackay CT score of 19.3 ± 2. Within the past year, 83.3% received over three courses of systemic corticosteroids for CRSwNP, and 50% had more than three polypectomies. After two weeks of dupilumab treatment, notable improvements were seen: reduced SNOT22 scores (week 2: 32.5, week 4: 18.1, week 16: 14, week 24: 13.8, week 52: 9.3), improved olfaction (weeks 4–16), reduced polyp size based on Lund-Mackay CT score (week 16: 13.3, week 52: 12.8), and enhanced lung function (FEV1 baseline: 3.15 L, week 16: 3.22 L, week 52: 3.22 L). Control was achieved by week 16 (ACT: 25/25). FeNO levels decreased [week 2: (18.2 ± 8.7) ppb, week 4: (16.5 ± 7.4) ppb, week 16: (16.9 ± 7.8) ppb, week 24: (13.7 ± 8.3) ppb, week 52: (13.4 ± 5.6) ppb]. No patients required nasal surgery.
Conclusions:
Dupilumab effectively targets interleukin 4 (IL4) and IL13, controlling type 2 inflammation spectrum, thus providing significant disease control for CRSwNP patients. Moreover, it improves asthma, even in mild to moderate cases, showcasing its broader therapeutic benefits.
Keywords
Asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), dupilumab, biologic agent, type 2 inflammationIntroduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is primarily characterized as a T2 inflammatory condition affecting the nasal and paranasal sinuses [1]. It results in the growth of soft tissue and the development of nasal polyps within the nasal and paranasal cavities [2]. This condition impacts approximately 1–4% of the adult population in Europe and the USA [3, 4]. The symptoms of CRSwNP encompass nasal blockage, rhinorrhea, loss of olfactory function, and sleep disturbances, and are associated with significant symptom burden, marked deterioration in health-related quality of life (HRQoL), and substantial economic consequences [5, 6]. Additionally, the overall disease burden can be further augmented as CRSwNP frequently coexists with other T2 inflammatory conditions such as asthma, nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (N-ERD), and allergic rhinitis [2, 7].
Treating CRSwNP is always a challenging task; intranasal corticosteroids are considered the milestone of treatment for many decades either before or after nasal surgery for long-term even lifelong use. In more severe cases, surgical removal of the inflamed polyp tissue is considered [8]. However, surgical interventions often provide only temporary relief, with a recurrence rate nearing 50% within a year [9]. Just recently, biologics have emerged as novel treatment options for severe and refractory CRSwNP, in cases where the standard of care fails to provide at least partial remission and a satisfactory QoL [10, 11].
Dupilumab is a fully human monoclonal antibody that blocks the shared receptor for interleukin 4 (IL4) and IL13, targeting thus the primary drivers of type 2 inflammation. It is approved for the treatment of CRSwNP, as well as severe asthma, eosinophilic esophagitis, and atopic dermatitis [12, 13]. Randomized clinical trials (RCTs) as SINUS 24 and SINUS 52 have demonstrated the safety and efficacy of dupilumab for severe CRSwNP. Besides, the results of these trials revealed that dupilumab significantly improved various parameters, including nasal polyp score, Lund-Mackay CT score, nasal obstruction or congestion score, as well as patient-reported outcomes and HRQoL assessments compared to the placebo [14].
In both RCTs and real-world studies, dupilumab has shown promise in enhancing lung function and asthma control among patients with severe asthma, irrespective of comorbid conditions such as CRSwNP [15–17]. Nevertheless, data remain scarce reading whether dupilumab exerts a similar effect in patients with CRSwNP and mild to moderate asthma. The primary aim of this study was to evaluate the response to dupilumab treatment in patients with severe CRSwNP and to assess its impact on concurrent non-severe asthma, Global Initiative for Asthma (GINA) steps 1–4.
Materials and methods
Study design, patient population, and data collection
We performed a single-center prospective study of patients > 18 years with severe uncontrolled CRSwNP who were authorized to initiate dupilumab treatment at a dose of 300 mg every two weeks at the Allergy unit “D. Kalogeromitros”, University General Hospital “Attikon” in Athens, Greece between May 2020 and July 2022.
Demographic characteristics (age, sex), data related to the disease characteristics [polypectomies, systemic steroid use, maintenance treatment, sensitization profile based on specific IgEs to common aeroallergens (ImmunoCapTM), and comorbidities (asthma, N-ERD, allergic rhinitis, atopic dermatitis)] were collected at baseline. Patients were prospectively assessed at baseline (week 0) and at 2, 4, 16, 24, and 52 weeks. Assessments encompassed a) the 22-item Sino-Nasal Outcome Test (SNOT22) total (0 to 110) and domain [ear/facial, emotion, function, nasal, and sleep, each computed as the average score of the corresponding items (0 to 5)] scores [18], b) peripheral blood eosinophil counts, c) fractional exhaled nitric oxide (FeNO) measurement, d) Lund-Mackay CT scores (at weeks 0, 16, and 52), e) Asthma Control Test (ACT) scores (at weeks 0, 16, and 52), and f) forced expiratory volume in one second (FEV1) measurements (at weeks 0, 16, and 52). Additionally, throughout the study’s duration, we closely monitored the usage of systemic corticosteroids, the occurrence of polypectomies, effect on smell, as well as the corticosteroid use during the previous year. All lung function tests and FeNO measurements adhered to the guidelines set forth by the European Respiratory Society (ERS) and the American Thoracic Society (ATS) [19, 20].
All data collected were strictly used for clinical purposes, and no personally identifiable information was accessible to clinicians who were not part of the clinical team. All patients received standard of care and signed a written informed consent for the initiation of dupilumab treatment. This study followed the Declaration of Helsinki and met the standards of Good Clinical Practice (GCP). The Ethics Committee at Attikon University Hospital (Protocol number: 312/9-5-2020) was notified and approved the study.
Statistical analysis
Data are presented as numbers and percentages for nominal parameters and as mean and SD for continuous variables. Comparisons between 2 groups were performed with Student t test or Mann-Whitney U test for continuous parametric or non-parametric variables and with chi-square test or Fisher exact test for categorical variables, each when appropriate. All statistical analyses were done with IBM, SPSS-24 (Armonk, NY) and GraphPad Prism 9 was used for the development of the graphs.
Results
Patient’s characteristics
Six patients (50% male) were included in the study. Baseline patient characteristics are presented in Table 1. Specifically, the mean age of the patients was (53.1 ± 7.4) years. Five patients had concomitant mild to moderate asthma (GINA steps 1–4), and three had concomitant N-ERD. The mean age of asthma onset was (31 ± 5) years. In the past year, five patients had received more than three courses of systemic corticosteroids for the management of CRSwNP, and three had undergone more than three polypectomies. Half of the patients were sensitized to aeroallergens and one patient had concomitant allergic rhinitis for which he had undergone 3 years of subcutaneous immunotherapy (SCIT), with moderate efficacy in rhinitis symptoms, before the initiation of dupilumab.
Baseline characteristics of the patients
| Patient characteristics | Dupilumab (n = 6) |
|---|---|
| Age (y), mean (SD) | 53.1 (7.4) |
| Sex, male; n (%) | 3 (50) |
| GINA steps 1–4, n (%) | 5 (83.3) |
| NSAID-exacerbated respiratory disease (N-ERD), n (%) | 3 (50) |
| Use of systemic steroids in the last year, n (%) | 5 (83.3) |
| Number of polypectomies in the last year, n (%) | |
| 3 or more | 3 (50) |
| 2 | 2 (33.3) |
| 1 | 1 (16.7) |
| Sensitization to aeroallergens, n (%) | 3 (50) |
| 3 years SCIT before treatment with dupilumab | 1 (16.7) |
GINA: Global Initiative for Asthma; NSAID: nonsteroidal anti-inflammatory drug; SCIT: subcutaneous immunotherapy
SNOT22
A reduction in SNOT22 was observed as early as 2 weeks after the first administration, with a significant decrease from a mean value of 71.6 ± 16.2 at baseline to 32.5 ± 30 at week 2 (Figure 1). Further reductions were observed at week 4 (18.1 ± 13), and low scores were maintained throughout the study (week 16: 14 ± 10.8, week 24: 13.8 ± 12.1, and week 52: 9.3 ± 4.9). Additionally, between week 4 and week 16, all patients reported an improvement in olfaction.
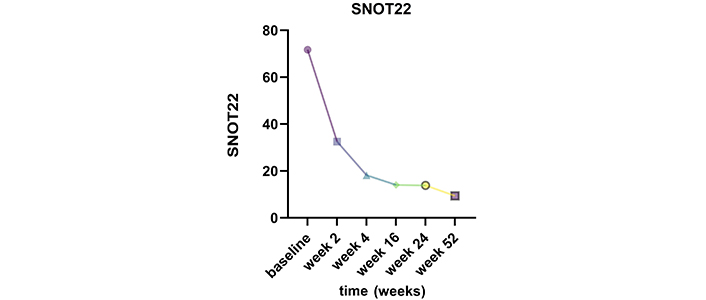
Change in SNOT22 mean scores from baseline to week 2, week 4, week 16, week 24, and week 52 while on dupilumab therapy. SNOT22: 22-item Sino-Nasal Outcome Test
Lund-Mackay CT score
The Lund-Mackay CT score was calculated at baseline (19.3 ± 2) and then at weeks 16 and 52. A reduction was observed at week 16 (mean value 13.3 ± 3.1), which was sustained at week 52 (12.8 ± 3.7) (Figure 2).
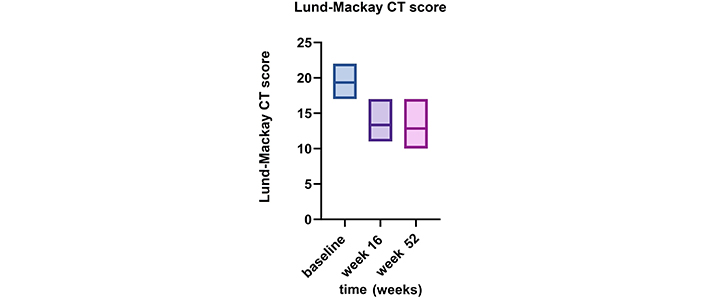
Alteration in Lund-Mackay CT score from baseline to weeks 16 and 52 while undergoing dupilumab therapy
Impact on asthma
The treatment with dupilumab improved all asthma parameters that have been recorded in all 5 asthmatic patients after 52 weeks. The ACT score improved from a mean of 21.1 ± 3 to 25 after 4 months of treatment and remained fully controlled by week 52 (Figure 3A). The lung function also benefited from dupilumab add-on treatment, with an improvement in FEV1 after 16 and 52 weeks [from (3.15 ± 0.76) L to (3.22 ± 0.77) L and (3.22 ± 0.78) L, respectively] (Figure 3B). FeNO also decreased from a mean value of (36.5 ± 16.1) ppb to (18.2 ± 8.7) ppb after 2 weeks and to (13.7 ± 8.3) ppb and (13.4 ± 5.6) ppb after 24 and 52 weeks, respectively (Figure 3C). In addition, all except for one patient were able to step down asthma treatment following GINA guidelines. Out of the three patients initially on GINA step 4 (track 1), two stepped down to GINA step 3, while the remaining two patients initially on GINA step 3 stepped down to steps 1–2 (as-needed low dose ICS/formoterol).
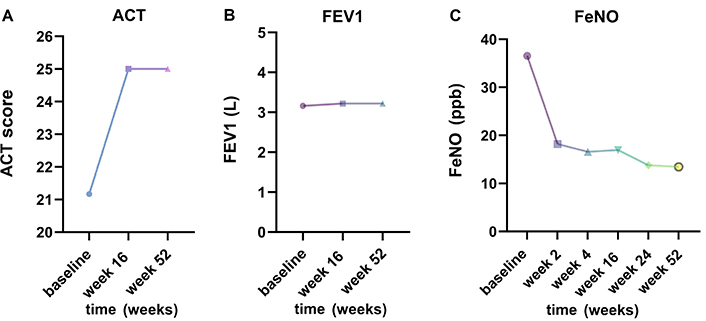
Impact on comorbid asthma. (A) Change in Asthma Control Test (ACT) over baseline to week 16 and 52; (B) demonstration of change in FEV1 from baseline to weeks 16 and 52 while on dupilumab therapy; (C) reduction in FeNO levels (mean values) from baseline to week 2, week 4, week 16, week 24, and 52 after initiation of dupilumab treatment. FEV1: forced expiratory volume in one second; FeNO: fractional exhaled nitric oxide
Impact on eosinophil counts
The mean blood eosinophil counts progressively decreased during the treatment, from 555 cells/μL (400–755) at baseline to 455 cells/μL (191–640) after 52 weeks of treatment. However, we observed an increase in this value from baseline in all patients 2 weeks after the administration of dupilumab [693 cells/μL (530–880)]. The elevation continued until week 16 [637 cells/μL (400–930)] and then gradually decreased [week 24: 530 (340–770) cells/μL] before returning to baseline values by week 52 [455 cells/μL (191–640)] (Figure 4). The elevation of blood eosinophils was asymptomatic and was not associated with any clinical symptoms.
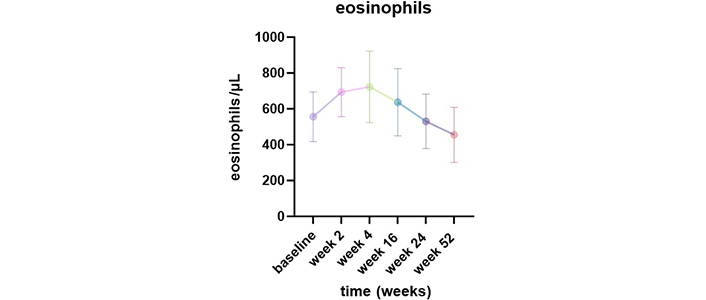
Change in peripheral eosinophils blood counts from baseline to week 52 after dupilumab initiation
Discussion
CRSwNP and asthma often coexist, making the management of these conditions challenging due to their interconnected nature. The inadequately controlled upper airways can contribute to poorly managed lower airways and vice versa [21]. Our study shows that patients who are treated with dupilumab for CRSwNP have an additional effect on their asthma control even if they suffer from mild-moderate and not severe asthma. The results of our study indicate that adding dupilumab to the treatment regimen significantly improved symptoms, airway function, and disease control in both upper and lower airways.
Addressing both components of this coexisting condition is crucial for effective treatment and improved patient outcomes. Dupilumab, a monoclonal antibody targeting type 2 inflammation, has been approved for the treatment of both type 2 severe asthma and CRSwNP [22]. Given that up to 67% of CRSwNP patients have comorbid asthma, and the rates are higher in severe CRSwNP cases, the potential impact of dupilumab on these coexisting conditions is of significant interest [23]. Given the European Medicines Agency’s approval for severe asthma, this recent endorsement for severe CRSwNP now allows for an indirect evaluation of how the biologic affects concurrent mild to moderate asthma [24].
The reduction in SNOT22 scores as early as 2 weeks post-initiation of dupilumab and sustained improvements at subsequent time points demonstrate its prompt and lasting efficacy in managing CRSwNP. Furthermore, the positive impact on Lund-Mackay CT scores affirms the potential of dupilumab in alleviating the radiological burden associated with CRSwNP.
In patients with comorbid asthma, the benefits of dupilumab were also evident. The improvements in ACT scores and pulmonary function, as indicated by increased FEV1, underline the favorable impact of dupilumab on asthma control. In agreement, a recent indirect treatment comparison has shown that dupilumab compared with omalizumab, mepolizumab, and benralizumab was the superior treatment for controlling nasal congestion when asthma comorbidity was present [25]. Additionally, the significant reduction in FeNO levels is indicative of well-controlled airway inflammation in asthma patients receiving dupilumab.
A notable finding was the effect of dupilumab on eosinophil levels in the blood. Although an initial increase in blood eosinophil count was observed after the administration of dupilumab, it gradually decreased and returned to baseline levels by week 52. Importantly, this elevation was asymptomatic and not associated with any clinical symptoms, suggesting that it may be a transient and benign response in accordance with other studies [26].
The small sample size, the single-center nature of the study, the lack of a control group as well as potential selection bias due to recruitment in an allergy center of a tertiary hospital are some limitations of our study. In addition, the fact that some validated patients’ related outcomes and assessment tools such as nasal polyp score, Sniffin’ Sticks for loss of smell assessment and Visual Analogue Scale (VAS) to assess the improvement in smell were not used in this study are another limitation. In addition, other tools as SNOT22, Lund-Mackay CT score, and ACT score, have their own limitations, including subjectivity, recall bias, and dependence on patient reporting constitutes additional limitations.
Despite the small group of patients and the above-mentioned additional limitations, our study contributes valuable insights into real-world applicability as data in the literature remain scarce regarding real-world evidence for the effectiveness of dupilumab in CRSwNP. Larger registries and other real-life studies are on the way to support our data [27]. In addition, the early assessment of efficacy as soon as week 2 and at many time points along with the assessment of asthma-related parameters constitute some of the strengths of the present study. The results contribute valuable insights regarding the effect on mild to moderate asthma and thus in an earlier asthma trajectory and the potential disease-modifying effect. Besides, our findings align with larger randomized controlled studies, affirming the positive impact of dupilumab in effectively managing both upper and lower airway symptoms in this population [21].
In conclusion, the evolving understanding of the shared inflammatory pathways in nasal polyps and asthma has broadened the eligibility for biologic treatments. Our study contributes to the growing body of evidence supporting the efficacy and benefits of dupilumab, in patients with nasal polyps and comorbid mild to moderate asthma. Data remain scarce regarding the effect a biologic agent like dupilumab could have on modifying the disease course, especially in the context of comorbid mild to moderate asthma. Early intervention with biologics in this patient population holds the promise of improved disease control, potentially modifying the course of both upper and lower airway diseases. Further research and long-term follow-up studies in large populations are needed to explore the cost-effectiveness ratio of biologic treatment in different subgroups of patients to optimize management in terms of a personalized treatment strategy.
Abbreviations
| ACT: | Asthma Control Test |
| CRSwNP: | chronic rhinosinusitis with nasal polyps |
| FeNO: | fractional exhaled nitric oxide |
| FEV1: | forced expiratory volume in one second |
| GINA: | Global Initiative for Asthma |
| IL4: | interleukin 4 |
| N-ERD: | nonsteroidal anti-inflammatory drug-exacerbated respiratory disease |
| SNOT22: | 22-item Sino-Nasal Outcome Test |
Declarations
Author contributions
NP and MM: Conceptualization, Validation, Writing—original draft, Writing—review & editing, Visualization. MM: Supervision. Both authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study followed the Declaration of Helsinki and met the standards of Good Clinical Practice (GCP). The Ethics Committee at Attikon University Hospital (Protocol number: 312/9-5-2020) was notified and approved the study.
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2024.